Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward
Abstract
:1. Introduction: A Brief Summary of Cystic Fibrosis Today
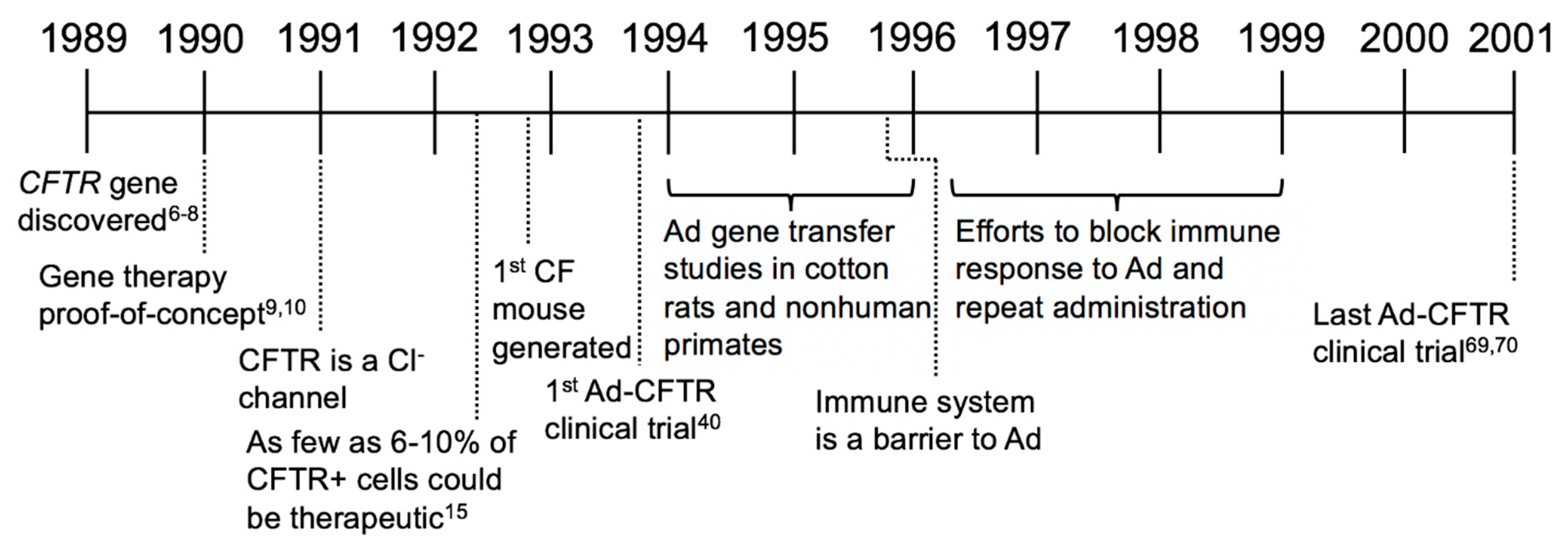
2. Establishing Benchmarks of Success and Adenovirus-Based Gene Therapy Trials (1989–2001)
3. Alternatives to Ad (1995–2008)
3.1. Adeno-Associated Virus
3.2. Nonviral Vectors
3.3. Retroviral and Lentiviral Vectors
3.4. Addressing Barriers to Gene Transfer
4. New Models for Preclinical Studies (2008–2018)
Gene Editing and the Era of Precision Medicine
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Condren, M.E.; Bradshaw, M.D. Ivacaftor: A novel gene-based therapeutic approach for cystic fibrosis. J. Pediatr. Pharmacol. Ther. 2013, 18, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, M.A.; Jain, M. Tezacaftor for the treatment of cystic fibrosis. Expert Rev. Respir. Med. 2018, 12, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Marson, F.A.L.; Bertuzzo, C.S.; Ribeiro, J.D. Classification of CFTR mutation classes. Lancet Respir. Med. 2016, 4, e37–e38. [Google Scholar] [CrossRef]
- Welsh, M.J.; Smith, A.E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993, 73, 1251–1254. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Konstan, M.W. Outcome measures for clinical trials assessing treatment of cystic fibrosis lung disease. Clin. Investig. 2012, 2, 163–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Kerem, B.; Rommens, J.M.; Buchanan, J.A.; Markiewicz, D.; Cox, T.K.; Chakravarti, A.; Buchwald, M.; Tsui, L.C. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Rommens, J.M.; Iannuzzi, M.C.; Kerem, B.; Drumm, M.L.; Melmer, G.; Dean, M.; Rozmahel, R.; Cole, J.L.; Kennedy, D.; Hidaka, N.; et al. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science 1989, 245, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Drumm, M.L.; Pope, H.A.; Cliff, W.H.; Rommens, J.M.; Marvin, S.A.; Tsui, L.C.; Collins, F.S.; Frizzell, R.A.; Wilson, J.M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell 1990, 62, 1227–1233. [Google Scholar] [CrossRef]
- Rich, D.P.; Anderson, M.P.; Gregory, R.J.; Cheng, S.H.; Paul, S.; Jefferson, D.M.; McCann, J.D.; Klinger, K.W.; Smith, A.E.; Welsh, M.J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 1990, 347, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Flotte, T.R.; Solow, R.; Owens, R.A.; Afione, S.; Zeitlin, P.L.; Carter, B.J. Gene expression from adeno-associated virus vectors in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 1992, 7, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.A.; Yoshimura, K.; Trapnell, B.C.; Yoneyama, K.; Rosenthal, E.R.; Dalemans, W.; Fukayama, M.; Bargon, J.; Stier, L.E.; Stratford-Perricaudet, L.; et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell 1992, 68, 143–155. [Google Scholar] [CrossRef]
- Yoshimura, K.; Rosenfeld, M.A.; Nakamura, H.; Scherer, E.M.; Pavirani, A.; Lecocq, J.P.; Crystal, R.G. Expression of the human cystic fibrosis transmembrane conductance regulator gene in the mouse lung after in vivo intratracheal plasmid-mediated gene transfer. Nucleic Acids Res. 1992, 20, 3233–3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, J.C.; Johnson, L.G.; Stutts, M.J.; Sarkadi, B.; Yankaskas, J.R.; Swanstrom, R.; Boucher, R.C. Correction of the apical membrane chloride permeability defect in polarized cystic fibrosis airway epithelia following retroviral-mediated gene transfer. Hum. Gene Ther. 1992, 3, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.G.; Olsen, J.C.; Sarkadi, B.; Moore, K.L.; Swanstrom, R.; Boucher, R.C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 1992, 2, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Schoumacher, R.A.; Ram, J.; Iannuzzi, M.C.; Bradbury, N.A.; Wallace, R.W.; Hon, C.T.; Kelly, D.R.; Schmid, S.M.; Gelder, F.B.; Rado, T.A.; et al. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc. Natl. Acad. Sci. USA 1990, 87, 4012–4016. [Google Scholar] [CrossRef] [PubMed]
- Cozens, A.L.; Yezzi, M.J.; Kunzelmann, K.; Ohrui, T.; Chin, L.; Eng, K.; Finkbeiner, W.E.; Widdicombe, J.H.; Gruenert, D.C. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994, 10, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Schwiebert, E.M.; Zeitlin, P.L.; Kuo, W.L.; Stanton, B.A.; Gruenert, D.C. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the delta F508 CFTR mutation. Am. J. Respir. Cell Mol. Biol. 1993, 8, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, P.L.; Lu, L.; Rhim, J.; Cutting, G.; Stetten, G.; Kieffer, K.A.; Craig, R.; Guggino, W.B. A cystic fibrosis bronchial epithelial cell line: Immortalization by adeno-12-SV40 infection. Am. J. Respir. Cell Mol. Biol. 1991, 4, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Dorin, J.R.; Dickinson, P.; Emslie, E.; Clarke, A.R.; Dobbie, L.; Hooper, M.L.; Halford, S.; Wainwright, B.J.; Porteous, D.J. Successful targeting of the mouse cystic fibrosis transmembrane conductance regulator gene in embryonal stem cells. Transgenic Res. 1992, 1, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Dorin, J.R.; Dickinson, P.; Alton, E.W.; Smith, S.N.; Geddes, D.M.; Stevenson, B.J.; Kimber, W.L.; Fleming, S.; Clarke, A.R.; Hooper, M.L.; et al. Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature 1992, 359, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dorin, J.R.; Porteous, D.J. Cystic fibrosis mice with disease-related changes in lung and reproductive tract. Lancet 1992, 340, 984. [Google Scholar] [CrossRef]
- Ratcliff, R.; Evans, M.J.; Doran, J.; Wainwright, B.J.; Williamson, R.; Colledge, W.H. Disruption of the cystic fibrosis transmembrane conductance regulator gene in embryonic stem cells by gene targeting. Transgenic Res. 1992, 1, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Snouwaert, J.N.; Brigman, K.K.; Latour, A.M.; Malouf, N.N.; Boucher, R.C.; Smithies, O.; Koller, B.H. An animal model for cystic fibrosis made by gene targeting. Science 1992, 257, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Colledge, W.H.; Ratcliff, R.; Foster, D.; Williamson, R.; Evans, M.J. Cystic fibrosis mouse with intestinal obstruction. Lancet 1992, 340, 680. [Google Scholar] [CrossRef]
- Dorin, J.R.; Farley, R.; Webb, S.; Smith, S.N.; Farini, E.; Delaney, S.J.; Wainwright, B.J.; Alton, E.W.; Porteous, D.J. A demonstration using mouse models that successful gene therapy for cystic fibrosis requires only partial gene correction. Gene Ther. 1996, 3, 797–801. [Google Scholar] [PubMed]
- Mastrangeli, A.; Danel, C.; Rosenfeld, M.A.; Stratford-Perricaudet, L.; Perricaudet, M.; Pavirani, A.; Lecocq, J.P.; Crystal, R.G. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J. Clin. Investig. 1993, 91, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pilewski, J.M.; Engelhardt, J.F.; Bavaria, J.E.; Kaiser, L.R.; Wilson, J.M.; Albelda, S.M. Adenovirus-mediated gene transfer to human bronchial submucosal glands using xenografts. Am. J. Physiol. 1995, 268, L657–L665. [Google Scholar] [CrossRef] [PubMed]
