Developmental Expression of HSP60 and HSP10 in the Coilia nasus Testis during Upstream Spawning Migration
Abstract
:1. Introduction
2. Results
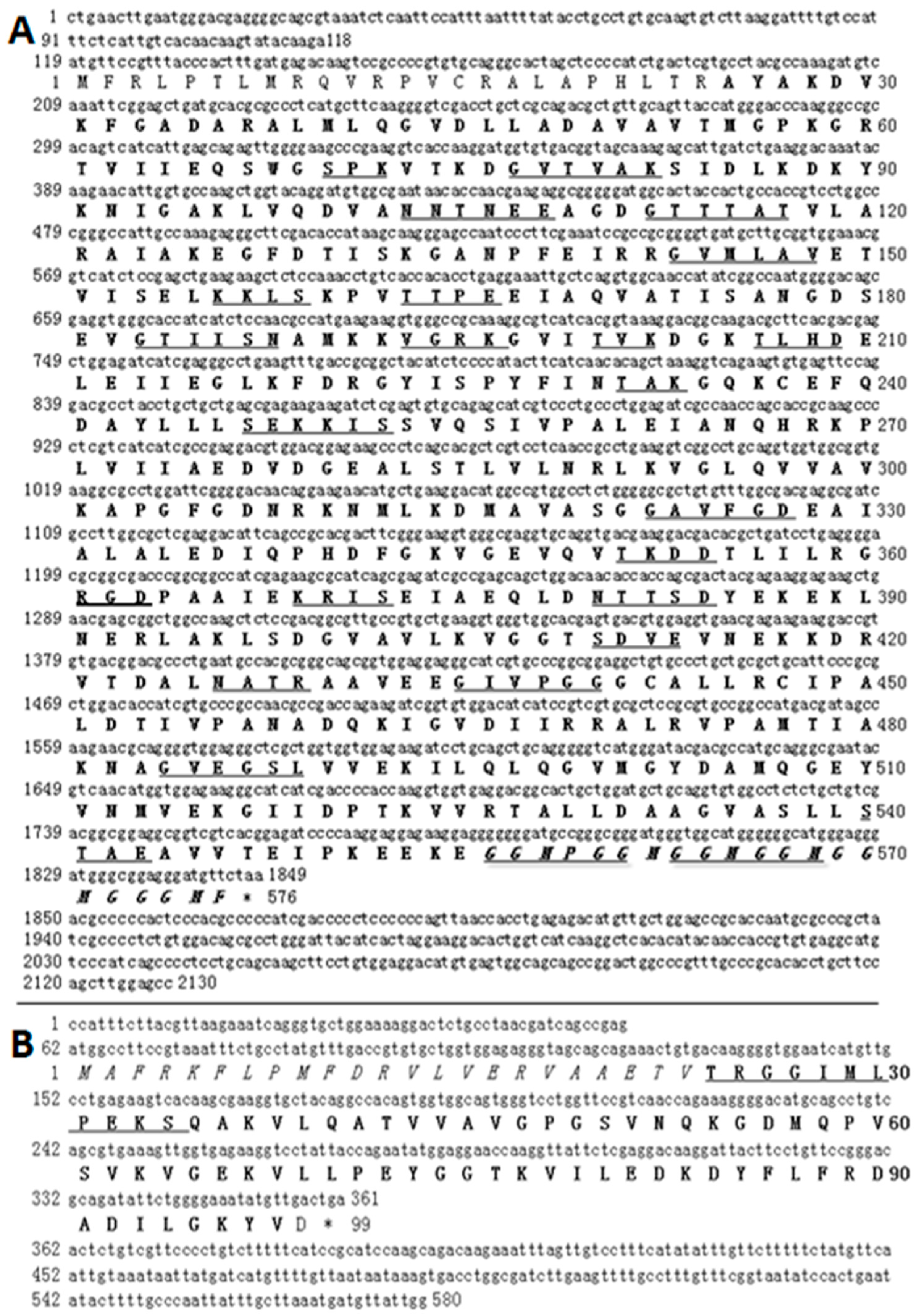
2.1. Characteristics of HSP60 and HSP10 cDNA
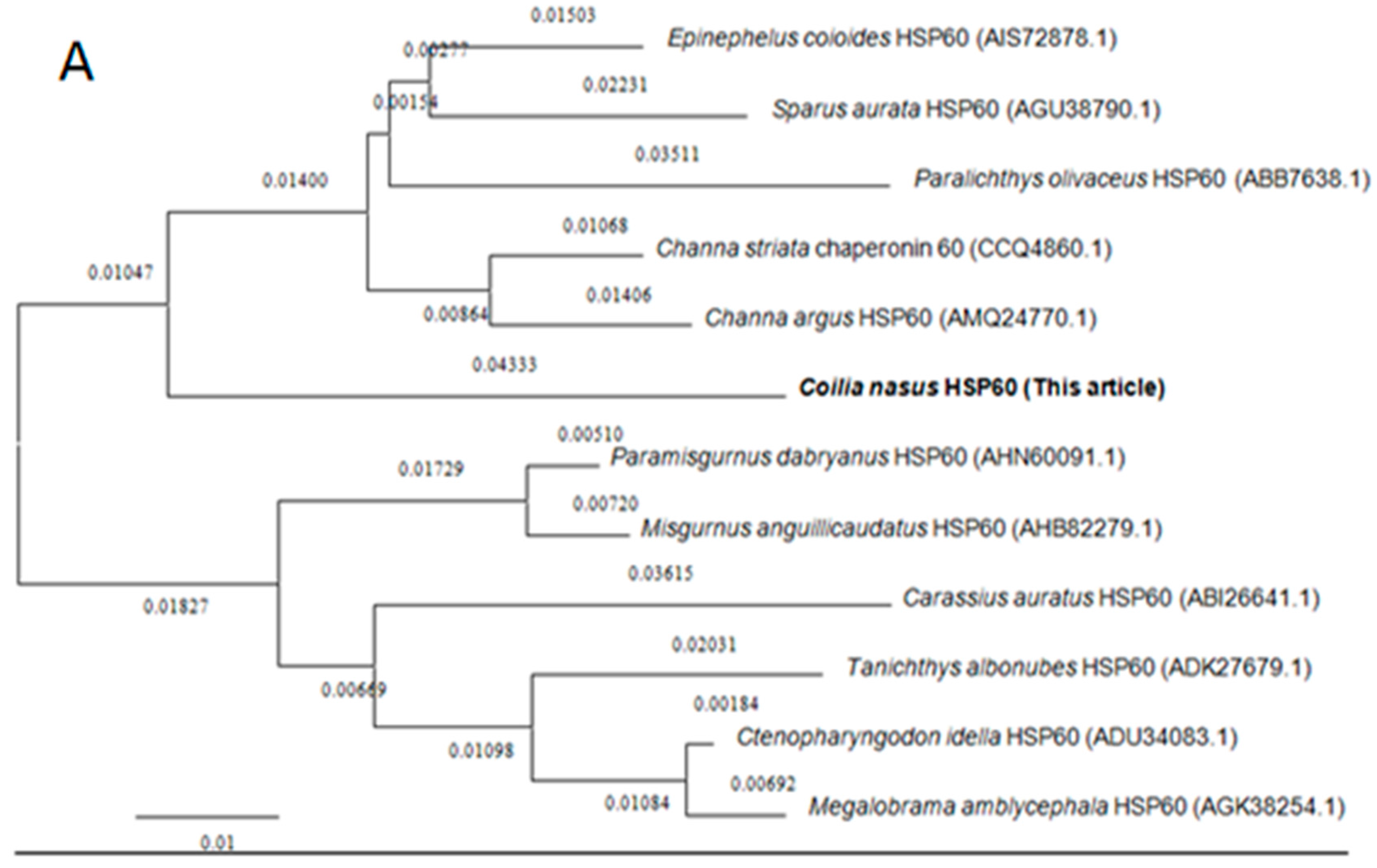
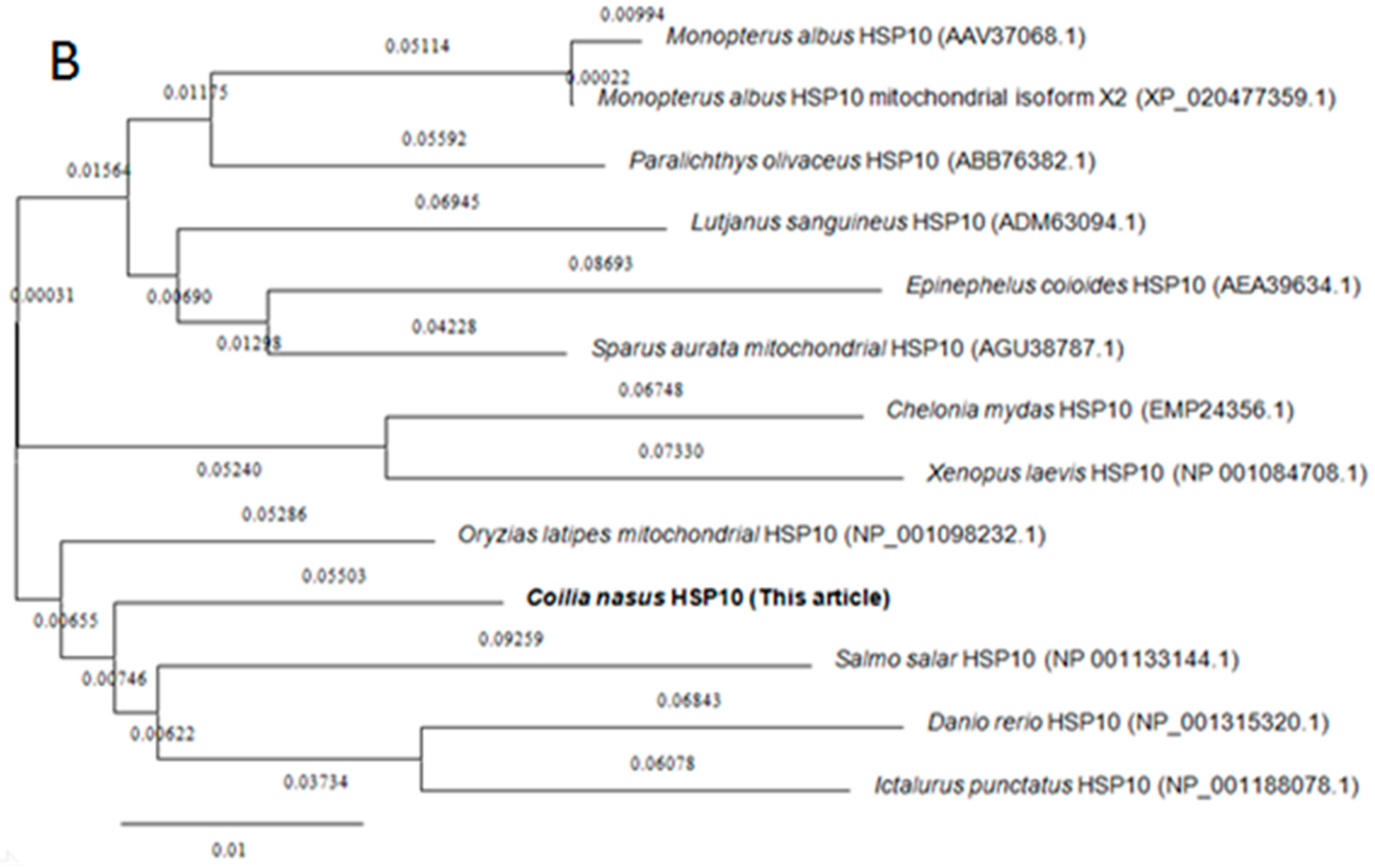
2.2. Homology and Phylogenetic Analyses of HSP60 and HSP10
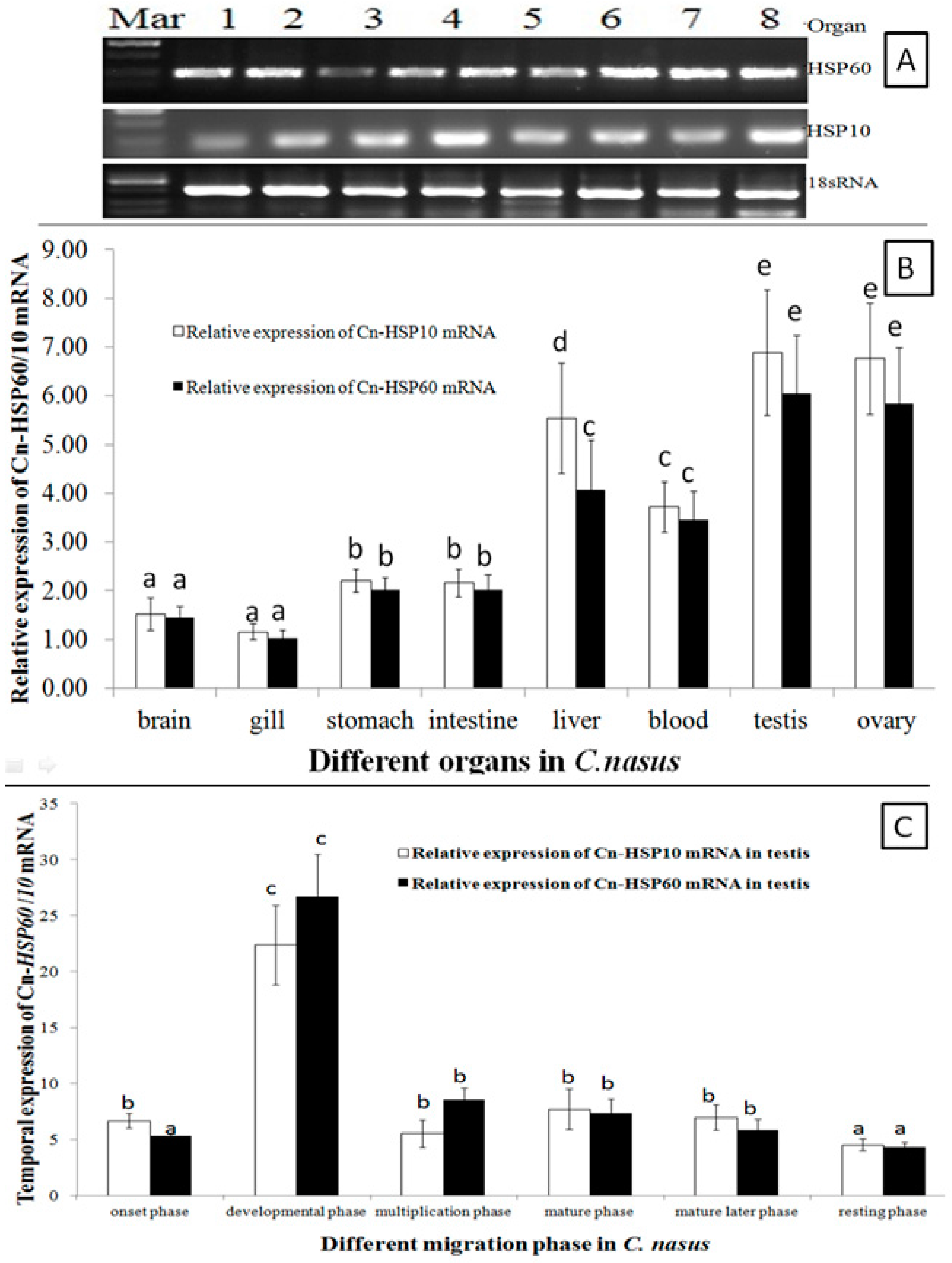
2.3. HSP10and HSP60 mRNA Expression Patterns
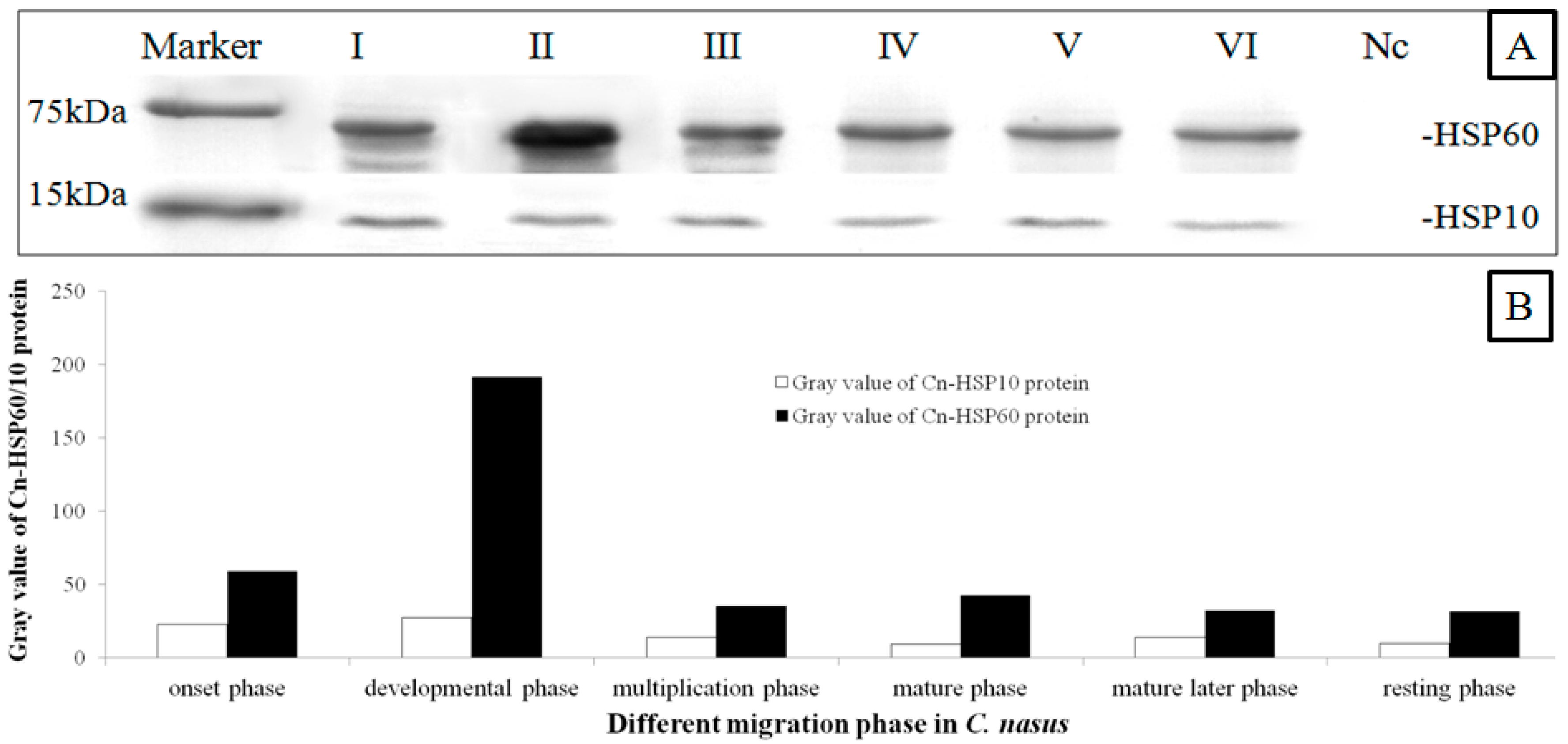
2.4. Western Blotting Results
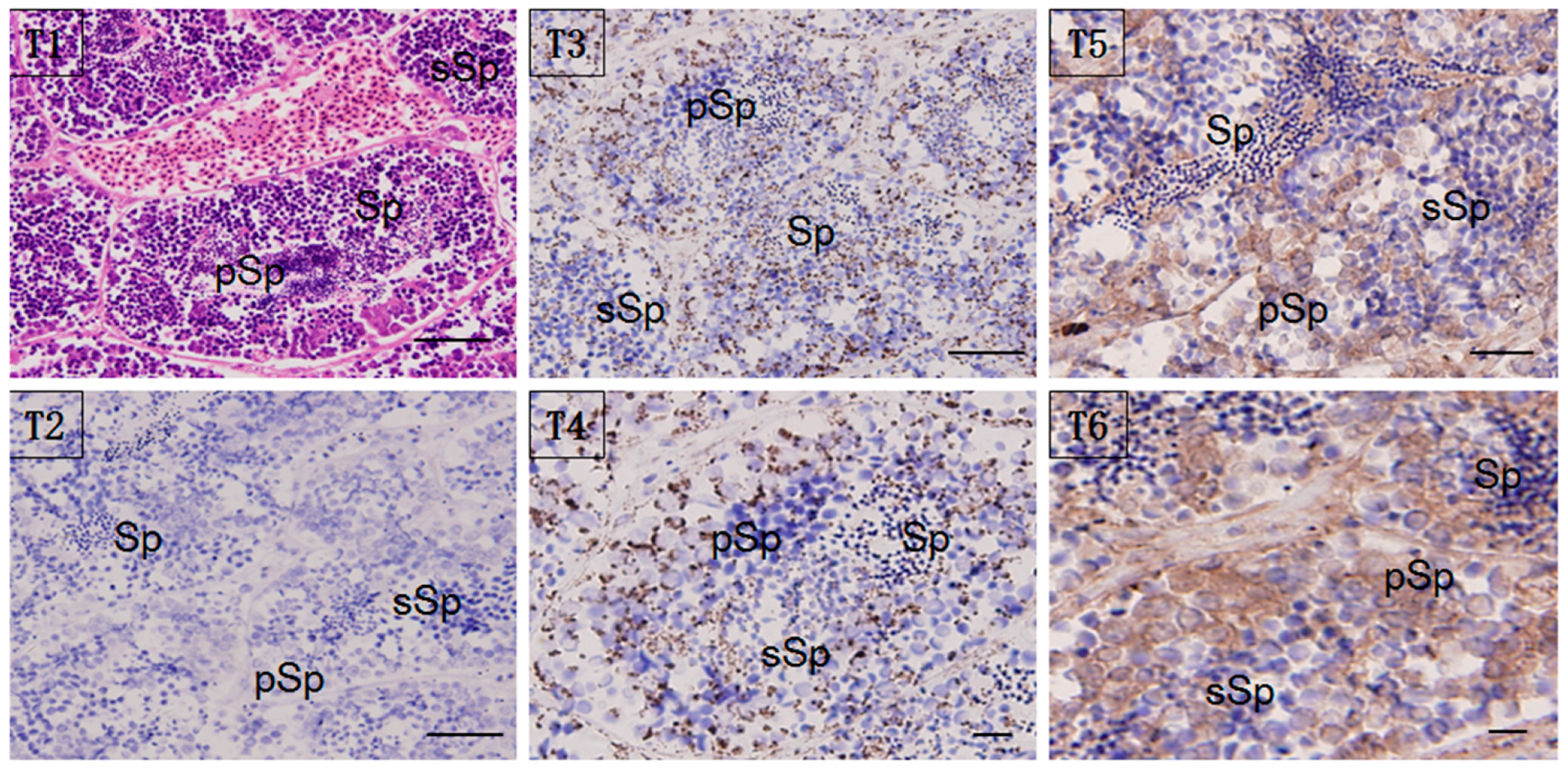
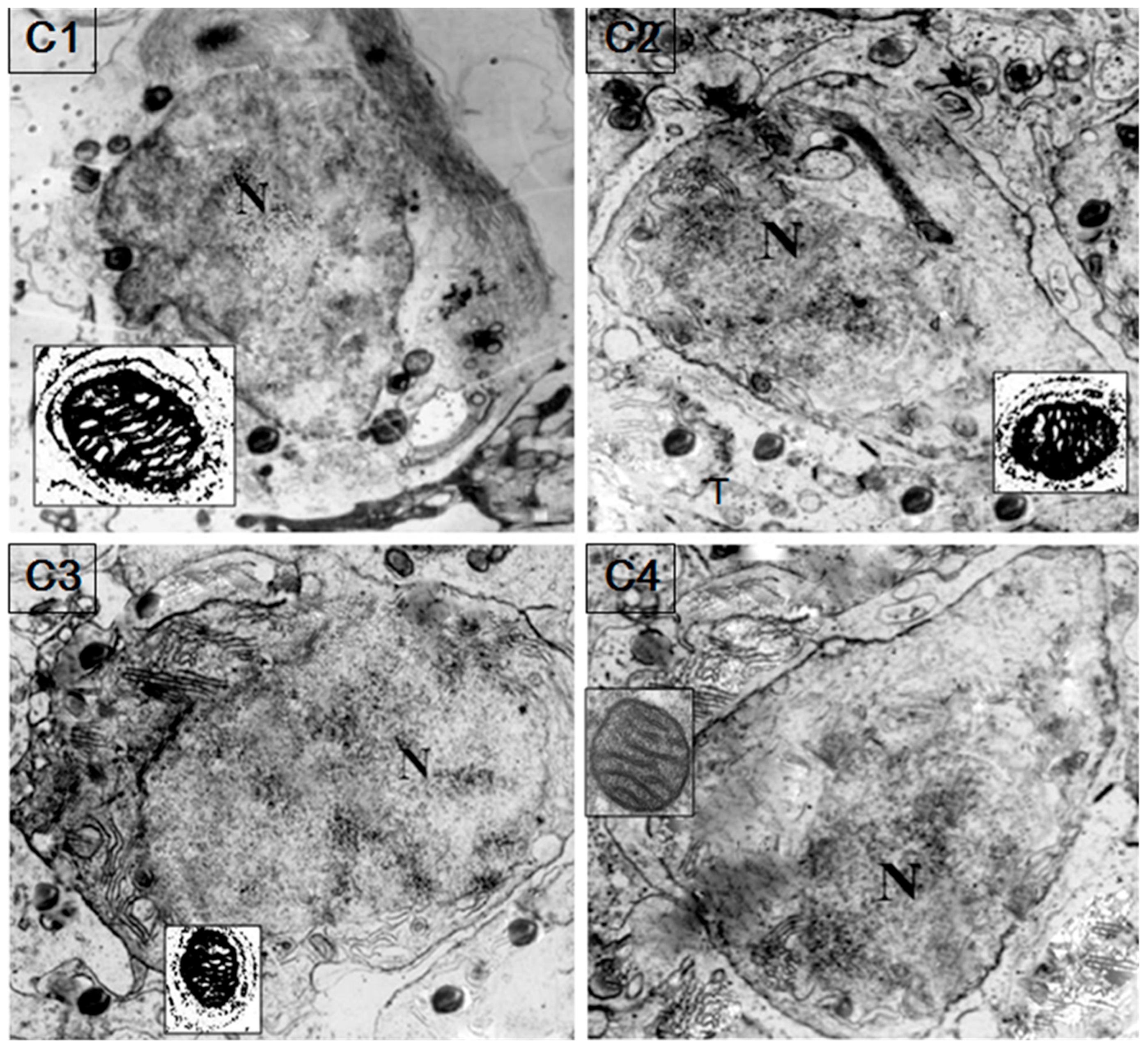
2.5. Localization of HSP60/HSP10
3. Discussion
4. Material and Methods
4.1. Fish Sampling and Organ Collection
4.2. Nucleic Acid Preparation
4.3. Gene Cloning of HSP60 and HSP10
4.4. Analysis for Expression Patterns
4.5. Western Blotting
4.6. Immunohistochemistry (IHC)
4.7. Immunoelectron Microscopy (IM)
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ando, S.; Hatano, M. Metabolic pathways of carotenoids in chum salmon Oncorhynchus keta during spawning migration. Comp. Biochem. Physiol. Part B: Comp. Biochem. 1987, 87, 411–416. [Google Scholar] [CrossRef]
- Charles, S.; Bravo De La Parra, R.; Mallet, J.P.; Persat, H.; Auger, P. Annual spawning migrations in modelling brown trout population dynamics inside an arborescent river network. Ecol. Model. 2000, 133, 15–31. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Liu, H.; Shen, X.-Q. Life history of Coilia nasus from the Yellow Sea inferred from otolith Sr: Ca ratios. Environ. Biol. Fishes 2012, 95, 503–508. [Google Scholar] [CrossRef]
