Chemical and Phase Reactions on the Contact between Refractory Materials and Slags, a Case from the 19th Century Zn-Pb Smelter in Ruda Śląska, Poland
Abstract
1. Introduction
2. Location and Historical Smelting Process
3. Materials and Methods
3.1. Sampling
3.2. Phase Composition and Chemistry of Phases
3.2.1. Microscopic Observations, SEM-EDS and EPMA
3.2.2. Raman Spectroscopy
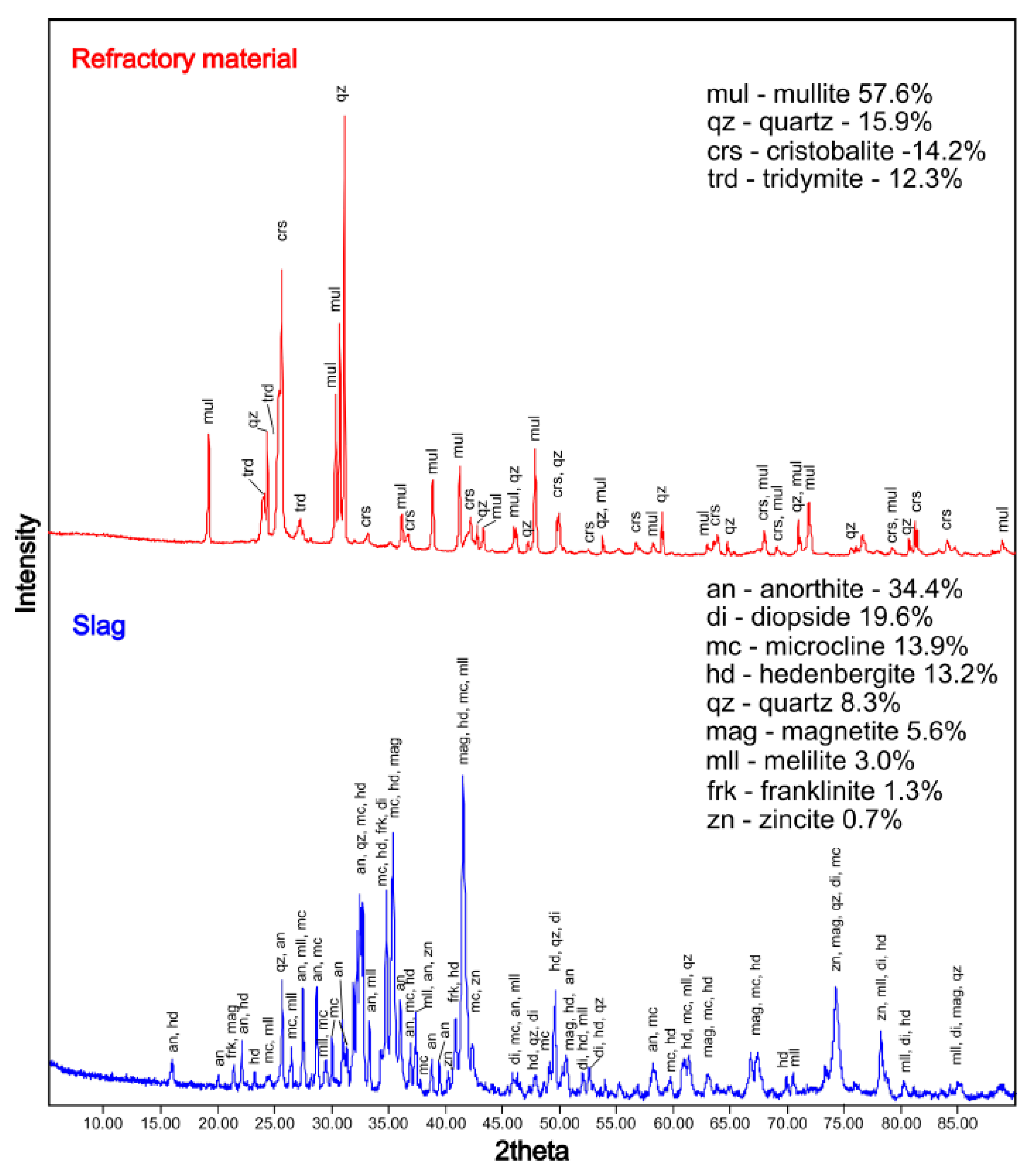
3.2.3. X-ray Diffraction
3.3. Bulk Chemical Composition
4. Results
4.1. Chemistry
4.2. Phase Composition
4.3. Chemistry of Phases
4.3.1. Oxides
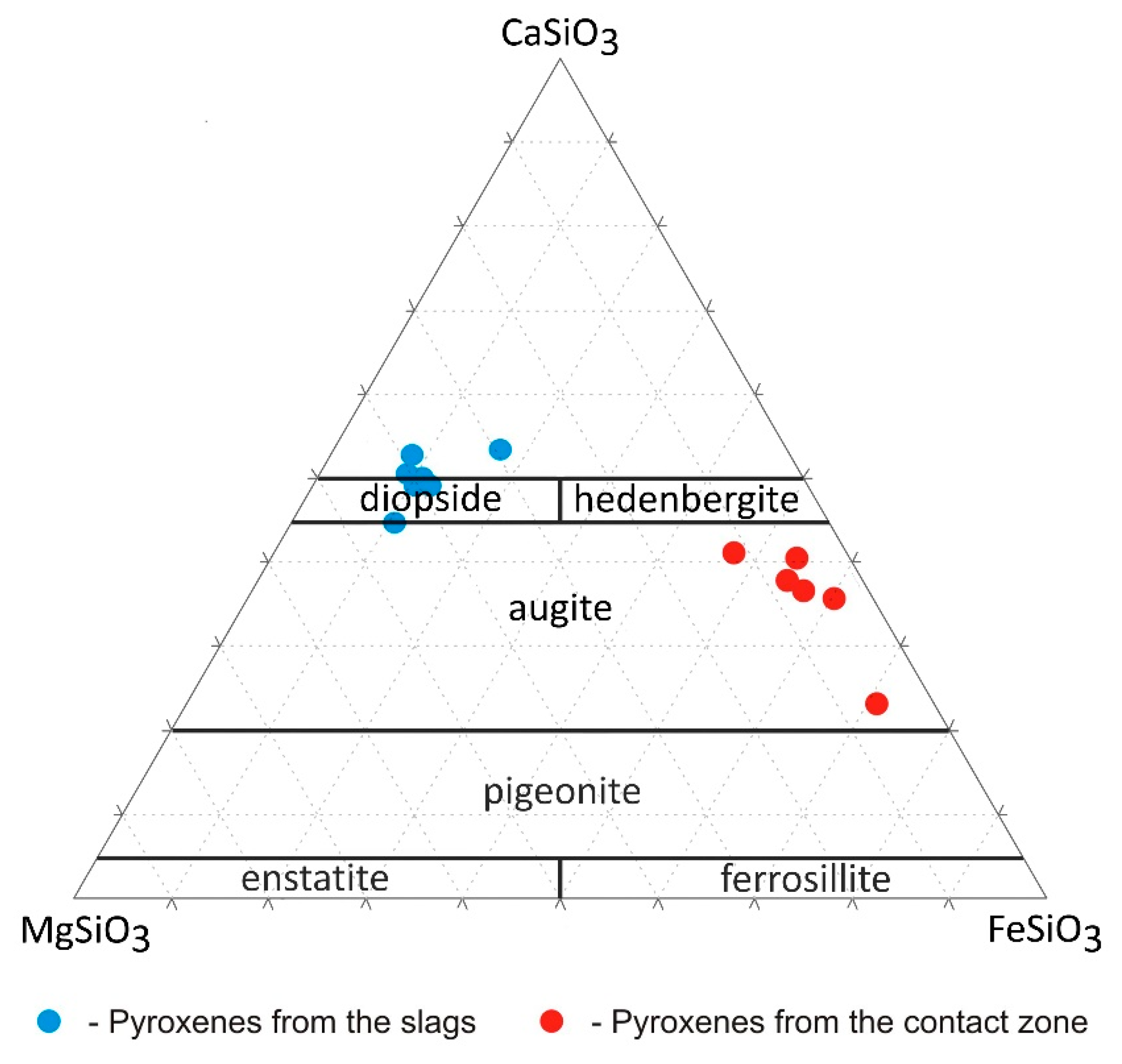
4.3.2. Silicates and Aluminosilicates
4.3.3. Glass
5. Discussion
5.1. Reactions of the Gas Phase with Refractory Materials
5.2. Chemical and Phase Reactions on the Contact between Melt and Refractory Elements
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Godzik, B.; Woch, M.W. History of mining in the Olkusz region. In Natural and Historical Values of the Olkusz Ore-Bearing Region; Polish Academy of Sciences: Krakow, Poland, 2015; pp. 29–37. [Google Scholar]
- De Windt, L.; Chaurand, P.; Rose, J. Kinetics of steel slag leaching: Batch tests and modeling. Waste Manag. 2011, 31, 225–235. [Google Scholar] [CrossRef]
- Ettler, V.; Legendre, O.; Bodean, F.; Touray, J.C. Primary phases and natural weathering of old lead-zinc pyrometallurgical slag from Príbram, Czech Republic. Can. Miner. 2001, 39, 873–888. [Google Scholar] [CrossRef]
- Ettler, V.; Mihaliewic, M.; Touray, J.C.; Piantone, P. Leaching of polished sections: An integrated approach for studying the liberation of heavy metals from lead-zinc metallurgical slags. Bull. Société Géologique Fr. 2002, 173, 161–169. [Google Scholar] [CrossRef]
- Kucha, H.; Martens, A.; Ottenburgs, R.; De Vos, W.; Viaene, W. Primary minerals of Zn-Pb mining and metallurgical dumps and their environmental behavior at Plombières, Belgium. Environ. Geol. 1996, 27, 1–15. [Google Scholar] [CrossRef]
- Liu, T.; Li, F.; Jin, Z.; Yang, Y. Acidic leaching of potentially toxic metals cadmium, cobalt, chromium, copper, nickel, lead, and zinc from two Zn smelting slag materials incubated in an acidic soil. Environ. Pollut. 2018, 238, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Faheem, M.; Linghao, Z.; Shan, L.; Xiao, H.; Binquan, J.; YanChyuan, S.; Dongwei, L. Solidification/stabilization of lead-zinc smelting slag in composite based geopolymer. J. Clean. Prod. 2019, 209, 1206–1215. [Google Scholar]