- Zabner, J.; Petersen, D.M.; Puga, A.P.; Graham, S.M.; Couture, L.A.; Keyes, L.D.; Lukason, M.J.; St George, J.A.; Gregory, R.J.; Smith, A.E.; et al. Safety and efficacy of repetitive adenovirus-mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat. Genet. 1994, 6, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yei, S.; Mittereder, N.; Tang, K.; O’Sullivan, C.; Trapnell, B.C. Adenovirus-mediated gene transfer for cystic fibrosis: Quantitative evaluation of repeated in vivo vector administration to the lung. Gene Ther. 1994, 1, 192–200. [Google Scholar] [PubMed]
- St George, J.A.; Pennington, S.E.; Kaplan, J.M.; Peterson, P.A.; Kleine, L.J.; Smith, A.E.; Wadsworth, S.C. Biological response of nonhuman primates to long-term repeated lung exposure to Ad2/CFTR-2. Gene Ther. 1996, 3, 103–116. [Google Scholar] [PubMed]
- Kaplan, J.M.; St George, J.A.; Pennington, S.E.; Keyes, L.D.; Johnson, R.P.; Wadsworth, S.C.; Smith, A.E. Humoral and cellular immune responses of nonhuman primates to long-term repeated lung exposure to Ad2/CFTR-2. Gene Ther. 1996, 3, 117–127. [Google Scholar] [PubMed]
- Mittereder, N.; Yei, S.; Bachurski, C.; Cuppoletti, J.; Whitsett, J.A.; Tolstoshev, P.; Trapnell, B.C. Evaluation of the efficacy and safety of in vitro, adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA. Hum. Gene Ther. 1994, 5, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Yei, S.; Mittereder, N.; Wert, S.; Whitsett, J.A.; Wilmott, R.W.; Trapnell, B.C. In vivo evaluation of the safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lung. Hum. Gene Ther. 1994, 5, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nunes, F.A.; Berencsi, K.; Gonczol, E.; Engelhardt, J.F.; Wilson, J.M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat. Genet. 1994, 7, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, J.F.; Litzky, L.; Wilson, J.M. Prolonged transgene expression in cotton rat lung with recombinant adenoviruses defective in E2a. Hum. Gene Ther. 1994, 5, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, J.F.; Ye, X.; Doranz, B.; Wilson, J.M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc. Natl. Acad. Sci. USA 1994, 91, 6196–6200. [Google Scholar] [CrossRef] [PubMed]
- Bout, A.; Imler, J.L.; Schultz, H.; Perricaudet, M.; Zurcher, C.; Herbrink, P.; Valerio, D.; Pavirani, A. In vivo adenovirus-mediated transfer of human CFTR cDNA to rhesus monkey airway epithelium: Efficacy, toxicity and safety. Gene Ther. 1994, 1, 385–394. [Google Scholar] [PubMed]
- McDonald, R.J.; Lukason, M.J.; Raabe, O.G.; Canfield, D.R.; Burr, E.A.; Kaplan, J.M.; Wadsworth, S.C.; St George, J.A. Safety of airway gene transfer with Ad2/CFTR2: Aerosol administration in the nonhuman primate. Hum. Gene Ther. 1997, 8, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Zabner, J.; Couture, L.A.; Gregory, R.J.; Graham, S.M.; Smith, A.E.; Welsh, M.J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 1993, 75, 207–216. [Google Scholar] [CrossRef]
- Rich, D.P.; Couture, L.A.; Cardoza, L.M.; Guiggio, V.M.; Armentano, D.; Espino, P.C.; Hehir, K.; Welsh, M.J.; Smith, A.E.; Gregory, R.J. Development and analysis of recombinant adenoviruses for gene therapy of cystic fibrosis. Hum. Gene Ther. 1993, 4, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G.; McElvaney, N.G.; Rosenfeld, M.A.; Chu, C.S.; Mastrangeli, A.; Hay, J.G.; Brody, S.L.; Jaffe, H.A.; Eissa, N.T.; Danel, C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat. Genet. 1994, 8, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Boucher, R.C.; Knowles, M.R.; Johnson, L.G.; Olsen, J.C.; Pickles, R.; Wilson, J.M.; Engelhardt, J.; Yang, Y.; Grossman, M. Gene Therapy for cystic fibrosis using E1-deleted adenovirus: A phase I trial in the nasal cavity. The University of North Carolina at Chapel Hill. Hum. Gene Ther. 1994, 5, 615–639. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Hohneker, K.W.; Zhou, Z.; Olsen, J.C.; Noah, T.L.; Hu, P.C.; Leigh, M.W.; Engelhardt, J.F.; Edwards, L.J.; Jones, K.R.; et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N. Engl. J. Med. 1995, 333, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Engelhardt, J.F.; Grossman, M.; Simon, R.H.; Yang, Y. Gene Therapy of cystic fibrosis lung disease using E1 deleted adenoviruses: A phase I trial. Hum. Gene Ther. 1994, 5, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.G.; McElvaney, N.G.; Herena, J.; Crystal, R.G. Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA adenovirus gene transfer vector. Hum. Gene Ther. 1995, 6, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G.; Jaffe, A.; Brody, S.; Mastrangeli, A.; McElvaney, N.G.; Rosenfeld, M.; Chu, C.S.; Danel, C.; Hay, J.; Eissa, T. A phase 1 study, in cystic fibrosis patients, of the safety, toxicity, and biological efficacy of a single administration of a replication deficient, recombinant adenovirus carrying the cDNA of the normal cystic fibrosis transmembrane conductance regulator gene in the lung. Hum. Gene Ther. 1995, 6, 643–666. [Google Scholar] [PubMed]
- Zabner, J.; Couture, L.A.; Smith, A.E.; Welsh, M.J. Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: Efficiency of adenovirus-mediated gene transfer in vitro. Hum. Gene Ther. 1994, 5, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.A.; Chu, C.S.; Seth, P.; Danel, C.; Banks, T.; Yoneyama, K.; Yoshimura, K.; Crystal, R.G. Gene transfer to freshly isolated human respiratory epithelial cells in vitro using a replication-deficient adenovirus containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum. Gene Ther. 1994, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.G.; Boyles, S.E.; Wilson, J.; Boucher, R.C. Normalization of raised sodium absorption and raised calcium-mediated chloride secretion by adenovirus-mediated expression of cystic fibrosis transmembrane conductance regulator in primary human cystic fibrosis airway epithelial cells. J. Clin. Investig. 1995, 95, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.H.; Engelhardt, J.F.; Yang, Y.; Zepeda, M.; Weber-Pendleton, S.; Grossman, M.; Wilson, J.M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: Toxicity study. Hum. Gene Ther. 1993, 4, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, J.F.; Simon, R.H.; Yang, Y.; Zepeda, M.; Weber-Pendleton, S.; Doranz, B.; Grossman, M.; Wilson, J.M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: Biological efficacy study. Hum. Gene Ther. 1993, 4, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Su, Q.; Wilson, J.M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J. Virol. 1996, 70, 7209–7212. [Google Scholar] [PubMed]
- Eissa, N.T.; Chu, C.S.; Danel, C.; Crystal, R.G. Evaluation of the respiratory epithelium of normals and individuals with cystic fibrosis for the presence of adenovirus E1a sequences relevant to the use of E1a-adenovirus vectors for gene therapy for the respiratory manifestations of cystic fibrosis. Hum. Gene Ther. 1994, 5, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiang, Z.; Ertl, H.C.; Wilson, J.M. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 7257–7261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Trinchieri, G.; Wilson, J.M. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat. Med. 1995, 1, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Scaria, A.; St George, J.A.; Gregory, R.J.; Noelle, R.J.; Wadsworth, S.C.; Smith, A.E.; Kaplan, J.M. Antibody to CD40 ligand inhibits both humoral and cellular immune responses to adenoviral vectors and facilitates repeated administration to mouse airway. Gene Ther. 1997, 4, 611–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirmule, N.; Truneh, A.; Haecker, S.E.; Tazelaar, J.; Gao, G.; Raper, S.E.; Hughes, J.V.; Wilson, J.M. Repeated administration of adenoviral vectors in lungs of human CD4 transgenic mice treated with a nondepleting CD4 antibody. J. Immunol. 1999, 163, 448–455. [Google Scholar] [PubMed]