- Liu, K.; Duan, J.-R.; Xu, D.-P.; Zhang, M.-Y.; Fang, D.-A.; Shi, W.-G. Present situation of Coilia nasus population features and yield in Yangtze river estuary waters in fishing season. Shengtaixue Zazhi 2012, 31, 3138–3143. [Google Scholar]
- Li, W.X.; Song, R.; Wu, S.G.; Zou, H.; Nie, P.; Wang, G.T. Seasonal occurrence of helminths in the anadromous fish Coilia nasus (engraulidae): Parasite indicators of fish migratory movements. J. Parasitol. 2010, 97, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.-Z.; Yokouchi, K.; Yu, X.; Cao, L.; Kuroki, M.; Otake, T.; Tsukamoto, K. The migratory history of anadromous and non-anadromous tapertail anchovy Coilia nasus in the Yangtze river estuary revealed by the otolith Sr: Ca ratio. Environ. Biol. Fishes 2012, 95, 481–490. [Google Scholar] [CrossRef]
- Du, F.; Xu, G.; Nie, Z.; Xu, P.; Gu, R. Transcriptome analysis gene expression in the liver of Coilia nasus during the stress response. BMC Genom. 2014, 15, 558. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Y.; Tang, W.; Yang, J.; Guo, H.; Zhu, G.; Li, H. Population structure of Coilia nasus in the Yangtze river revealed by insertion of short interspersed elements. Biochem. Syst. Ecol. 2014, 54, 103–112. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Duan, J.-R.; Liu, K.; Xu, D.-P.; Zhang, M.-Y.; Fang, D.-A.; Xu, P. Testes transcriptome profiles of the anadromous fish Coilia nasus during the onset of spermatogenesis. Mar. Genom. 