Unintentional Genomic Changes Endow Cupriavidus metallidurans with an Augmented Heavy-Metal Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Synthetic Construct Generation
2.3. Estimation of Bacterial Tolerance to Metals
2.4. Plasmid Copy Number Determination
2.5. Illumina Sequencing and Assembly
2.6. Total RNA Isolation and Microarray
3. Results and Discussion
3.1. The Influence of Plasmid pTP6 on Increased Heavy Metal Resistance in MSR33
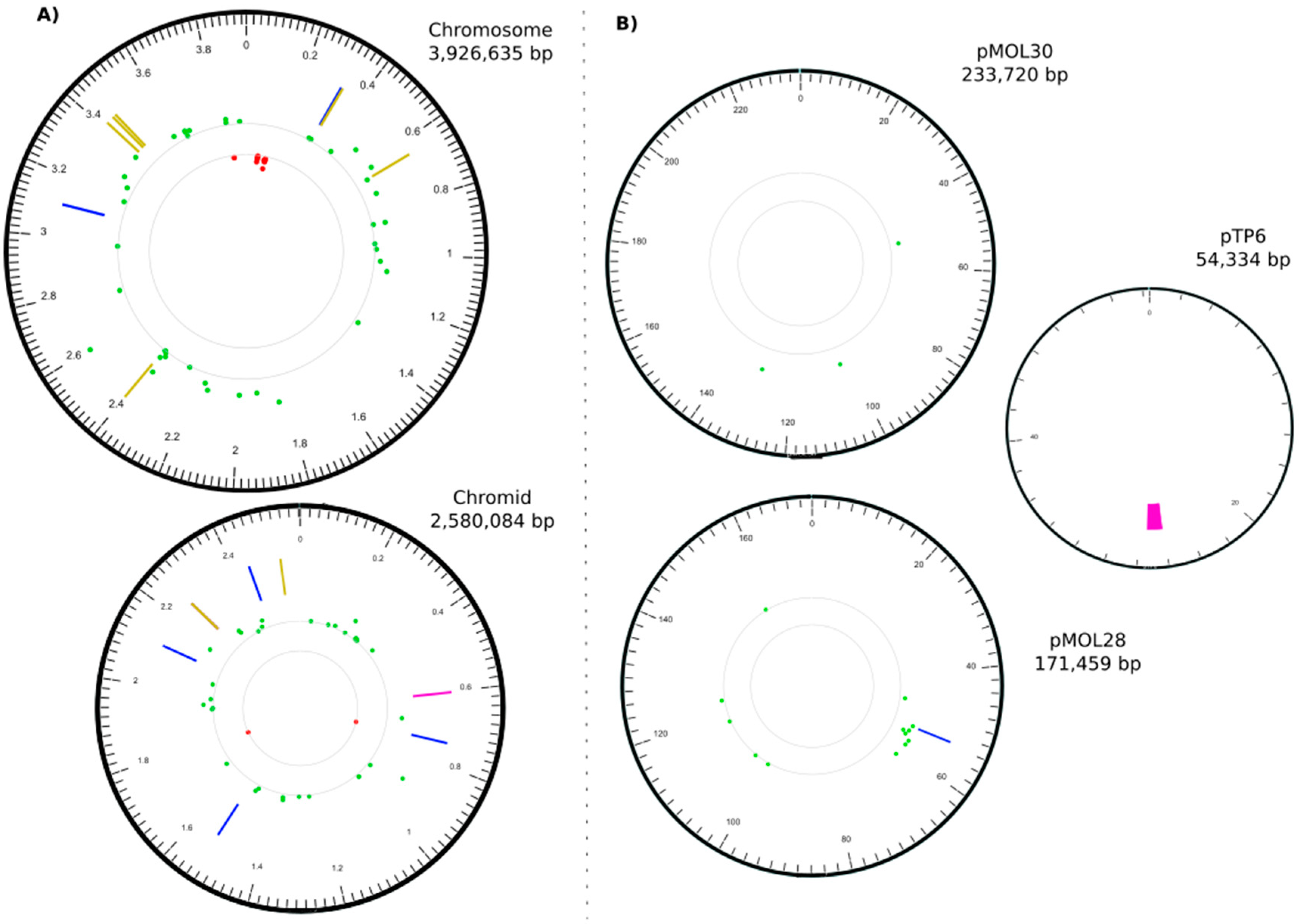
3.2. The C. metallidurans MSR33 Genome Showed Multiple Insertions or Deletions and Single Nucleotide Polymorphisms
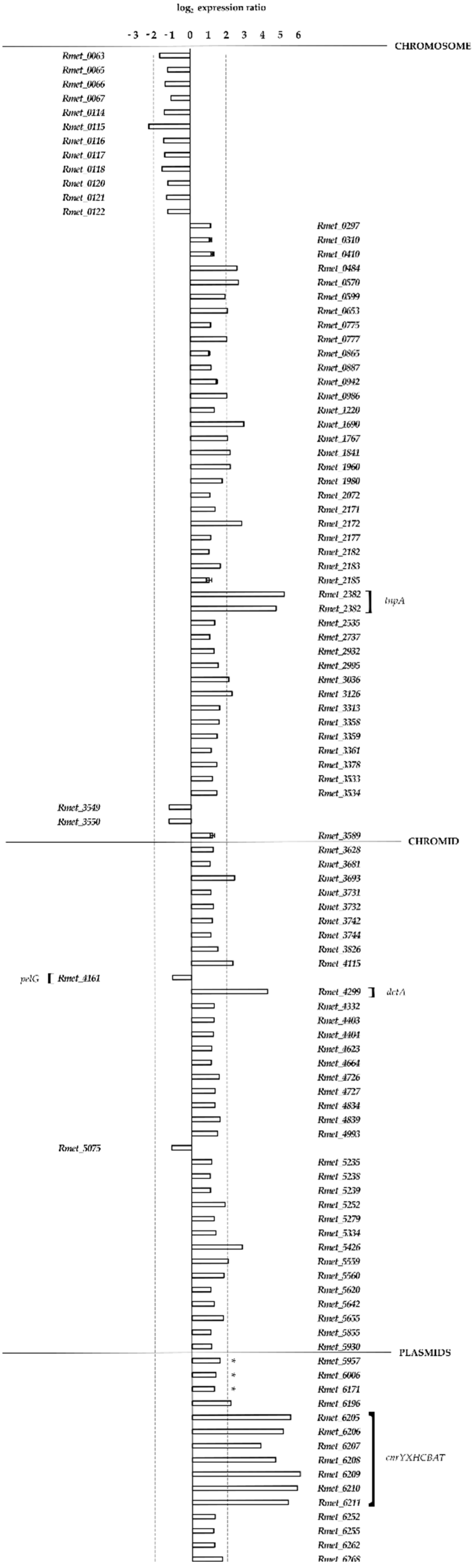
3.3. Transcriptional Analysis of Strain MSR33
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakermans, C. Microbial Evolution under Extreme Conditions; De Gruyter: Altoona, PA, USA, 2015; Volume 2. [Google Scholar]
- Knoll, A.H. Paleobiological perspectives on early microbial evolution. CSH Perspect. Biol. 2015, 7, a018093. [Google Scholar] [CrossRef] [PubMed]
- Springael, D.; Top, E.M. Horizontal gene transfer and microbial adaptation to xenobiotics: New types of mobile genetic elements and lessons from ecological studies. Trends Microbiol. 2004, 12, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, H.; Zhang, H.; Huang, K.; Gu, T.; Ni, H.; Hong, Q.; Li, S. Characterization of a newly isolated highly effective 3,5,6-trichloro-2-pyridinol degrading strain Cupriavidus pauculus P2. Curr. Microbiol. 2012, 65, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ilori, M.O.; Picardal, F.W.; Aramayo, R.; Adebusoye, S.A.; Obayori, O.S.; Benedik, M.J. Catabolic plasmid specifying polychlorinated biphenyl degradation in Cupriavidus sp. strain SK-4: Mobilization and expression in a pseudomonad. J. Basic Microb. 2015, 55, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M.; Van Houdt, R. Adaptation to xenobiotics and toxic compounds by Cupriavidus and Ralstonia with special reference to Cupriavidus metallidurans CH34 and mobile genetic elements. In Biodegradative Bacteria: How Bacteria Degrade, Survive, Adapt, and Evolve; Nojiri, H., Tsuda, M., Fukuda, M., Kamagata, Y., Eds.; Springer: Tokyo, Japan, 2014; pp. 105–127. [Google Scholar]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M. Bacteria adapted to industrial biotopes: The metal resistant Ralstonia. In Bacterial Stress Responses; Storz, G., Hengge-Aronis, R., Eds.; ASM Press: Washington, DC, USA, 2000; pp. 403–414. [Google Scholar]
- Sobecky, P.A.; Coombs, J.M. Horizontal gene transfer in metal and radionuclide contaminated soils. In Horizontal Gene Transfer: Genomes in Flux; Gogarten, M.B., Gogarten, J.P., Olendzenski, L.C., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 455–472. [Google Scholar]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Potter, M.; Schwartz, E.; et al. Genome sequence of the bioplastic-producing “knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amadou, C.; Pascal, G.; Mangenot, S.; Glew, M.; Bontemps, C.; Capela, D.; Carrere, S.; Cruveiller, S.; Dossat, C.; Lajus, A.; et