- Welsh, M.J.; Smith, A.E.; Zabner, J.; Rich, D.P.; Graham, S.M.; Gregory, R.J.; Pratt, B.M.; Moscicki, R.A. Cystic fibrosis gene therapy using an adenovirus vector: In vivo safety and efficacy in nasal epithelium. Hum. Gene Ther. 1994, 5, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.J.; Zabner, J.; Graham, S.M.; Smith, A.E.; Moscicki, R.; Wadsworth, S. Adenovirus-mediated gene transfer for cystic fibrosis: Part A. Safety of dose and repeat administration in the nasal epithelium. Part B. Clinical efficacy in the maxillary sinus. Hum. Gene Ther. 1995, 6, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Zabner, J.; Ramsey, B.W.; Meeker, D.P.; Aitken, M.L.; Balfour, R.P.; Gibson, R.L.; Launspach, J.; Moscicki, R.A.; Richards, S.M.; Standaert, T.A.; et al. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J. Clin. Investig. 1996, 97, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Bellon, G.; Calmard, L.; Thouvenot, D.; Levrey, H.; Jagneaux, V.; Poitevin, F.; Malcus, C.; Accart, N.; Sene, C.; Layani, M.P.; et al. Aerosol administration of a replication defective recombinant adenovirus expressing normal human cDNA-CFTR in the respiratory tractus in patients with cystic fibrosis. C R Seances Soc. Biol. Fil. 1996, 190, 109–142. [Google Scholar] [PubMed]
- Bellon, G.; Michel-Calemard, L.; Thouvenot, D.; Jagneaux, V.; Poitevin, F.; Malcus, C.; Accart, N.; Layani, M.P.; Aymard, M.; Bernon, H.; et al. Aerosol administration of a recombinant adenovirus expressing CFTR to cystic fibrosis patients: A phase I clinical trial. Hum. Gene Ther. 1997, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.G.; Leopold, P.L.; Hackett, N.R.; Grasso, T.M.; Williams, P.M.; Tucker, A.L.; Kaner, R.J.; Ferris, B.; Gonda, I.; Sweeney, T.D.; et al. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J. Clin. Investig. 1999, 104, 1245–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuckerman, J.B.; Robinson, C.B.; McCoy, K.S.; Shell, R.; Sferra, T.J.; Chirmule, N.; Magosin, S.A.; Propert, K.J.; Brown-Parr, E.C.; Hughes, J.V.; et al. A phase I study of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis. Hum. Gene Ther. 1999, 10, 2973–2985. [Google Scholar] [CrossRef] [PubMed]
- Lehrman, S. Virus treatment questioned after gene therapy death. Nature 1999, 401, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E. Gene therapy death prompts review of adenovirus vector. Science 1999, 286, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Raper, S.E.; Wilson, J.M.; Yudkoff, M.; Robinson, M.B.; Ye, X.; Batshaw, M.L. Developing adenoviral-mediated in vivo gene therapy for ornithine transcarbamylase deficiency. J. Inherit. Metab. Dis. 1998, 21 (Suppl. 1), 119–137. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.M.; O’Sullivan, B.P.; Lapey, A.; Dorkin, H.; Oren, J.; Balfour, R.; Perricone, M.A.; Rosenberg, M.; Wadsworth, S.C.; Smith, A.E.; et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. I. Methods, safety, and clinical implications. Hum. Gene Ther. 2001, 12, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.A.; Morris, J.E.; Pavelka, K.; Plog, M.S.; O’Sullivan, B.P.; Joseph, P.M.; Dorkin, H.; Lapey, A.; Balfour, R.; Meeker, D.P.; et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. II. Transfection efficiency in airway epithelium. Hum. Gene Ther. 2001, 12, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Wendtner, C.M. Recombinant adeno-associated virus (rAAV) vectors for somatic gene therapy: Recent advances and potential clinical applications. Cytokines Mol. Ther. 1996, 2, 69–79. [Google Scholar] [PubMed]
- Kearns, W.G.; Afione, S.A.; Fulmer, S.B.; Pang, M.C.; Erikson, D.; Egan, M.; Landrum, M.J.; Flotte, T.R.; Cutting, G.R. Recombinant adeno-associated virus (AAV-CFTR) vectors do not integrate in a site-specific fashion in an immortalized epithelial cell line. Gene Ther. 1996, 3, 748–755. [Google Scholar] [PubMed]
- Afione, S.A.; Conrad, C.K.; Kearns, W.G.; Chunduru, S.; Adams, R.; Reynolds, T.C.; Guggino, W.B.; Cutting, G.R.; Carter, B.J.; Flotte, T.R. In vivo model of adeno-associated virus vector persistence and rescue. J. Virol. 1996, 70, 3235–3241. [Google Scholar] [PubMed]
- Conrad, C.K.; Allen, S.S.; Afione, S.A.; Reynolds, T.C.; Beck, S.E.; Fee-Maki, M.; Barrazza-Ortiz, X.; Adams, R.; Askin, F.B.; Carter, B.J.; et al. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther. 1996, 3, 658–668. [Google Scholar] [PubMed]
- Zeitlin, P.L.; Chu, S.; Conrad, C.; McVeigh, U.; Ferguson, K.; Flotte, T.R.; Guggino, W.B. Alveolar stem cell transduction by an adeno-associated viral vector. Gene Ther. 1995, 2, 623–631. [Google Scholar] [PubMed]
- Halbert, C.L.; Standaert, T.A.; Aitken, M.L.; Alexander, I.E.; Russell, D.W.; Miller, A.D. Transduction by adeno-associated virus vectors in the rabbit airway: Efficiency, persistence, and readministration. J. Virol. 1997, 71, 5932–5941. [Google Scholar] [PubMed]
- Flotte, T.R.; Afione, S.A.; Conrad, C.; McGrath, S.A.; Solow, R.; Oka, H.; Zeitlin, P.L.; Guggino, W.B.; Carter, B.J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. USA 1993, 90, 10613–10617. [Google Scholar] [CrossRef] [PubMed]
- Flotte, T.R.; Afione, S.A.; Solow, R.; Drumm, M.L.; Markakis, D.; Guggino, W.B.; Zeitlin, P.L.; Carter, B.J. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J. Biol. Chem. 1993, 268, 3781–3790. [Google Scholar] [PubMed]
- Rubenstein, R.C.; McVeigh, U.; Flotte, T.R.; Guggino, W.B.; Zeitlin, P.L. CFTR gene transduction in neonatal rabbits using an adeno-associated virus (AAV) vector. Gene Ther. 1997, 4, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Halbert, C.L.; Standaert, T.A.; Wilson, C.B.; Miller, A.D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J. Virol. 1998, 72, 9795–9805. [Google Scholar] [PubMed]