- Mouni, L.; Belkhiri, L.; Bouzaza, A.; Bollinger, J.C. Chemical associations and sorption capacity of Pb and Zn: Column experiments on a polluted soil from the Amizour mining district (Algeria). Environ. Earth Sci. 2016, 75, 96. [Google Scholar] [CrossRef]
- Piatak, N.M.; Seal, R., II. Mineralogy and the release of trace elements from slag from the Hegeler Zinc smelter, Illinois (USA). Appl. Geochem. 2010, 25, 302–320. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R., II. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Tyszka, R.; Kierczak, J.; Pietranik, A.; Ettler, V.; Mihaljevic, M. Extensive weathering of zinc smelting slag in a heap in Upper Silesia (Poland): Potential environmental risks posed by mechanical disturbance of slag deposits. Appl. Geochem. 2014, 40, 70–81. [Google Scholar] [CrossRef]
- Warchulski, R.; Mendecki, M.; Gawęda, A.; Sołtysiak, M.; Gadowski, M. Rainwater-induced migration of potentially toxic elements from a Zn–Pb slag dump in Ruda Śląska in light of mineralogical, geochemical and geophysical investigations. Appl. Geochem. 2019, 109, 104396. [Google Scholar] [CrossRef]
- Ettler, V.; Mihaljevič, M.; Jarošíková, A.; Culka, A.; Kříbek, B.; Majer, V.; Vaněk, A.; Penížek, V.; Sracek, O.; Mapani, B.; et al. Vanadium-rich slags from the historical processing of Zn–Pb–V ores at Berg Aukas (Namibia): Mineralogy and environmental stability. Appl. Geochem. 2020, 114, 1935–2016. [Google Scholar] [CrossRef]
- Mendecki, M.; Warchulski, R.; Szczuka, M.; Środek, D.; Pierwoła, J. Geophysical and petrological studies of the former lead smelting waste dump in Sławków, Poland. J. Appl. Geophys. 2020, 179, 104080. [Google Scholar] [CrossRef]
- Geseler, J. Use of stellworks slag in Europe. Waste Manag. 1996, 16, 59–63. [Google Scholar] [CrossRef]
- Alwaeli, M. Application of granulated lead–zinc slag in concrete as an opportunity to save natural resources. Radiat. Phys. Chem. 2013, 83, 54–60. [Google Scholar] [CrossRef]
- Warchulski, R.; Gawęda, A.; Janeczek, J.; Kądziołka-Gaweł, M. Mineralogy and origin of coarse-grained segregations in the pyrometallurgical Zn-Pb slags from Katowice-Wełnowiec (Poland). Mineral. Petrol. 2016, 110, 681–692. [Google Scholar] [CrossRef]