al. Genome sequence of the beta-rhizobium Cupriavidus taiwanensis and comparative genomics of rhizobia. Genome Res. 2008, 18, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.J.; Van Houdt, R.; Moors, H.; Monsieurs, P.; Morin, N.; Michaux, A.; Benotmane, M.A.; Leys, N.; Vallaeys, T.; Lapidus, A.; et al. The complete genome sequence of Cupriavidus metallidurans strain CH34, a master survivalist in harsh and anthropogenic environments. PLoS ONE 2010, 5, e10433. [Google Scholar] [CrossRef] [PubMed]
- Lykidis, A.; Perez-Pantoja, D.; Ledger, T.; Mavromatis, K.; Anderson, I.J.; Ivanova, N.N.; Hooper, S.D.; Lapidus, A.; Lucas, S.; Gonzalez, B.; et al. The complete multipartite genome sequence of Cupriavidus necator JMP134, a versatile pollutant degrader. PLoS ONE 2010, 5, e9729. [Google Scholar] [CrossRef] [PubMed]
- Poehlein, A.; Kusian, B.; Friedrich, B.; Daniel, R.; Bowien, B. Complete genome sequence of the type strain Cupriavidus necator N-1. J. Bacteriol. 2011, 193, 5017. [Google Scholar] [CrossRef] [PubMed]
- Cserhati, M.; Kriszt, B.; Szoboszlay, S.; Toth, A.; Szabo, I.; Tancsics, A.; Nagy, I.; Horvath, B.; Nagy, I.; Kukolya, J. De novo genome project of Cupriavidus basilensis OR16. J. Bacteriol. 2012, 194, 2109–2110. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.W.; Thinagaran, D.A.L.; Gan, H.M.; Yin, W.F.; Chan, K.G. Whole-genome squence of Cupriavidus sp. strain BIS7, a heavy-metal-resistant bacterium. J. Bacteriol. 2012, 194, 6324. [Google Scholar] [CrossRef] [PubMed]
- Li, L.G.; Cai, L.; Zhang, T. Genome of Cupriavidus sp. HMR-1, a heavy metal-resistant bacterium. Genome Announc. 2013, 1, e00202-12. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.; Waters, R.J.; Skerker, J.M.; Kuehl, J.V.; Price, M.N.; Huang, J.; Chakraborty, R.; Arkin, A.P.; Deutschbauer, A. Complete genome sequence of Cupriavidus basilensis 4G11, isolated from the oak ridge field research center site. Genome Announc. 2015, 3, e00322-15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Chen, M.L.; Xiao, J.F.; Hao, L.R.; Crowley, D.E.; Zhang, Z.W.; Yu, J.; Huang, N.; Huo, M.X.; Wu, J.Y. Genome sequence analysis of the naphthenic acid degrading and metal resistant bacterium Cupriavidus gilardii CR3. PLoS ONE 2015, 10, e0132881. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.C.; Chen, Y.F.; Zhou, Y.L.; Wang, D.S.; Sun, L.N.; Tang, X.Y.; Hua, R.M. Complete genome sequence of a novel chlorpyrifos degrading bacterium, Cupriavidus nantongensis X1. J. Biotechnol. 2016, 227, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M.; Monchy, S.; Vallaeys, T.; Auquier, V.; Benotmane, A.; Bertin, P.; Taghavi, S.; Dunn, J.; van der Lelie, D.; Wattiez, R. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: Towards a catalogue of metal-responsive genes. FEMS Microbiol. Rev. 2003, 27, 385–410. [Google Scholar] [CrossRef]
- Monchy, S.; Benotmane, M.A.; Janssen, P.; Vallaeys, T.; Taghavi, S.; van der Lelie, D.; Mergeay, M. Plasmids pMOL28 and pMOL30 of Cupriavidus metallidurans are specialized in the maximal viable response to heavy metals. J. Bacteriol. 2007, 189, 7417–7425. [Google Scholar] [CrossRef] [PubMed]