- Fischer, A.C.; Beck, S.E.; Smith, C.I.; Laube, B.L.; Askin, F.B.; Guggino, S.E.; Adams, R.J.; Flotte, T.R.; Guggino, W.B. Successful transgene expression with serial doses of aerosolized rAAV2 vectors in rhesus macaques. Mol. Ther. 2003, 8, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.C.; Smith, C.I.; Cebotaru, L.; Zhang, X.; Askin, F.B.; Wright, J.; Guggino, S.E.; Adams, R.J.; Flotte, T.; Guggino, W.B. Expression of a truncated cystic fibrosis transmembrane conductance regulator with an AAV5-pseudotyped vector in primates. Mol. Ther. 2007, 15, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Limberis, M.P.; Wilson, J.M. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. USA 2006, 103, 12993–12998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Luo, M.; Guo, C.; Yan, Z.; Wang, Y.; Engelhardt, J.F. Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther. 2007, 14, 1543–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Luo, M.; Zhang, L.N.; Yan, Z.; Zak, R.; Ding, W.; Mansfield, S.G.; Mitchell, L.G.; Engelhardt, J.F. Spliceosome-mediated RNA trans-splicing with recombinant adeno-associated virus partially restores cystic fibrosis transmembrane conductance regulator function to polarized human cystic fibrosis airway epithelial cells. Hum. Gene Ther. 2005, 16, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, Z.; Luo, M.; Engelhardt, J.F. Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction. Am. J. Respir. Cell Mol. Biol. 2006, 34, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Halbert, C.L.; Lam, S.L.; Miller, A.D. High-efficiency promoter-dependent transduction by adeno-associated virus type 6 vectors in mouse lung. Hum. Gene Ther. 2007, 18, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Flotte, T.R.; Schwiebert, E.M.; Zeitlin, P.L.; Carter, B.J.; Guggino, W.B. Correlation between DNA transfer and cystic fibrosis airway epithelial cell correction after recombinant adeno-associated virus serotype 2 gene therapy. Hum. Gene Ther. 2005, 16, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Messner, A.H.; Moran, M.L.; Daifuku, R.; Kouyama, K.; Desch, J.K.; Manley, S.; Norbash, A.M.; Conrad, C.K.; Friborg, S.; et al. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 1999, 109, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Nepomuceno, I.B.; Messner, A.H.; Moran, M.L.; Batson, E.P.; Dimiceli, S.; Brown, B.W.; Desch, J.K.; Norbash, A.M.; Conrad, C.K.; et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum. Gene Ther. 2002, 13, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Flotte, T.R.; Zeitlin, P.L.; Reynolds, T.C.; Heald, A.E.; Pedersen, P.; Beck, S.; Conrad, C.K.; Brass-Ernst, L.; Humphries, M.; Sullivan, K.; et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: A two-part clinical study. Hum. Gene Ther. 2003, 14, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.L.; Moss, R.B.; Waltz, D.A.; Dovey, M.E.; Tonelli, M.R.; McNamara, S.C.; Gibson, R.L.; Ramsey, B.W.; Carter, B.J.; Reynolds, T.C. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 2001, 12, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.B.; Milla, C.; Colombo, J.; Accurso, F.; Zeitlin, P.L.; Clancy, J.P.; Spencer, L.T.; Pilewski, J.; Waltz, D.A.; Dorkin, H.L.; et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: A randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 2007, 18, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.B.; Rodman, D.; Spencer, L.T.; Aitken, M.L.; Zeitlin, P.L.; Waltz, D.; Milla, C.; Brody, A.S.; Clancy, J.P.; Ramsey, B.; et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: A multicenter, double-blind, placebo-controlled trial. Chest 2004, 125, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Fan, P.D.; Frizzell, R.A. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum. Gene Ther. 1996, 7, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, D.; Fischer, H.; Fan, P.D.; Widdicombe, J.H.; Kan, Y.W.; Dong, J.Y. Efficient expression of CFTR function with adeno-associated virus vectors that carry shortened CFTR genes. Proc. Natl. Acad. Sci. USA 1998, 95, 10158–10163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Fischer, H.; Zhang, L.; Fan, P.; Ding, R.X.; Dong, J. Efficient CFTR expression from AAV vectors packaged with promoters—The second generation. Gene Ther. 1999, 6, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ostedgaard, L.S.; Zabner, J.; Vermeer, D.W.; Rokhlina, T.; Karp, P.H.; Stecenko, A.A.; Randak, C.; Welsh, M.J. CFTR with a partially deleted R domain corrects the cystic fibrosis chloride transport defect in human airway epithelia in vitro and in mouse nasal mucosa in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 3093–3098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostedgaard, L.S.; Rokhlina, T.; Karp, P.H.; Lashmit, P.; Afione, S.; Schmidt, M.; Zabner, J.; Stinski, M.F.; Chiorini, J.A.; Welsh, M.J. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc. Natl. Acad. Sci. USA 2005, 102, 2952–2957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostedgaard, L.S.; Meyerholz, D.K.; Vermeer, D.W.; Karp, P.H.; Schneider, L.; Sigmund, C.D.; Welsh, M.J. Cystic fibrosis transmembrane conductance regulator with a shortened R domain rescues the intestinal phenotype of CFTR−/− mice. Proc. Natl. Acad. Sci. USA 2011, 108, 2921–2926. [Google Scholar] [CrossRef] [PubMed]
- Zabner, J.; Fasbender, A.J.; Moninger, T.; Poellinger, K.A.; Welsh, M.J. Cellular and molecular barriers to gene transfer by a cationic lipid. J. Biol. Chem. 1995, 270, 18997–19007. [Google Scholar] [CrossRef] [PubMed]
- Kollen, W.J.; Midoux, P.; Erbacher, P.; Yip, A.; Roche, A.C.; Monsigny, M.; Glick, M.C.; Scanlin, T.F. Gluconoylated and glycosylated polylysines as vectors for gene transfer into cystic fibrosis airway epithelial cells. Hum. Gene Ther. 1996, 7, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; Liggitt, H.D.; Roche, L.; Nguyen, H.T.; Pearlman, R.; Raabe, O.G.; Bussey, L.B.; Gorman, C.M. Aerosol delivery of lipid:DNA complexes to lungs of rhesus monkeys. Pharm. Res. 1998, 15, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Fasbender, A.; Marshall, J.; Moninger, T.O.; Grunst, T.; Cheng, S.; Welsh, M.J. Effect of co-lipids in enhancing cationic lipid-mediated gene transfer in vitro and in vivo. Gene Ther. 1997, 4, 716–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, P.G.; Geddes, D.M.; Alton, E.W. Protocols for in vivo measurement of the ion transport defects in cystic fibrosis nasal epithelium. Eur. Respir. J. 1994, 7, 2050–2056. [Google Scholar] [PubMed]
- Middleton, P.G.; Caplen, N.J.; Gao, X.; Huang, L.; Gaya, H.; Geddes, D.M.; Alton, E.W. Nasal application of the cationic liposome DC-Chol:DOPE does not alter ion transport, lung function or bacterial growth. Eur. Respir. J. 1994, 7, 442–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplen, N.J.; Alton, E.W.; Middleton, P.G.; Dorin, J.R.; Stevenson, B.J.; Gao, X.; Durham, S.R.; Jeffery, P.K.; Hodson, M.E.; Coutelle, C.; et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat. Med. 1995, 1, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hyde, S.C.; Southern, K.W.; Gileadi, U.; Fitzjohn, E.M.; Mofford, K.A.; Waddell, B.E.; Gooi, H.C.; Goddard, C.A.; Hannavy, K.; Smyth, S.E.; et al. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 2000, 7, 1156–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noone, P.G.; Hohneker, K.W.; Zhou, Z.; Johnson, L.G.; Foy, C.; Gipson, C.; Jones, K.; Noah, T.L.; Leigh, M.W.; Schwartzbach, C.; et al. Safety and biological efficacy of a lipid-CFTR complex for gene transfer in the nasal epithelium of adult patients with cystic fibrosis. Mol. Ther. 2000, 1, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.R.; Southern, K.W.; Mofford, K.A.; Seddon, T.; Huang, L.; Sorgi, F.; Thomson, A.; MacVinish, L.J.; Ratcliff, R.; Bilton, D.; et al. A placebo-controlled study of liposome-mediated gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 1997, 4, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porteous, D.J.; Dorin, J.R.; McLachlan, G.; Davidson-Smith, H.; Davidson, H.; Stevenson, B.J.; Carothers, A.D.; Wallace, W.A.; Moralee, S.; Hoenes, C.; et al. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 1997, 4, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, G.; Ho, L.P.; Davidson-Smith, H.; Samways, J.; Davidson, H.; Stevenson, B.J.; Carothers, A.D.; Alton, E.W.; Middleton, P.G.; Smith, S.N.; et al. Laboratory and clinical studies in support of cystic fibrosis gene therapy using pCMV-CFTR-DOTAP. Gene Ther. 1996, 3, 1113–1123. [Google Scholar] [PubMed]