2015, 24, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Song, L.; Weng, Z.; Liu, S.; Liu, Z. HSP90, HSP60 and sHSP families of heat shock protein genes in channel catfish and their expression after bacterial infections. Fish Shellfish Immunol. 2015, 44, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.M.; Schulze, A.D.; Ginther, N.; Li, S.; Patterson, D.A.; Farrell, A.P.; Hinch, S.G. Salmon spawning migration: Metabolic shifts and environmental triggers. Comp. Biochem. Physiol. Part D: Genom. Proteom. 2009, 4, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Koll, H.; Guiard, B.; Rassow, J.; Ostermann, J.; Horwich, A.L.; Neupert, W.; Hartl, F.U. Antifolding activity of HSP60 couples protein import into the mitochondrial matrix with export to the intermembrane space. Cell 1992, 68, 1163. [Google Scholar] [CrossRef]
- Zeilstraryalls, J.; Fayet, O.; Georgopoulos, C. The universally conserved groE (HSP60) chaperonins. Annu. Rev. Microbiol. 1991, 45, 301. [Google Scholar] [CrossRef] [PubMed]
- Paranko, J.; Seitz, J.; Meinhardt, A. Developmental expression of heat shock protein 60 (HSP60) in the rat testis and ovary. Differentiation 1996, 60, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Nogales, A.; Nederlof, M.; Benedito-Palos, L.; Ballester-Lozano, G.F.; Folkedal, O.; Olsen, R.E.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Metabolic and transcriptional responses of gilthead sea bream (Sparus aurata L.) to environmental stress: New insights in fish mitochondrial phenotyping. Gen. Comp. Endocrinol. 2014, 205, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.T.; Naylor, D.J.; Høj, P.B.; Clark, M.S.; Hoogenraad, N.J. The role of molecular chaperones in mitochondrial protein import and folding. In International Review of Cytology; Kwang, W.J., Ed.; Academic Press: New York, NY, USA, 1997; Volume 174, pp. 127–193. [Google Scholar]
