Algal Lipids as Biocollector for Recovery of Coal from Fine Coal Waste by Froth Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Cultivation
2.2. Algae Lipid Extraction
2.3. Waste Coal
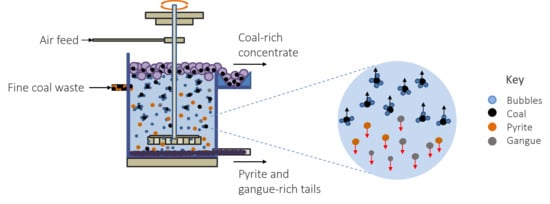
2.4. Flotation Experiments
3. Results
3.1. Site 1 Sample—Flotation Results
3.1.1. Effect of Collector Type
3.1.2. Effect of Collector Dosage
3.1.3. Effect of pH
3.2. Site 2 Sample—Flotation Results
3.2.1. Effect of Collector Type
3.2.2. Effect of Collector Dosage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Department of Energy South Africa. Coal Resources. Available online: http://www.energy.gov.za/files/esources/coal/coal_discards.html (accessed on 6 November 2018).
- Kalenga, P.M.; Cukrowska, E.; Tutu, H.; Chimuka, L. Characterization of South African coal for metals, inorganic and organic sulfur compounds. S. Afr. J. Chem. 2011, 64, 254–262. [Google Scholar]
- Kgabi, N.A.; Waanders, F.B.; Taole, S.H. Iron-Sulphur compounds of South African coal. Eur. J. Sci. Res. 2009, 34, 190–195. [Google Scholar]
- Maledi, N.B. Characterisation of Mineral Matter in South African Coals Using Micro-Raman Spectroscopy and Other Techniques. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2017. [Google Scholar]
- Chugh, Y.P.; Behum, P.T. Coal waste management practices in the USA: An overview. Int. J. Coal Sci. Technol. 2014, 1, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Komnitsas, K.; Paspaliaris, I.; Zilberchmidt, M.; Groudev, S. Environmental impacts at coal waste disposal sites-efficiency of desulfurization technologies. Glob. NEST J. 2001, 3, 109–116. [Google Scholar]
- Iroala, O.J. Combining Froth Flotation with Reflux Classification to Mitigate ARD Generating Potential of the Waterberg and Witbank Coal Ultrafines via Sulfide Removal. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2014. [Google Scholar]
- Kazadi Mbamba, C.; Harrison, S.T.L.; Franzidis, J.-P.; Broadhurst, J.L. Mitigating acid rock drainage risks while recovering low-Sulfur coal from ultrafine colliery wastes using froth flotation. Miner. Eng. 2012, 29, 13–21. [Google Scholar] [CrossRef]
- Kotsiopoulos, A.; Harrison, S. Enhancing ARD mitigation by application of benign tailings to reduce the permeability of waste rock dumps. Adv. Mater. Res. 2015, 1130, 560–563. [Google Scholar] [CrossRef]
- Harrison, S.T.L.; Broadhurst, J.L.; van Hille, R.P.; Oyekola, O.O.; Bryan, C.; Hesketh, A.; Opitz, A. A Systematic Approach to Sulphidic Waste Rock and Tailings Management to Minimise ARD Formation; Report No. 1831/1/10; South African Water Research Commission: Pretoria, South Africa, 2010. [Google Scholar]
- Amaral Filho, J.R.; Firpo, B.; Broadhurst, J.L.; Harrison, S.T.L. The feasibility of South African coal waste for production of “FabSoil’, a technosol. Miner. Eng. 2020, 146, 106059. [Google Scholar] [CrossRef]
- Firpo, B.; Amaral Filho, J.; André Homrich Schneider, I. A brief procedure to fabricate soils from coal mine wastes based on mineral processing, agricultural, and environmental concepts. Miner. Eng. 2015, 76, 81–86. [Google Scholar] [CrossRef]
- Harrison, S.T.L.; Franzidis, J.-P.; Van Hille, R.P.; Mokone, T.; Broadhurst, J.; Kazadi Mbamba, C.; Opitz, A.; Chiume, R.; Vries, E.; Stander, H.; et al. Evaluating Approaches to and Benefits of Minimising the Formation of Acid Rock Drainage through Management of the Disposal of Sulphidic Waste Rock and Tailings; Report No. 2015/1/13; South African Water Research Commission: Pretoria, South Africa, 2013. [Google Scholar]
- Harrison, S.T.L.; Broadhurst, J.L.; Opitz, A.; Fundikwe, B.; Stander, H.M.; Mostert, L.; Amaral Filho, J.; Kotsiopoulos, A. An Industrial Ecology Approach to Sulphide-Containing Mineral Wastes to Minimize ARD Formation Part 2: Design for Disposal and Extraction of Products of Value; Report No. WRC K5/2231b; South African Water Research Commission: Pretoria, South Africa, in press.