- Zabner, J.; Cheng, S.H.; Meeker, D.; Launspach, J.; Balfour, R.; Perricone, M.A.; Morris, J.E.; Marshall, J.; Fasbender, A.; Smith, A.E.; et al. Comparison of DNA-lipid complexes and DNA alone for gene transfer to cystic fibrosis airway epithelia in vivo. J. Clin. Investig. 1997, 100, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.W.; Stern, M.; Farley, R.; Jaffe, A.; Chadwick, S.L.; Phillips, J.; Davies, J.; Smith, S.N.; Browning, J.; Davies, M.G.; et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: A double-blind placebo-controlled trial. Lancet 1999, 353, 947–954. [Google Scholar] [CrossRef]
- Ruiz, F.E.; Clancy, J.P.; Perricone, M.A.; Bebok, Z.; Hong, J.S.; Cheng, S.H.; Meeker, D.P.; Young, K.R.; Schoumacher, R.A.; Weatherly, M.R.; et al. A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis. Hum. Gene Ther. 2001, 12, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Davis, P.B.; Wagener, J.S.; Hilliard, K.A.; Stern, R.C.; Milgram, L.J.; Kowalczyk, T.H.; Hyatt, S.L.; Fink, T.L.; Gedeon, C.R.; et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum. Gene Ther. 2004, 15, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.W.; Boyd, A.C.; Cheng, S.H.; Cunningham, S.; Davies, J.C.; Gill, D.R.; Griesenbach, U.; Higgins, T.; Hyde, S.C.; Innes, J.A.; et al. A randomised, double-blind, placebo-controlled phase IIB clinical trial of repeated application of gene therapy in patients with cystic fibrosis. Thorax 2013, 68, 1075–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alton, E.; Armstrong, D.K.; Ashby, D.; Bayfield, K.J.; Bilton, D.; Bloomfield, E.V.; Boyd, A.C.; Brand, J.; Buchan, R.; Calcedo, R.; et al. A Randomised, Double-Blind, Placebo-Controlled Trial of Repeated Nebulisation of Non-Viral Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Gene Therapy in Patients with Cystic Fibrosis; NIHR Journals Library: Southampton, UK, 2016. [Google Scholar] [CrossRef]
- Wang, G.; Slepushkin, V.A.; Bodner, M.; Zabner, J.; van Es, H.H.; Thomas, P.; Jolly, D.J.; Davidson, B.L.; McCray, P.B., Jr. Keratinocyte growth factor induced epithelial proliferation facilitates retroviral-mediated gene transfer to distal lung epithelia in vivo. J. Gene Med. 1999, 1, 22–30. [Google Scholar] [CrossRef]
- Wang, G.; Davidson, B.L.; Melchert, P.; Slepushkin, V.A.; van Es, H.H.; Bodner, M.; Jolly, D.J.; McCray, P.B., Jr. Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J. Virol. 1998, 72, 9818–9826. [Google Scholar] [PubMed]
- Duan, D.; Yue, Y.; Yan, Z.; McCray, P.B., Jr.; Engelhardt, J.F. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum. Gene Ther. 1998, 9, 2761–2776. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.W.; van’t Hof, W.; Yi, S.M.; Schroth, M.K.; Zabner, J.; Crystal, R.G.; Welsh, M.J. Apical localization of the coxsackie-adenovirus receptor by glycosyl-phosphatidylinositol modification is sufficient for adenovirus-mediated gene transfer through the apical surface of human airway epithelia. J. Virol. 2001, 75, 7703–7711. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sinn, P.L.; McCray, P.B., Jr. Development of retroviral vectors for gene transfer to airway epithelia. Curr. Opin. Mol. Ther. 2000, 2, 497–506. [Google Scholar] [PubMed]
- Johnson, L.G.; Olsen, J.C.; Naldini, L.; Boucher, R.C. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 2000, 7, 568–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halbert, C.L.; Aitken, M.L.; Miller, A.D. Retroviral vectors efficiently transduce basal and secretory airway epithelial cells in vitro resulting in persistent gene expression in organotypic culture. Hum. Gene Ther. 1996, 7, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Blomer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Lee, P.S.; Yang, J.S.; Wilson, J.M. Lentiviral vectors for gene therapy of cystic fibrosis. Hum. Gene Ther. 1997, 8, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Slepushkin, V.; Zabner, J.; Keshavjee, S.; Johnston, J.C.; Sauter, S.L.; Jolly, D.J.; Dubensky, T.W., Jr.; Davidson, B.L.; McCray, P.B., Jr. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J. Clin. Investig. 1999, 104, R55–R62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinn, P.L.; Goreham-Voss, J.D.; Arias, A.C.; Hickey, M.A.; Maury, W.; Chikkanna-Gowda, C.P.; McCray, P.B., Jr. Enhanced gene expression conferred by stepwise modification of a nonprimate lentiviral vector. Hum. Gene Ther. 2007, 18, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peeples, M.E.; Boucher, R.C.; Collins, P.L.; Pickles, R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 2002, 76, 5654–5666. [Google Scholar] [CrossRef] [PubMed]
- Sinn, P.L.; Hickey, M.A.; Staber, P.D.; Dylla, D.E.; Jeffers, S.A.; Davidson, B.L.; Sanders, D.A.; McCray, P.B., Jr. Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor α. J. Virol. 2003, 77, 5902–5910. [Google Scholar] [CrossRef] [PubMed]
- Kobinger, G.P.; Weiner, D.J.; Yu, Q.C.; Wilson, J.M. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat. Biotechnol. 2001, 19, 225–230. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.; Patel, M.; Pickles, R.J.; Johnson, L.G.; Olsen, J.C. Influenza M2 envelope protein augments avian influenza hemagglutinin pseudotyping of lentiviral vectors. Gene Ther. 2006, 13, 715–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobinger, G.P.; Limberis, M.P.; Somanathan, S.; Schumer, G.; Bell, P.; Wilson, J.M. Human immunodeficiency viral vector pseudotyped with the spike envelope of severe acute respiratory syndrome coronavirus transduces human airway epithelial cells and dendritic cells. Hum. Gene Ther. 2007, 18, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Sinn, P.L.; Penisten, A.K.; Burnight, E.R.; Hickey, M.A.; Williams, G.; McCoy, D.M.; Mallampalli, R.K.; McCray, P.B. Gene transfer to respiratory epithelia with lentivirus pseudotyped with Jaagsiekte sheep retrovirus envelope glycoprotein. Hum. Gene Ther. 2005, 16, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; DeMartini, J.C.; Miller, A.D. Retrovirus vectors bearing jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J. Virol. 2000, 74, 4698–4704. [Google Scholar] [CrossRef] [PubMed]