- Zieliński, K.; Rzeszowski, M. Wykorzystanie odpadów hutniczych w przemyśle cementowym. Hut. Wiadomości. Hut. 2007, 74, 610–615. [Google Scholar]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Fečko, P.; Zechner, V.; Guziurek, M.; Lyčková, B.; Pertile, E. Zastosowanie ługowania bakteryjnego do odpadów ze starych składowisk ekologicznych usytuowanych w regionie Karlove Vary. Inżynieria Miner. 2012, 13, 55–64. [Google Scholar]
- Hołda, A.; Kisielowska, E. Biological removal of Cr(VI) ions from aqueous solutions by Trichoderma viride. Physicochem. Probl. Miner. Process. 2013, 49, 47–60. [Google Scholar]
- Chiarantini, L.; Benvenuti, M.; Costagliola, P.; Fedi, M.E.; Guideri, S.; Romualdi, A. Copper production at Baratti (Populonia, southern Tuscany) in the early Etruscan period (9th–8th centuries BC). J. Archaeol. Sci. 2009, 36, 1626–1636. [Google Scholar] [CrossRef]
- Ettler, V.; Cervinka, R.; Johan, Z. Mineralogy of medieval slags from lead and silver smelting (Bohutín, Příbram district, Czech Republic): Towards estimation of historical smelting conditions. Archaeometry 2009, 51, 987–1007. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z.; Zavřel, J.; Wallisová, M.S.; Mihaljevič, M.; Šebek, O. Slag remains from the Na Slupi site (Prague, Czech Republic): Evidence for early medieval non-ferrous metal smelting. J. Archaeol. Sci. 2015, 53, 72–83. [Google Scholar] [CrossRef]
- Toffolo, L.; Addis, A.; Martin, S.; Nimis, P.; Rottoli, M.; Godard, G. The Misérègne slag deposit (Valle d’Aosta, Western Alps, Italy): Insights into (pre-)Roman copper metallurgy. J. Archaeol. Sci. Rep. 2018, 19, 248–260. [Google Scholar] [CrossRef]
- Warchulski, R.; Juszczuk, P.; Gawęda, A. Geochemistry, petrology and evolutionary computations in the service of archaeology: Restoration of the historical smelting process at the Katowice–Szopienice site. Archaeol. Anthropol. Sci. 2018, 10, 1023–1035. [Google Scholar] [CrossRef]
- Cabała, J.; Warchulski, R.; Rozmus, D.; Środek, D.; Szełęg, E. Pb-Rich slags, minerals, and pollution resulted from a medieval Ag-Pb smelting and mining operation in the Silesian-Cracovian region (Southern Poland). Minerals 2020, 10, 28. [Google Scholar] [CrossRef]