- El-Midany, A.A.; Abdel-Khalek, M.A. Reducing sulfur and ash from coal using Bacillus subtilis and Paenibacillus polymyxa. Fuel 2014, 115, 589–595. [Google Scholar] [CrossRef]
- Misra, M.; Smith, R.W.; Raichur, A.M.; Mouat, A.M.Y.; Bukka, K. Novel Microorganism for Selective Separation of Coal from Pyrite and Ash; DOE Grant No.: DE-FG22-93PC93215; The Department of Energy: Pittsburgh, PA, USA, 1995. [Google Scholar]
- Vasumathi, N.; Kumar, T.V.V.; Rao, S.S.; Prabhakar, S.; Raju, G.B.; Kumar, S.S.; Raman, U. Eco-Friendly and cost-Effective reagent for coal flotation. Int. J. Eng. Res. 2013, 2, 418–423. [Google Scholar]
- Yi, Q.; Li, W.; Zhang, X.; Feng, J.; Zhang, J.; Wu, J. Tech-Economic evaluation of waste cooking oil to bio-Flotation agent technology in the coal flotation industry. J. Clean. Prod. 2015, 95, 131–141. [Google Scholar] [CrossRef]
- Dube, R.M. Collectors for Enabling Flotation of Oxidized Coal. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2012. [Google Scholar]
- Xia, W.; Yang, J.; Liang, C. Improving oxidized coal flotation using biodiesel as a collector. Int. J. Coal Prep. Util. 2013, 33, 181–187. [Google Scholar] [CrossRef]
- Kazadi Mbamba, C.; Franzidis, J.-P.; Harrison, S.T.L.; Broadhurst, J.L. Flotation of coal and sulphur from South African ultrafine colliery wastes. J. South. Afr. Inst. Min. Metall. 2013, 113, 399–405. [Google Scholar]
- Jera, M.K. An Economic Analysis of Coal Desulphurisation by Froth Flotation to Prevent Acid Rock Drainage (ARD) and an Economic Review of Capping Covers and ARD Treatment Processes. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2013. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Schlagermann, P.; Göttlicher, G.; Dillschneider, R.; Rosello-Sastre, R.; Posten, C. Composition of algal oil and its potential as biofuel. J. Combust. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.J.; Makhost, Z.; Barzana, E.; Karel, M. Lipid content and fatty acid composition of green algae Scenedesmus obliquus grown in a constant cell density apparatus. Food Biotechnol. 1987, 1, 117–128. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Dominguez, H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013. [Google Scholar]
- Axelsson, M.; Gentili, F. A single-Step method for rapid extraction of total lipids from green microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef] [Green Version]
- Ramuedzisi, H.E. Challenges in Recycling Used Cooking Oil to Produce Biodiesel in Polokwane. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2016. [Google Scholar]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J. Appl. Phycol. 2012, 24, 989–1001. [Google Scholar] [CrossRef]
- Naghdi, F.G.; Gonzalez, L.M.G.; Chan, W.; Schenk, P.M. Progress on lipid extraction from wet algal biomass for biodiesel production. Microb. Biotechnol. 2016, 9, 718–726. [Google Scholar] [CrossRef]
- Langley, N.; Harrison, S.T.L.; Van Hille, R.P. A critical evaluation of CO2 supplementation to algal systems by direct injection. Biochem. Eng. J. 2012, 68, 70–75. [Google Scholar] [CrossRef]
- Griffiths, M.J. Optimising Microalgal Lipid Productivity for Biodiesel Production. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2011. [Google Scholar]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. Selection of direct transesterification as the preferred method for assay of fatty acid content of microalgae. Lipids 2010, 45, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.J. Mixing, Mass Transfer and Energy Analysis across Bioreactor Types in Microalgal Cultivation and Lipid Production. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2015. [Google Scholar]