- Kremer, K.L.; Dunning, K.R.; Parsons, D.W.; Anson, D.S. Gene delivery to airway epithelial cells in vivo: A direct comparison of apical and basolateral transduction strategies using pseudotyped lentivirus vectors. J. Gene Med. 2007, 9, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Mitomo, K.; Griesenbach, U.; Inoue, M.; Somerton, L.; Meng, C.; Akiba, E.; Tabata, T.; Ueda, Y.; Frankel, G.M.; Farley, R.; et al. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Griesenbach, U.; Inoue, M.; Meng, C.; Farley, R.; Chan, M.; Newman, N.K.; Brum, A.; You, J.; Kerton, A.; Shoemark, A.; et al. Assessment of F/HN-pseudotyped lentivirus as a clinically relevant vector for lung gene therapy. Am. J. Respir. Crit. Care Med. 2012, 186, 846–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, A.G.; Kremer, K.L.; Koldej, R.; Miller, D.S.; Anson, D.S.; Parsons, D.W. Single-dose lentiviral gene transfer for lifetime airway gene expression. J. Gene Med. 2009, 11, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Cmielewski, P.; Anson, D.S.; Parsons, D.W. Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: Effect of acyl chain length. Respir. Res. 2010, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Sinn, P.L.; Cooney, A.L.; Oakland, M.; Dylla, D.E.; Wallen, T.J.; Pezzulo, A.A.; Chang, E.H.; McCray, P.B., Jr. Lentiviral vector gene transfer to porcine airways. Mol. Ther. Nucleic Acids 2012, 1, e56. [Google Scholar] [CrossRef] [PubMed]
- Farrow, N.; Miller, D.; Cmielewski, P.; Donnelley, M.; Bright, R.; Parsons, D.W. Airway gene transfer in a non-human primate: Lentiviral gene expression in marmoset lungs. Sci. Rep. 2013, 3, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cmielewski, P.; Donnelley, M.; Parsons, D.W. Long-term therapeutic and reporter gene expression in lentiviral vector treated cystic fibrosis mice. J. Gene Med. 2014, 16, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.W.; Grunst, T.; Bergelson, J.M.; Finberg, R.W.; Welsh, M.J.; Zabner, J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 1999, 274, 10219–10226. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; St George, J.A.; Lukason, M.; Cheng, S.H.; Scheule, R.K.; Eastman, S.J. EGTA enhancement of adenovirus-mediated gene transfer to mouse tracheal epithelium in vivo. Hum. Gene Ther. 2001, 12, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zabner, J.; Deering, C.; Launspach, J.; Shao, J.; Bodner, M.; Jolly, D.J.; Davidson, B.L.; McCray, P.B., Jr. Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am. J. Respir. Cell Mol. Biol. 2000, 22, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Seiler, M.P.; Luner, P.; Moninger, T.O.; Karp, P.H.; Keshavjee, S.; Zabner, J. Thixotropic solutions enhance viral-mediated gene transfer to airway epithelia. Am. J. Respir. Cell Mol. Biol. 2002, 27, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sinn, P.L.; Shah, A.J.; Donovan, M.D.; McCray, P.B., Jr. Viscoelastic gel formulations enhance airway epithelial gene transfer with viral vectors. Am. J. Respir. Cell Mol. Biol. 2005, 32, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J. Detergent-lentiviral combination gives gene therapy hope for cystic fibrosis. Lancet 2002, 360, 1306. [Google Scholar] [CrossRef]
- Fasbender, A.; Lee, J.H.; Walters, R.W.; Moninger, T.O.; Zabner, J.; Welsh, M.J. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J. Clin. Investig. 1998, 102, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Zabner, J.; Welsh, M.J. Delivery of an adenovirus vector in a calcium phosphate coprecipitate enhances the therapeutic index of gene transfer to airway epithelia. Hum. Gene Ther. 1999, 10, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.; Welsh, M. Mechanism by which calcium phosphate coprecipitation enhances adenovirus-mediated gene transfer. Gene Ther. 1999, 6, 1845–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Welsh, M.J. Enhancement of calcium phosphate-mediated transfection by inclusion of adenovirus in coprecipitates. Gene Ther. 1999, 6, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otake, K.; Ennist, D.L.; Harrod, K.; Trapnell, B.C. Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses. Hum. Gene Ther. 1998, 9, 2207–2222. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.W.; Grubb, B.R.; Johnson, L.G.; Boucher, R.C. Enhanced in vivo airway gene transfer via transient modification of host barrier properties with a surface-active agent. Hum. Gene Ther. 1998, 9, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Drapkin, P.T.; O’Riordan, C.R.; Yi, S.M.; Chiorini, J.A.; Cardella, J.; Zabner, J.; Welsh, M.J. Targeting the urokinase plasminogen activator receptor enhances gene transfer to human airway epithelia. J. Clin. Investig. 2000, 105, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabner, J.; Wadsworth, S.C.; Smith, A.E.; Welsh, M.J. Adenovirus-mediated generation of cAMP-stimulated Cl-transport in cystic fibrosis airway epithelia in vitro: Effect of promoter and administration method. Gene Ther. 1996, 3, 458–465. [Google Scholar] [PubMed]
- Zabner, J.; Zeiher, B.G.; Friedman, E.; Welsh, M.J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J. Virol. 1996, 70, 6994–7003. [Google Scholar] [PubMed]
- Alton, E.W.; Armstrong, D.K.; Ashby, D.; Bayfield, K.J.; Bilton, D.; Bloomfield, E.V.; Boyd, A.C.; Brand, J.; Buchan, R.; Calcedo, R.; et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015, 3, 684–691. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, X.; Feng, Z.; Li, G.; Fisher, J.T.; Stewart, Z.A.; Engelhardt, J.F. Optimization of Recombinant Adeno-Associated Virus-Mediated Expression for Large Transgenes, Using a Synthetic Promoter and Tandem Array Enhancers. Hum. Gene Ther. 2015, 26, 334–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Singh, R.N.; Crystal, R.G. Regulatable promoters for use in gene therapy applications: Modification of the 5’-flanking region of the CFTR gene with multiple cAMP response elements to support basal, low-level gene expression that can be upregulated by exogenous agents that raise intracellular levels of cAMP. Hum. Gene Ther. 1996, 7, 1883–1893. [Google Scholar] [PubMed]
- Suzuki, M.; Singh, R.N.; Crystal, R.G. Ability of a chimeric cAMP-responsive promoter to confer pharmacologic control of CFTR cDNA expression and cAMP-mediated Cl− secretion. Gene Ther. 1997, 4, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; O’Connor, S.P.; Armentano, D.; Berthelette, P.B.; Schiavi, S.C.; Jefferson, D.M.; Smith, A.E.; Wadsworth, S.C.; Cheng, S.H. Ability of adenovirus vectors containing different CFTR transcriptional cassettes to correct ion transport defects in CF cells. Am. J. Physiol. 1996, 271, L527–L537. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.H.; O’Brodovich, H.; Plumb, J.; Wen, Y.; Sohn, K.J.; Lu, Z.; Zhang, F.; Lukacs, G.L.; Tanswell, A.K.; Hui, C.C.; et al. Development of an epithelium-specific expression cassette with human DNA regulatory elements for transgene expression in lung airways. Proc. Natl. Acad. Sci. USA 1997, 94, 14695–14700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Zak, R.; Zhang, Y.; Ding, W.; Godwin, S.; Munson, K.; Peluso, R.; Engelhardt, J.F. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J. Virol. 2004, 78, 2863–2874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Karp, P.; Gerard, C.J.; Pastor, E.; Laux, D.; Munson, K.; Yan, Z.; Liu, X.; Godwin, S.; Thomas, C.P.; et al. Dual therapeutic utility of proteasome modulating agents for pharmaco-gene therapy of the cystic fibrosis airway. Mol. Ther. 2004, 10, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Excoffon, K.J.; Koerber, J.T.; Dickey, D.D.; Murtha, M.; Keshavjee, S.; Kaspar, B.K.; Zabner, J.; Schaffer, D.V. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc. Natl. Acad. Sci. USA 2009, 106, 3865–3870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhang, L.; Johnson, J.S.; Zhijian, W.; Grieger, J.C.; Ping-Jie, X.; Drouin, L.M.; Agbandje-McKenna, M.; Pickles, R.J.; Samulski, R.J. Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium. Mol. Ther. 2009, 17, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Flotte, T.R.; Fischer, A.C.; Goetzmann, J.; Mueller, C.; Cebotaru, L.; Yan, Z.; Wang, L.; Wilson, J.M.; Guggino, W.B.; Engelhardt, J.F. Dual reporter comparative indexing of rAAV pseudotyped vectors in chimpanzee airway. Mol. Ther. 2010, 18, 594–600. [Google Scholar] [CrossRef] [PubMed]