- Avoscan, L.; Untereiner, G.; Degrouard, J.; Carriere, M.; Gouget, B. Uranium and selenium resistance in Cupriavidus metallidurans CH34. Toxicol. Lett. 2007, 172, S157. [Google Scholar] [CrossRef]
- Monsieurs, P.; Moors, H.; Van Houdt, R.; Janssen, P.J.; Janssen, A.; Coninx, I.; Mergeay, M.; Leys, N. Heavy metal resistance in Cupriavidus metallidurans CH34 is governed by an intricate transcriptional network. Biometals 2011, 24, 1133–1151. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, I.; Sghaier, H.; Monsieurs, P.; Moors, H.; Van Houdt, R.; Fattouch, S.; Saidi, M.; Landolsi, A.; Leys, N. Strontium-induced genomic responses of Cupriavidus metallidurans and strontium bioprecipitation as strontium carbonate. Ann. Microbiol. 2013, 63, 833–844. [Google Scholar] [CrossRef]
- Van Houdt, R.; Mergeay, M. Genomic context of metal response genes in Cupriavidus metallidurans with a focus on strain CH34. In Metal Response in Cupriavidus Metallidurans: Volume I: From Habitats to Genes and Proteins; Mergeay, M., Van Houdt, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 21–44. [Google Scholar]
- Vandenbussche, G.; Mergeay, M.; Van Houdt, R. Metal response in Cupriavidus metallidurans: Insights into the structure-function relationship of proteins. In Metal Response in Cupriavidus Metallidurans: Volume II: Insights into the Structure-Function Relationship of Proteins; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–70. [Google Scholar]
- Nies, D.H. The biological chemistry of the transition metal “transportome” of Cupriavidus metallidurans. Metallomics 2016, 8, 481–507. [Google Scholar] [CrossRef] [PubMed]
- Millacura, F.A.; Cardenas, F.; Mendez, V.; Seeger, M.; Rojas, L.A. Degradation of benzene by the heavy-metal resistant bacterium Cupriavidus metallidurans CH34 reveals its catabolic potential for aromatic compounds. bioRxiv 2017. [Google Scholar] [CrossRef]
- Smalla, K.; Haines, A.S.; Jones, K.; Krogerrecklenfort, E.; Heuer, H.; Schloter, M.; Thomas, C.M. Increased abundance of IncP-1 beta plasmids and mercury resistance genes in mercury-polluted river sediments: First discovery of IncP-1 beta plasmids with a complex mer transposon as the sole accessory element. Appl. Environ. Microb. 2006, 72, 7253–7259. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Yanez, C.; Gonzalez, M.; Lobos, S.; Smalla, K.; Seeger, M. Characterization of the metabolically modified heavy metal-resistant Cupriavidus metallidurans strain MSR33 generated for mercury bioremediation. PLoS ONE 2011, 6, e17555. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Monchy, S.; Leys, N.; Mergeay, M. New mobile genetic elements in Cupriavidus metallidurans CH34, their possible roles and occurrence in other bacteria. Antonie Leeuwenhoek 2009, 96, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Mijnendonckx, K.; Provoost, A.; Monsieurs, P.; Leys, N.; Mergeay, M.; Mahillon, J.; Van Houdt, R. Insertion sequence elements in Cupriavidus metallidurans CH34: Distribution and role in adaptation. Plasmid 2011, 65, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Monsieurs, P.; Mijnendonckx, K.; Provoost, A.; Janssen, A.; Mergeay, M.; Leys, N. Variation in genomic islands contribute to genome plasticity in Cupriavidus metallidurans. BMC Genom. 