- Savina, M.V.; Gamper, N.L. Respiration and adenine nucleotides of baltic lamprey (Lampetra fluviatilis L.) hepatocytes during spawning migration. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 1998, 120, 375–383. [Google Scholar] [CrossRef]
- Khalil, A.A.; Kabapy, N.F.; Deraz, S.F.; Smith, C. Heat shock proteins in oncology: Diagnostic biomarkers or therapeutic targets? Biochim. Biophys. Acta (BBA) - Rev. Cancer 2011, 1816, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Banh, S.; Wiens, L.; Sotiri, E.; Treberg, J.R. Mitochondrial reactive oxygen species production by fish muscle mitochondria: Potential role in acute heat-induced oxidative stress. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2016, 191, 99–107. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, K.M. Mitochondrial biogenesis in cold-bodied fishes. J. Exp. Biol. 2011, 214, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Deswal, R. Low temperature stress modulated secretome analysis and purification of antifreeze protein from Hippophae rhamnoides, a himalayan wonder plant. J. Proteome Res. 2012, 11, 2684–2696. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Horwich, A.L.; Sigler, P.B. The crystal structure of the asymmetric groEL-groES-(ADP)7 chaperonin complex. Nature 1997, 388, 741–750. [Google Scholar] [PubMed]
- Gupta, R.S. Evolution of the chaperonin families (HSP60, HSP10 and TCP-1) of proteins and the origin of eukaryotic cells. Mol. Microbiol. 1995, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Sun, L.; Liu, S.; Zhang, L.; Ru, X.; Zhao, Y.; Yang, H. Molecular cloning of heat shock protein 10 (HSP10) and 60 (HSP60) cDNAs and their expression analysis under thermal stress in the sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2014, 171, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.C.; Chang, H.C.; Roeben, A.; Wischnewski, D.; Wischnewski, N.; Kerner, M.J.; Hartl, F.U.; Hayer-Hartl, M. Structural features of the groEL-groES nano-cage required for rapid folding of encapsulated protein. Cell 2006, 125, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Silva, R.; Rössle, S.C.; Bisch, P.M.; Rondinelli, E.; Ürményi, T.P. Gene characterization and predicted protein structure of the mitochondrial chaperonin HSP10 of Trypanosoma cruzi. Gene 2005, 349, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Todgham, A.; Ackerman, P.; Bibeau, M.; Nakano, K.; Schulte, P.; Iwama, G. Heat Shock Protein Genes and Their Functional Significance in Fish. Gene 2002, 295, 173–183. [Google Scholar]
- Neuer, A.; Spandorfer, S.D.; Giraldo, P.; Jeremias, J.; Dieterle, S.; Korneeva, I.; Liu, H.C.; Rosenwaks, Z.; Witkin, S.S. Heat shock protein expression during gametogenesis and embryogenesis. Infect. Dis. Obstet. Gynecol. 1999, 7, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, A.; Wettstein, R.; Benavente, R. Stage-specific gene expression during rat spermatogenesis: Application of the mRNA differential display method. Int. J. Dev. Biol. 1996, 40, 385–388. [Google Scholar] [PubMed]