- White, A.F.; Mazur, M.; Sorscher, E.J.; Zinn, K.R.; Ponnazhagan, S. Genetic modification of adeno-associated viral vector type 2 capsid enhances gene transfer efficiency in polarized human airway epithelial cells. Hum. Gene Ther. 2008, 19, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Chen, L.; Anton, M.; Sankar, U.; Rudnicki, M.A.; Graham, F.L. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 1996, 93, 13565–13570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, K.J.; Choi, H.; Burda, J.; Chen, S.J.; Wilson, J.M. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology 1996, 217, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gary, H.; McKinney, R.L.; Rosengart, T.; Lesser, M.L.; Crystal, R.G. Systemic interleukin-6 responses following administration of adenovirus gene transfer vectors to humans by different routes. Mol. Ther. 2002, 6, 287–297. [Google Scholar] [PubMed]
- Toietta, G.; Koehler, D.R.; Finegold, M.J.; Lee, B.; Hu, J.; Beaudet, A.L. Reduced inflammation and improved airway expression using helper-dependent adenoviral vectors with a K18 promoter. Mol. Ther. 2003, 7, 649–658. [Google Scholar] [CrossRef]
- Van Heeckeren, A.M.; Scaria, A.; Schluchter, M.D.; Ferkol, T.W.; Wadsworth, S.; Davis, P.B. Delivery of CFTR by adenoviral vector to cystic fibrosis mouse lung in a model of chronic Pseudomonas aeruginosa lung infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L717–L726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Heeckeren, A.M.; Schluchter, M.D.; Drumm, M.L.; Davis, P.B. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L944–L952. [Google Scholar] [CrossRef] [PubMed]
- Koehler, D.R.; Sajjan, U.; Chow, Y.H.; Martin, B.; Kent, G.; Tanswell, A.K.; McKerlie, C.; Forstner, J.F.; Hu, J. Protection of Cftr knockout mice from acute lung infection by a helper-dependent adenoviral vector expressing Cftr in airway epithelia. Proc. Natl. Acad. Sci. USA 2003, 100, 15364–15369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oceandy, D.; McMorran, B.J.; Smith, S.N.; Schreiber, R.; Kunzelmann, K.; Alton, E.W.; Hume, D.A.; Wainwright, B.J. Gene complementation of airway epithelium in the cystic fibrosis mouse is necessary and sufficient to correct the pathogen clearance and inflammatory abnormalities. Hum. Mol. Genet. 2002, 11, 1059–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, D.R.; Martin, B.; Corey, M.; Palmer, D.; Ng, P.; Tanswell, A.K.; Hu, J. Readministration of helper-dependent adenovirus to mouse lung. Gene Ther. 2006, 13, 773–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushwah, R.; Cao, H.; Hu, J. Potential of helper-dependent adenoviral vectors in modulating airway innate immunity. Cell. Mol. Immunol. 2007, 4, 81–89. [Google Scholar] [PubMed]
- Rogers, C.S.; Abraham, W.M.; Brogden, K.A.; Engelhardt, J.F.; Fisher, J.T.; McCray, P.B., Jr.; McLennan, G.; Meyerholz, D.K.; Namati, E.; Ostedgaard, L.S.; et al. The porcine lung as a potential model for cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L240–L263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, C.S.; Stoltz, D.A.; Meyerholz, D.K.; Ostedgaard, L.S.; Rokhlina, T.; Taft, P.J.; Rogan, M.P.; Pezzulo, A.A.; Karp, P.H.; Itani, O.A.; et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 2008, 321, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Klymiuk, N.; Mundhenk, L.; Kraehe, K.; Wuensch, A.; Plog, S.; Emrich, D.; Langenmayer, M.C.; Stehr, M.; Holzinger, A.; Kroner, C.; et al. Sequential targeting of CFTR by BAC vectors generates a novel pig model of cystic fibrosis. J. Mol. Med. 2012, 90, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, D.A.; Rokhlina, T.; Ernst, S.E.; Pezzulo, A.A.; Ostedgaard, L.S.; Karp, P.H.; Samuel, M.S.; Reznikov, L.R.; Rector, M.V.; Gansemer, N.D.; et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J. Clin. Investig. 2013, 123, 2685–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Sui, H.; Fisher, J.T.; Yan, Z.; Liu, X.; Cho, H.J.; Joo, N.S.; Zhang, Y.; Zhou, W.; Yi, Y.; et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Investig. 2010, 120, 3149–3160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Yan, Z.; Yi, Y.; Li, Z.; Lei, D.; Rogers, C.S.; Chen, J.; Zhang, Y.; Welsh, M.J.; Leno, G.H.; et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J. Clin. Investig. 2008, 118, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Olivier, A.K.; Liang, B.; Yi, Y.; Sui, H.; Evans, T.I.; Zhang, Y.; Zhou, W.; Tyler, S.R.; Fisher, J.T.; et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am. J. Respir. Cell Mol. Biol. 2014, 50, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Feng, Z.; Sun, X.; Zhang, Y.; Zou, W.; Wang, Z.; Jensen-Cody, C.; Liang, B.; Park, S.Y.; Qiu, J.; et al. Human Bocavirus Type-1 Capsid Facilitates the Transduction of Ferret Airways by Adeno-Associated Virus Genomes. Hum. Gene Ther. 2017, 28, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Tuggle, K.L.; Birket, S.E.; Cui, X.; Hong, J.; Warren, J.; Reid, L.; Chambers, A.; Ji, D.; Gamber, K.; Chu, K.K.; et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS ONE 2014, 9, e91253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birket, S.E.; Davis, J.M.; Fernandez, C.M.; Tuggle, K.L.; Oden, A.M.; Chu, K.K.; Tearney, G.J.; Fanucchi, M.V.; Sorscher, E.J.; Rowe, S.M. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight 2018, 3, 97199. [Google Scholar] [CrossRef] [PubMed]
- Navis, A.; Bagnat, M. Loss of cftr function leads to pancreatic destruction in larval zebrafish. Dev. Biol. 2015, 399, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Perisse, I.V.; Cotton, C.U.; Regouski, M.; Meng, Q.; Domb, C.; Van Wettere, A.J.; Wang, Z.; Harris, A.; White, K.L.; et al. A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI Insight 2018, 3, 123529. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, N.A.; Kardia, E.; Kamaldin, N.; Latahir, A.Z.; Yahaya, B.H. The rabbit as a model for studying lung disease and stem cell therapy. BioMed Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.R.; Steele, M.S.; Valerio, D.M.; Miron, A.; Mann, R.J.; LePage, D.F.; Conlon, R.A.; Cotton, C.U.; Drumm, M.L.; Hodges, C.A. A G542X cystic fibrosis mouse model for examining nonsense mutation directed therapies. PLoS ONE 2018, 13, e0199573. [Google Scholar] [CrossRef] [PubMed]