2012, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M.; Nies, D.; Schlegel, H.G.; Gerits, J.; Charles, P.; Van Gijsegem, F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 1985, 162, 328–334. [Google Scholar] [PubMed]
- Maniatis, T.; Fritsch, E.F.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1982. [Google Scholar]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop, R.M.; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef]
- Taghavi, S.; Vanderlelie, D.; Mergeay, M. Electroporation of Alcaligenes eutrophus with (mega) plasmids and genomic DNA fragments. Appl. Environ. Microb. 1994, 60, 3585–3591. [Google Scholar]
- Lee, C.; Kim, J.; Shin, S.G.; Hwang, S. Absolute and relative qPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 2006, 123, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R. BEDTools: The Swiss-army tool for genome feature analysis. Curr. Protoc. Bioinform. 2014, 47, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv, 2013; arXiv:1303.3997. [Google Scholar]
- Norberg, P.; Bergstrom, M.; Jethava, V.; Dubhashi, D.; Hermansson, M. The IncP-1 plasmid backbone adapts to different host bacterial species and evolves through homologous recombination. Nat. Commun. 2011, 2, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Grosse, C.; Nies, D.H. Regulation of the cnr cobalt and nickel resistance determinant from Ralstonia sp. strain CH34. J. Bacteriol. 2000, 182, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Tibazarwa, C.; Wuertz, S.; Mergeay, M.; Wyns, L.; van Der Lelie, D. Regulation of the cnr cobalt and nickel resistance determinant of Ralstonia eutropha (Alcaligenes eutrophus) CH34. J. Bacteriol. 2000, 182, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Nies, D.H.; McEvoy, M.M.; Rensing, C. Switch or funnel: How RND-type transport systems control periplasmic metal homeostasis. J. Bacteriol. 2011, 193, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Collard, J.M.; Provoost, A.; Taghavi, S.; Mergeay, M. A new type of Alcaligenes eutrophus CH34 zinc resistance generated by mutations affecting regulation of the cnr cobalt-nickel resistance system. J. Bacteriol. 1993, 175, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Vandecraen, J.; Monsieurs, P.; Mergeay, M.; Leys, N.; Aertsen, A.; Van Houdt, R. Zinc-induced transposition of insertion sequence elements contributes to increased adaptability of Cupriavidus metallidurans. Front. Microbiol. 2016, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chruszcz, M.; Lasota, P.; Lebioda, L.; Minor, W. Data mining of metal ion environments present in protein structures. J. Inorg. Biochem. 