- Wegele, H.; Muller, L.; Buchner, J. HSP70 and HSP90 - a relay team for protein folding. Rev. Physiol. Biochem. Pharmacol. 2004, 151, 1–44. [Google Scholar] [PubMed]
- Mollapour, M.; Neckers, L. Post-translational modifications of HSP90 and their contributions to chaperone regulation. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 2012, 1823, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Hatano, M.; Zama, K. Deterioration of chum salmon muscle during spawning migration—VI. Changes in serum protease inhibitory activity d during spawning migration of chum salmon (Oncorhynchus keta). Comp. Biochem. Physiol. Part B: Comp. Biochem. 1985, 82, 111–115. [Google Scholar] [CrossRef]
- Mommsen, T.P. Salmon spawning migration and muscle protein metabolism: The august krogh principle at work. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2004, 139, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Behrens, J.W.; Præbel, K.; Steffensen, J.F. Swimming energetics of the barents sea capelin (Mallotus villosus) during the spawning migration period. J. Exp. Mar. Biol. Ecol. 2006, 331, 208–216. [Google Scholar] [CrossRef]
- Iwama, G.K.; Thomas, P.T.; Forsyth, R.B.; Vijayan, M.M. Heat shock protein expression in fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.-J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Chalmel, F.; Rolland, A.D.; Niederhauser-Wiederkehr, C.; Chung, S.S.W.; Demougin, P.; Gattiker, A.; Moore, J.; Patard, J.-J.; Wolgemuth, D.J.; Jégou, B.; et al. The conserved transcriptome in human and rodent male gametogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 8346–8351. [Google Scholar] [CrossRef] [PubMed]