- Darrah, R.J.; Mitchell, A.L.; Campanaro, C.K.; Barbato, E.S.; Litman, P.; Sattar, A.; Hodges, C.A.; Drumm, M.L.; Jacono, F.J. Early pulmonary disease manifestations in cystic fibrosis mice. J. Cyst. Fibros. 2016, 15, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, C.A.; Cotton, C.U.; Palmert, M.R.; Drumm, M.L. Generation of a conditional null allele for Cftr in mice. Genesis 2008, 46, 546–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzulo, A.A.; Tang, X.X.; Hoegger, M.J.; Abou Alaiwa, M.H.; Ramachandran, S.; Moninger, T.O.; Karp, P.H.; Wohlford-Lenane, C.L.; Haagsman, H.P.; van Eijk, M.; et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012, 487, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooney, A.L.; Abou Alaiwa, M.H.; Shah, V.S.; Bouzek, D.C.; Stroik, M.R.; Powers, L.S.; Gansemer, N.D.; Meyerholz, D.K.; Welsh, M.J.; Stoltz, D.A.; et al. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight 2016, 1, e88730. [Google Scholar] [CrossRef] [PubMed]
- Steines, B.; Dickey, D.D.; Bergen, J.; Excoffon, K.J.; Weinstein, J.R.; Li, X.; Yan, Z.; Abou Alaiwa, M.H.; Shah, V.S.; Bouzek, D.C.; et al. CFTR gene transfer with AAV improves early cystic fibrosis pig phenotypes. JCI Insight 2016, 1, e88728. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.L.; Singh, B.K.; Loza, L.M.; Thornell, I.M.; Hippee, C.E.; Powers, L.S.; Ostedgaard, L.S.; Meyerholz, D.K.; Wohlford-Lenane, C.; Stoltz, D.A.; et al. Widespread airway distribution and short-term phenotypic correction of cystic fibrosis pigs following aerosol delivery of piggyBac/adenovirus. Nucleic Acids Res. 2018, 46, 9561–9600. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.S.; Ernst, S.; Tang, X.X.; Karp, P.H.; Parker, C.P.; Ostedgaard, L.S.; Welsh, M.J. Relationships among CFTR expression, HCO3− secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies. Proc. Natl. Acad. Sci. USA 2016, 113, 5382–5387. [Google Scholar] [CrossRef] [PubMed]
- Porteus, M.H.; Carroll, D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 2005, 23, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Tan, S.; Qiao, G.; Barlow, K.A.; Wang, J.; Xia, D.F.; Meng, X.; Paschon, D.E.; Leung, E.; Hinkley, S.J.; et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 2011, 29, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Epinat, J.C.; Arnould, S.; Chames, P.; Rochaix, P.; Desfontaines, D.; Puzin, C.; Patin, A.; Zanghellini, A.; Paques, F.; Lacroix, E. A novel engineered meganuclease induces homologous recombination in yeast and mammalian cells. Nucleic Acids Res. 2003, 31, 2952–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Flynn, R.; Hollywood, J.A.; Scallan, M.F.; Harrison, P.T. Correction of the DeltaF508 Mutation in the Cystic Fibrosis Transmembrane Conductance Regulator Gene by Zinc-Finger Nuclease Homology-Directed Repair. Biores. Open Access 2012, 1, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Thibodeau-Beganny, S.; Osiak, A.; Wright, D.A.; Anthony, R.M.; Eichtinger, M.; Jiang, T.; Foley, J.E.; Winfrey, R.J.; Townsend, J.A.; et al. Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol. Cell 2008, 31, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Annaluru, N.; Kandavelou, K.; Chandrasegaran, S. TALEN-mediated generation and genetic correction of disease-specific human induced pluripotent stem cells. Curr. Gene Ther. 2014, 14, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Hollywood, J.A.; Lee, C.M.; Scallan, M.F.; Harrison, P.T. Analysis of gene repair tracts from Cas9/gRNA double-stranded breaks in the human CFTR gene. Sci. Rep. 2016, 6, 32230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnight, E.R.; Staber, J.M.; Korsakov, P.; Li, X.; Brett, B.T.; Scheetz, T.E.; Craig, N.L.; McCray, P.B., Jr. A Hyperactive Transposase Promotes Persistent Gene Transfer of a piggyBac DNA Transposon. Mol. Ther. Nucleic Acids 2012, 1, e50. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.L.; Singh, B.K.; Sinn, P.L. Hybrid nonviral/viral vector systems for improved piggyBac DNA transposon in vivo delivery. Mol. Ther. 2015, 23, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ouyang, H.; Ip, W.; Du, K.; Duan, W.; Avolio, J.; Wu, J.; Duan, C.; Yeger, H.; Bear, C.E.; et al. Testing gene therapy vectors in human primary nasal epithelial cultures. Mol. Ther. Methods Clin. Dev. 2015, 2, 15034. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Machuca, T.N.; Yeung, J.C.; Wu, J.; Du, K.; Duan, C.; Hashimoto, K.; Linacre, V.; Coates, A.L.; Leung, K.; et al. Efficient gene delivery to pig airway epithelia and submucosal glands using helper-dependent adenoviral vectors. Mol. Ther. Nucleic Acids 2013, 2, e127. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yang, T.; Li, X.F.; Wu, J.; Duan, C.; Coates, A.L.; Hu, J. Readministration of helper-dependent adenoviral vectors to mouse airway mediated via transient immunosuppression. Gene Ther. 2011, 18, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wu, J.; Duan, C.; Du, K.; Lee, C.M.; Yeger, H.; Hu, J. Long-Term Expression of the Human CFTR Gene in Mouse Airway via Helper-Dependent Adenoviral Vector Delivery and Transient Immunosuppression. Hum. Gene Ther. 2016, 27, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Ashtari, M.; Nikonova, E.S.; Marshall, K.A.; Young, G.J.; Aravand, P.; Pan, W.; Ying, G.S.; Willett, A.E.; Mahmoudian, M.; Maguire, A.M.; et al. The Role of the Human Visual Cortex in Assessment of the Long-Term Durability of Retinal Gene Therapy in Follow-on RPE65 Clinical Trial Patients. Ophthalmology 2017, 124, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.D.; Bachmeier, C.A.; Phuoc, V.H.; Chavez, J.C. Axicabtagene ciloleucel (KTE-C19), an anti-CD19 CAR T therapy for the treatment of relapsed/refractory aggressive B-cell non-Hodgkin’s lymphoma. Ther. Clin. Risk Manag. 2018, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Kolte, A.; Patil, S.; Lesimple, P.; Hanrahan, J.W.; Misra, A. PEGylated composite nanoparticles of PLGA and polyethylenimine for safe and efficient delivery of pDNA to lungs. Int. J. Pharm. 2017, 524, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.W.; Beekman, J.M.; Boyd, A.C.; Brand, J.; Carlon, M.S.; Connolly, M.M.; Chan, M.; Conlon, S.; Davidson, H.E.; Davies, J.C.; et al. Preparation for a first-in-man lentivirus trial in patients with cystic fibrosis. Thorax 2017, 72, 137–147. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooney, A.L.; McCray, P.B., Jr.; Sinn, P.L. Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward. Genes 2018, 9, 538. https://doi.org/10.3390/genes9110538
Cooney AL, McCray PB Jr., Sinn PL. Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward. Genes. 2018; 9(11):538. https://doi.org/10.3390/genes9110538
Chicago/Turabian StyleCooney, Ashley L., Paul B. McCray, Jr., and Patrick L. Sinn. 2018. "Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward" Genes 9, no. 11: 538. https://doi.org/10.3390/genes9110538
APA StyleCooney, A. L., McCray, P. B., Jr., & Sinn, P. L. (2018). Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward. Genes, 9(11), 538. https://doi.org/10.3390/genes9110538