2008, 102, 1765–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilakaraj, R.; Raghunathan, K.; Anishetty, S.; Pennathur, G. In silico identification of putative metal binding motifs. Bioinformatics 2007, 23, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, A.; Herzberg, M.; Voigt, A.; Seravalli, J.; Grass, G.; Scherer, J.; Nies, D.H. Contributions of five secondary metal uptake systems to metal homeostasis of Cupriavidus metallidurans CH34. J. Bacteriol. 2011, 193, 4652–4663. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Grosse, C.; Reissmann, J.; Pribyl, T.; Nies, D.H. Czcd is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J. Bacteriol. 1999, 181, 6876–6881. [Google Scholar] [PubMed]
- Munkelt, D.; Grass, G.; Nies, D.H. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 2004, 186, 8036–8043. [Google Scholar] [CrossRef] [PubMed]
- Barber-Zucker, S.; Shaanan, B.; Zarivach, R. Transition metal binding selectivity in proteins and its correlation with the phylogenomic classification of the cation diffusion facilitator protein family. Sci. Rep. 2017, 7, 16381. [Google Scholar] [CrossRef] [PubMed]
- Scherer, J.; Nies, D.H. CzcP is a novel efflux system contributing to transition metal resistance in Cupriavidus metallidurans CH34. Mol. Microbiol. 2009, 73, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Delmar, J.A.; Su, C.-C.; Yu, E.W. Bacterial multi-drug efflux transporters. Annu. Rev. Biophys. 2014, 43, 93–117. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Lee, J.K.; O’ Connell, J.D.; Miercke, L.J.W.; Verschueren, K.H.; Srinivasan, V.; Bauvois, C.; Govaerts, C.; Robbins, R.A.; Ruysschaert, J.M.; et al. Metal-induced conformational changes in ZneB suggest an active role of membrane fusion proteins in efflux resistance systems. Proc. Natl. Acad. Sci. USA 2010, 107, 11038–11043. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Su, C.C.; Lei, H.T.; Bolla, J.R.; Do, S.V.; Yu, E.W. Structure and mechanism of the tripartite CusCBA heavy-metal efflux complex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, J.E.; Ekende, E.N.; Kifle, E.G.; O’Connell, J.D.; De Angelis, F.; Tessema, M.B.; Derfoufi, K.M.; Robles-Colmenares, Y.; Robbins, R.A.; Goormaghtigh, E.; et al. Structures of intermediate transport states of ZneA, a Zn(II)/proton antiporter. Proc. Natl. Acad. Sci. USA 2013, 110, 18484–18489. [Google Scholar] [CrossRef] [PubMed]
- Sota, M.; Tsuda, M.; Yano, H.; Suzuki, H.; Forney, L.J.; Top, E.M. Region-specific insertion of transposons in combination with selection for high plasmid transferability and stability accounts for the structural similarity of IncP-1 plasmids. J. Bacteriol. 2007, 189, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Leys, N.; Baatout, S.; Rosier, C.; Dams, A.; s’ Heeren, C.; Wattiez, R.; Mergeay, M. The response of Cupriavidus metallidurans CH34 to spaceflight in the international space station. Antonie Leeuwenhoek 2009, 96, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Mijnendonckx, K.; Provoost, A.; Ott, C.M.; Venkateswaran, K.; Mahillon, J.; Leys, N.; Van Houdt, R. Characterization of the survival ability of Cupriavidus metallidurans and Ralstonia pickettii from space-related environments. Microb. Ecol. 2013, 65, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Byloos, B.; Coninx, I.; Van Hoey, O.; Cockell, C.; Nicholson, N.; Ilyin, V.; Van Houdt, R.; Boon, N.; Leys, N. The impact of space flight on survival and interaction of Cupriavidus metallidurans CH34 with basalt, a volcanic moon analog rock. Front. Microbiol. 