- Fishelson, L.; Delarea, Y.; Gon, O. Testis structure, spermatogenesis, spermatocytogenesis, and sperm structure in cardinal fish (apogonidae, perciformes). Brain Struct. Funct. 2006, 211, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.A.; Duan, J.R.; Zhou, Y.F.; Zhang, M.Y.; Xu, D.P.; Liu, K.; Xu, P. Molecular characteristic, protein distribution and potential regulation of HSP90AA1 in the anadromous fish Coilia nasus. Genes 2016, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.-B.; Zhang, C.-X.; Xu, G.-C.; Gu, R.-B.; Xu, P. Development of gonads in Coilia nasus from the yangtze river and artificial pond. Chin. J. Zool. 2009, 44, 111–117. [Google Scholar]
- Kaiser, U.B.; Zhao, D.; Cardona, G.R.; Chin, W.W. Isolation and characterization of cDNAsnas encoding the rat pituitary gonadotropin-releasing hormone receptor. Biochem. Biophys. Res. Commun. 1993, 189, 1645–1652. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped blast and psi-blast: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G. Heat shock protein 70 (HSP70): Membrane location, export and immunological relevance. Methods 2007, 43, 229–237. [Google Scholar] [CrossRef] [PubMed]
| Primer Name F—Forward/R—Reverse | DNA-Sequence 5′-3′ | Annealing Temperature (°C) | Fragment Size (bp) |
|---|---|---|---|
| Gene-specific Primer pairs for RACE (GP) | |||
| GpHSP60-5′ | 5′-CTCCACCCCTGCGTTCTTGGCTATCG-3′ | 74.4 | - |