- Burger, E.; Bourgarit, D.; Wattiaux, A.; Fialin, M. The reconstruction of the first copper-smeltingprocesses in Europe during the 4th and the 3rd millennium BC: Where does the oxygen come from? Appl. Phys. A 2010, 100, 713–724. [Google Scholar] [CrossRef]
- Kierczak, J.; Pietranik, A. Mineralogy and composition of historical Cu slags from the Rudawy Janowickie mountains, southwestern Poland. Can. Mineral. 2011, 49, 1281–1296. [Google Scholar] [CrossRef]
- Piatak, N.; Seal, R., II. Mineralogy and environmental geochemistry of historical iron slag, Hopewell Furnace National Historic Site, Pennsylvania, USA. Appl. Geochem. 2012, 27, 623–643. [Google Scholar] [CrossRef]
- Ströbele, F.; Wenzel, T.; Kronz, A.; Hildebrandt, L.H.; Markl, G. Mineralogical and geochemical characterization of high-medieval lead–silver smelting slags from Wiesloch near Heidelberg (Germany)—An approach to process reconstruction. Archaeol. Anthropol. Sci. 2010, 2, 191–215. [Google Scholar] [CrossRef][Green Version]
- Warchulski, R.; Gawęda, A.; Kądziołka-Gaweł, M.; Szopa, K. Composition and element mobilization in pyrometallurgical slags from the Orzeł Biały smelting plant in the Bytom–Piekary Śląskie area, Poland. Mineral. Mag. 2015, 79, 459–483. [Google Scholar] [CrossRef]
- Warchulski, R. Zn-Pb slag crystallization: Evaluating temperature conditions on the basis of geothermometry. Eur. J. Mineral. 2016, 28, 375–384. [Google Scholar] [CrossRef]
- Warchulski, R.; Gawęda, A.; Kupczak, K.; Banasik, K.; Krzykawski, T. Slags from Ruda Śląska, Poland as a large-scale laboratory for the crystallization of rare natural rocks: Melilitolites and paralavas. Lithos 2020, 372–373, 105666. [Google Scholar] [CrossRef]
- De Wilde, E.; Bellemans, I.; Campforts, M.; Guo, M.; Blanpain, B.; Moelans, N.; Verbeken, K. Investigation of high-temperature slag/copper/spinel interactions. Metall. Mater. Trans. B 2016, 47, 3421–3434. [Google Scholar] [CrossRef]
- Puziewicz, J.; Zainoun, K.; Bril, H. Primary phases in pyrometallurgical slags from a zinc-smelting waste dump, Świętochłowice, Upper Silesia, Poland. Can. Mineral. 2007, 45, 1189–1200. [Google Scholar] [CrossRef]