- Han, C. Coal Cleaning by Froth Floatation. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1983. [Google Scholar]
- Salentinig, S.; Sagalowicz, L.; Glatter, O. Self-Assembled structures and pKa value of oleic acid in systems of biological relevance. Langmuir 2010, 26, 11670–11679. [Google Scholar] [CrossRef] [PubMed]
- Pashkovskaya, A.A.; Vazdar, M.; Zimmermann, L.; Jovanovic, O.; Pohl, P.; Poh, E.E. Mechanism of long-Chain free fatty acid protonation at the membrane-Water interface. Biophys. J. 2018, 114, 2142–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, X.; Meng, Y.; Wan, L.; Li, G.; Yang, M.; Jin, W. pH-Responsive aqueous foams of oleic acid/oleate solution. J. Dispers. Sci. Technol. 2014, 35, 293–300. [Google Scholar] [CrossRef]
- Kurniawan, J.; Suga, K.; Kuhl, T.L. Interaction forces and membrane charge tunability: Oleic acid containing membranes in different pH conditions. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 211–217. [Google Scholar] [CrossRef]
- Humeres, E.; Debacher, N.A. Kinetics and mechanism of coal flotation. Colloid Polym. Sci. 2001, 280, 365–371. [Google Scholar] [CrossRef]











| Collector Type | Overall Yield (%) | Combustibles Recovery (%) | Ash Recovery (%) | Sulphur Recovery (%) | Flotation Efficiency Index (%) |
|---|---|---|---|---|---|
| RALs | 34.1 ± 1.2 | 47.1 ± 1.6 | 19.2 ± 0.7 | 25.3 ± 3.4 | 27.8 ± 1.8 |
| FAMEs | 35.3 ± 0.7 | 50.3 ± 1.4 | 18.4 ± 0.6 | 24.1 ± 2.4 | 31.7 ± 1.6 |
| Oleic Acid | 34.3 ± 0.6 | 50.7 ± 1.2 | 16.7 ± 0.6 | 18.9 ± 1.7 | 34.0 ± 1.3 |
| Dodecane | 15.4 ± 0.1 | 21.4 ± 0.2 | 8.8 ± 0.1 | 11.1 ± 0.6 | 12.6 ± 0.3 |
| Collector Type | Ash Content (%) | Sulphur Content (%) |
|---|---|---|
| (Feed) | (49.0) | (5.71) |
| RALs | 26.1 ± 0.3 | 2.6 ± 0.3 |
| FAMEs | 24.1 ± 0.1 | 2.8 ± 0.3 |
| Oleic Acid | 23.5 ± 0.7 | 2.4 ± 0.2 |
| Dodecane | 27.0 ± 0.3 | 2.6 ± 0.1 |
| Collector Type | Overall Yield (%) | Combustibles Recovery (%) | Ash Recovery (%) | Sulphur Recovery (%) | Flotation Efficiency Index (%) |
|---|---|---|---|---|---|
| RALs | 95.2 ± 0.1 | 97.6 ± 0.2 | 87.8 ± 0.4 | 86.7 ± 5.3 | 9.8 ± 0.5 |
| FAMEs | 96.0 ± 0.1 | 97.8 ± 0.2 | 90.3 ± 0.4 | 97.7 ± 5.3 | 7.5 ± 0.01 |
| Oleic Acid | 86.2 ± 0.3 | 91.0 ± 0.3 | 71.3 ± 0.4 | 73.5 ± 11.1 | 19.7 ± 0.01 |
| Dodecane | 61.7 ± 0.9 | 68.9 ± 1.1 | 40.0 ± 1.2 | - | 28.9 ± 0.03 |
| Collector Type | Ash Content (%) | Sulphur Content (%) |
|---|---|---|
| (Feed) | (26) | (0.91) |
| RALs | 22.8 ± 0.1 | 0.8 ± 0.04 |
| FAMEs | 23.2 ± 0.1 | 0.7 ± 0.04 |
| Oleic Acid | 20.2 ± 0.1 | 0.7 ± 0.05 |
| Dodecane | 16.1 ± 0.4 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiodza, K.G.; Harrison, S.T.L.; Fagan-Endres, M.A. Algal Lipids as Biocollector for Recovery of Coal from Fine Coal Waste by Froth Flotation. Minerals 2020, 10, 70. https://doi.org/10.3390/min10010070
Chiodza KG, Harrison STL, Fagan-Endres MA. Algal Lipids as Biocollector for Recovery of Coal from Fine Coal Waste by Froth Flotation. Minerals. 2020; 10(1):70. https://doi.org/10.3390/min10010070
Chicago/Turabian StyleChiodza, Kudzai G., Susan T. L. Harrison, and Marijke A. Fagan-Endres. 2020. "Algal Lipids as Biocollector for Recovery of Coal from Fine Coal Waste by Froth Flotation" Minerals 10, no. 1: 70. https://doi.org/10.3390/min10010070
APA StyleChiodza, K. G., Harrison, S. T. L., & Fagan-Endres, M. A. (2020). Algal Lipids as Biocollector for Recovery of Coal from Fine Coal Waste by Froth Flotation. Minerals, 10(1), 70. https://doi.org/10.3390/min10010070