2017, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, P.; Vallet-Gely, I.; Soscia, C.; Genin, S.; Filloux, A. The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 2005, 151, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.G.; Fox, M.S. Transposon stability and a role for conjugational transfer in adaptive mutability. Proc. Natl. Acad. Sci. USA 2000, 97, 7393–7398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie-Oleza, J.A.; Lanfranconi, M.P.; Nogales, B.; Lalucat, J.; Bosch, R. Conjugative interaction induces transposition of ISPst9 in Pseudomonas stutzeri AN10. J. Bacteriol. 2009, 191, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Baharoglu, Z.; Bikard, D.; Mazel, D. Conjugative DNA transfer induces the bacterial SOS response and promotes antibiotic resistance development through integron activation. PLoS Genet. 2010, 6, e1001165. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Srivastava, P. Broad host range plasmids. FEMS Microbiol. Lett. 2013, 348, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obranic, S.; Babic, F.; Maravic-Vlahovicek, G. Improvement of pBBR1MCS plasmids, a very useful series of broad-host-range cloning vectors. Plasmid 2013, 70, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Wang, J.; Shen, X.; Rey, J.F.; Yuan, Q.; Yan, Y. Fundamental CRISPR-cas9 tools and current applications in microbial systems. Synth. Syst. Biotechnol. 2017, 2, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.B.; Rand, J.M.; Nurani, W.; Courtney, D.K.; Liu, S.A.; Pfleger, B.F. Genetic tools for reliable gene expression and recombineering in Pseudomonas putida. J. Ind. Microbiol. Biotechnol. 2018, 45, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Li, Z.; Liu, L.; Zhao, D.; Zhang, X.; Bi, C. Genome editing of Ralstonia eutropha using an electroporation-based CRISPR-cas9 technique. Biotechnol. Biofuels 2018, 11, 172. [Google Scholar] [CrossRef] [PubMed]
| Strain/Metal in mM | Hg2+ | Cd2+ | Ni2+ | Co2+ |
|---|---|---|---|---|
| C. metallidurans CH34 | 0.01 | 2.0 | 10.0 | 11.0 |
| C. metallidurans MSR33 | 0.10 | 4.0 | 12.0 | 20.0 |
| C. metallidurans CH34 (pBBR::merTPAGB1) | 0.10 | 2.0 | ND 1 | ND 1 |
| Type | Replicon (#) | Start | End | Size (bp) | Description | Targeted Gene(s) | Function (MaGe Annotation) (*) |
|---|---|---|---|---|---|---|---|
| IN | CHR1 | 328395 | 328395 | 1102 | IS1088 | Rmet_0312 | putative transporter |
| IN | CHR1 | 3106728 | 3106728 | 1102 | IS1088 | Rmet_2857 (tauB) | taurine ABC transporter ATP-binding protein |
| DEL | CHR2 | 602035 | 602490 | 455 | Rmet_4033 | LysR family transcriptional regulator | |