- Tyszka, R.; Pietranik, A.; Kierczak, J.; Zieliński, G.; Darling, J. Cadmium distribution in Pb-Zn slags from Upper Silesia, Poland: Implications for cadmium mobility from slag phases to the environment. J. Geochem. Explor. 2018, 186, 215–224. [Google Scholar] [CrossRef]
- Jonczy, I.; Gawor, Ł. Coal mining and post-metallurgic dumping grounds and their connections with exploitation of raw materials in the region of Ruda Śląska. Arch. Min. Sci. 2017, 62, 301–311. [Google Scholar] [CrossRef]
- Geoportal. Available online: geoportal.gov.pl (accessed on 10 July 2020).
- Dębicki, J. Przemysł Cynkowy. Szkic Historyczno-Gospodarczy; Skład główny; Gebethner i Wolff: Warszawa, Poland, 1927. [Google Scholar]
- Dobis, N. Przemysł Cynku i Ołowiu w Polsce; Chrześcijańska Drukarnia “Nakładowa”: Będzin, Poland, 1938. [Google Scholar]
- Domański, W.; Krupkowski, A. Metalurgia Cynku i Kadmu; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1954. [Google Scholar]
- Piernikarczyk, J. Historia Górnictwa i Hutnictwa na Górnym Śląsku. Cz. 2; Śląski Związek Akademicki: Katowice, Poland, 1936. [Google Scholar]
- Morimoto, N. Nomenclature of Pyroxenes. Mineral. Petrol. 1988, 39, 55–76. [Google Scholar] [CrossRef]
- Brawer, S.A.; White, W.B. Raman spectroscopic investigation of the structure of silicate glasses (II). Soda-alkaline earth-alumina ternary and quaternary glasses. J. Non Cryst. Solids 1977, 23, 261–278. [Google Scholar] [CrossRef]
- McMillan, P. Structural studies of silicate glasses and melts—Applications and limitations of Raman spectroscopy. Am. Mineral. 1984, 69, 622–644. [Google Scholar]
- Mysen, B.O.; Finger, L.W.; Virgo, D.; Seifert, F.A. Curve-fitting of Raman spectra of silicate glasses. Am. Mineral. 1982, 67, 686–695. [Google Scholar]
- Neuville, D.R.; De Ligny, D.; Henderson, G.S. Advances in Raman spectroscopy applied to earth and material sciences. Rev. Mineral. Geochem. 2014, 78, 509–541. [Google Scholar] [CrossRef]
- Seifert, F.A.; Mysen, B.O.; Virgo, D. Three-dimensional network structure of quenched melts (glass) in the systems SiO2–NaAlO2, SiO2–CaAl2O4 and SiO2–MgAl2O4. Am. Mineral. 1982, 67, 696–717. [Google Scholar]
- Konijnendijk, W.L.; Buster, J.H.J.M. Raman-scattering measurements of silicate glasses containing sulphate. J. Non-Cryst. Solids 1977, 23, 401–418. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Jahanshahi, S.; Wright, S. Kinetics of reduction of CaO-FeOx-MgO-PbO-SiO2 slags by CO-CO2 gas mixtures. Metall. Mater. Trans. B 2017, 48, 2057–2066. [Google Scholar] [CrossRef]