| GpHSP60-3′ | 5′-ATGACGATAGCCAAGAACGCAGGGGT-3′ | 65.5 | - |
| GpHSP10-5′ | 5′-CCATTTCTTACGTTAAGAAATCAGG-3′ | 69.3 | - |
| GpHSP10-3′ | 5′-TTTCATCCGCATCCAAGCAGACAAGA-3′ | 70.8 | - |
| Primers for RT-qPCR | |||
| HSP60-F | 5′-GAATAACACCAACGAAGAGGCGG-3′ | 73.3 | 384 |
| HSP60-R | 5′-TTGATGAAGTATGGGGAGATGTAGCC-3′ | 64.9 | |
| HSP10-F | 5′-GTGGCAGTGGGTCCTGGTTCCGTC-3′ | 62.7 | 222 |
| HSP10-R | 5′-TCTTGTCTGCTTGGATGCGGATGA-3′ | 70.8 | |
| 18sRNA primers | |||
| 18sRNA-R | 5′-TGATTGGGACTGGGGATTGAA-3′ | 59.2 | 232 |
| 18sRNA-F | 5′-TAGCGACGGGCGGTGTGT-3′ | 62.4 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, D.-A.; Zhou, Y.-F.; Zhang, M.-Y.; Xu, D.-P.; Liu, K.; Duan, J.-R. Developmental Expression of HSP60 and HSP10 in the Coilia nasus Testis during Upstream Spawning Migration. Genes 2017, 8, 189. https://doi.org/10.3390/genes8070189
Fang D-A, Zhou Y-F, Zhang M-Y, Xu D-P, Liu K, Duan J-R. Developmental Expression of HSP60 and HSP10 in the Coilia nasus Testis during Upstream Spawning Migration. Genes. 2017; 8(7):189. https://doi.org/10.3390/genes8070189
Chicago/Turabian StyleFang, Di-An, Yan-Feng Zhou, Min-Ying Zhang, Dong-Po Xu, Kai Liu, and Jin-Rong Duan. 2017. "Developmental Expression of HSP60 and HSP10 in the Coilia nasus Testis during Upstream Spawning Migration" Genes 8, no. 7: 189. https://doi.org/10.3390/genes8070189
APA StyleFang, D. -A., Zhou, Y. -F., Zhang, M. -Y., Xu, D. -P., Liu, K., & Duan, J. -R. (2017). Developmental Expression of HSP60 and HSP10 in the Coilia nasus Testis during Upstream Spawning Migration. Genes, 8(7), 189. https://doi.org/10.3390/genes8070189