| IN | CHR2 | 741818 | 741818 | 1102 | IS1088 | Rmet_4160 (pelF) | EPS biosynthesis, biofilm formation |
| IN | CHR2 | 1529231 | 1529231 | 1102 | IS1088 | Rmet_4867 (acrA) | membrane fusion protein, multidrug efflux |
| IN | CHR2 | 2113815 | 2113815 | 256 | tniA/Tn3 | Rmet_5388 (apbE) | ApbE-like lipoprotein |
| IN | CHR2 | 2253560 | 2253560 | 3 | +CTT | Rmet_5508 | long-chain-fatty-acid-CoA ligase |
| DEL | CHR2 | 2253566 | 2253568 | 3 | −CGG | Rmet_5508 | long-chain-fatty-acid-CoA ligase |
| IN | CHR2 | 2440975 | 2440975 | 1104 | IS1088 | Rmet_5682 (nimB) | membrane fusion protein, heavy metal transport |
| IN | pMOL28 | 53484 | 53484 | 1104 | IS1088 | Rmet_6205 (cnrY) | antisigma factor |
| DEL | pTP6 | 26155 | 27385 | 1230 | upf30.5, upf31.0, parA | respectively an outer membrane protein, a DNA methylase, and a plasmid partition protein |
| Replicon (#) | Position | SNP | SNP Type | Affected Gene | Gene Description * |
|---|---|---|---|---|---|
| CHR1 | 333850 | A→G | intergenic (+201/−75) | Rmet_0314 →/→ ssb1 | putative transporter, major facilitator family/single-stranded DNA-binding protein (helix-destabilizing protein) |
| CHR1 | 645608 | A→G | A23A (GCA→GCG) | Rmet_0598 → | Ser/Thr protein phosphatase family protein |
| CHR1 | 2400725 | G→A | intergenic (−77/+116) | greA ←/← carB | transcription elongation factor/carbamoyl-phosphate synthase large subunit |
| CHR1 | 3418147 | A→G | V295A (GTC→GCC) | dppF ← | dipeptide transporter; ATP-binding component of ABC superfamily |
| CHR1 | 3444412 | A→G | V17A (GTC→GCC) | NirJ ← | heme d1 biosynthesis protein |
| CHR1 | 3456113 | A→G | V76A (GTG→GCG) | acyP ← | acylphosphatase |
| CHR2 | 2253543 | A→T | V158E (GTG→GAG) | Rmet_5508 ← | long-chain-fatty-acid-CoA ligase |
| CHR2 | 2253553 | G→T | P155T (CCG→ACG) | Rmet_5508 ← | long-chain-fatty-acid-CoA ligase |
| CHR2 | 2529357 | T→C | G264G (GGA→GGG) | Rmet_5769 ← | esterase |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millacura, F.A.; Janssen, P.J.; Monsieurs, P.; Janssen, A.; Provoost, A.; Van Houdt, R.; Rojas, L.A. Unintentional Genomic Changes Endow Cupriavidus metallidurans with an Augmented Heavy-Metal Resistance. Genes 2018, 9, 551. https://doi.org/10.3390/genes9110551
Millacura FA, Janssen PJ, Monsieurs P, Janssen A, Provoost A, Van Houdt R, Rojas LA. Unintentional Genomic Changes Endow Cupriavidus metallidurans with an Augmented Heavy-Metal Resistance. Genes. 2018; 9(11):551. https://doi.org/10.3390/genes9110551
Chicago/Turabian StyleMillacura, Felipe A., Paul J. Janssen, Pieter Monsieurs, Ann Janssen, Ann Provoost, Rob Van Houdt, and Luis A. Rojas. 2018. "Unintentional Genomic Changes Endow Cupriavidus metallidurans with an Augmented Heavy-Metal Resistance" Genes 9, no. 11: 551. https://doi.org/10.3390/genes9110551
APA StyleMillacura, F. A., Janssen, P. J., Monsieurs, P., Janssen, A., Provoost, A., Van Houdt, R., & Rojas, L. A. (2018). Unintentional Genomic Changes Endow Cupriavidus metallidurans with an Augmented Heavy-Metal Resistance. Genes, 9(11), 551. https://doi.org/10.3390/genes9110551