- Shevchenko, M.; Jak, E. Experimental phase equilibria studies of the PbO–SiO2 system. J. Am. Ceram. Soc. 2018, 101, 458–471. [Google Scholar] [CrossRef]
- Cullers, R.L. Magmatic processes. In Geochemistry. Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1998; pp. 373–414. [Google Scholar]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 2 September 2020).
- Perry, R.H.; Chilton, C.H. Chemical Engineers’ Handbook, 5th ed.; McGraw-Hill: New York, NY, USA, 1973; p. 50. [Google Scholar]
- Gavrichev, K.; Bolshakov, A.; Kondakov, D.; Khoroshilov, A.; Denisov, S. Thermal transformations of lead oxides. J. Therm. Anal. Calorim. 2008, 92, 857–863. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, Y.; Zou, Z.; Zeilstra, C.; Meijer, K.; Boom, R. Thermal decomposition behaviour of fine iron ore particles. ISIJ Int. 2014, 54, 2196–2205. [Google Scholar] [CrossRef]
- Helsen, L.; Van den Bulck, E.; Van Bael, M.K.; Vanhoyland, G.; Mullens, J. Thermal behaviour of arsenic oxides (As2O5 and As2O3) and the influence of reducing agents (glucose and activated carbon). Thermochim. Acta 2004, 414, 145–153. [Google Scholar] [CrossRef]





| Element | Standard | Element | Standard | Element | Standard |
|---|---|---|---|---|---|
| Na | NaAlSi3O8 | Al | KAlSi3O8 | Mn | MnSiO3 |
| As | GaAs | K | KAlSi3O8 | S | BaSO4 |
| Si | CaMgSi2O6 | Fe | Fe2O3 | Ti | TiO2 |
| Mg | CaMgSi2O6 | Zn | ZnS | Pb | PbCrO4 |
| Ca | CaMgSi2O6 | Ni | NiO | P | YPO4 |
| Cr | Cr2O3 | Cl | Na4BeAlSi4O12Cl | Ba | BaSO4 |
| Co | CoO | Sr | SrSO4 | V | V2O5 |
| Oxide/Element | Concentration | Slag | Refractory Material |
|---|---|---|---|
| SiO2 | % | 38.17 | 65.70 |
| TiO2 | % | 0.69 | 0.73 |
| Al2O3 | % | 15.81 | 29.08 |
| Fe2O3 | % | 22.71 | 2.38 |
| Cr2O3 | % | 0.02 | 0.03 |
| MnO | % | 0.41 | 0.02 |
| BaO | % | 0.71 | 0.02 |
| MgO | % | 5.73 | 0.30 |
| CaO | % | 10.56 | 0.41 |
| K2O | % | 1.58 | 0.59 |
| Na2O | % | 0.27 | 0.06 |
| P2O5 | % | 0.25 | 0.08 |
| TOT/C | % | 0.05 | 0.02 |
| TOT/S | % | 0.13 | 0.03 |
| LOI | % | −0.3 | 0.0 |
| V | PPM | 141 | 97 |
| Ni | PPM | 141 | 135 |
| Zn | PPM | >10,000 | 223 |
| As | PPM | 2155 | 23 |
| Sr | PPM | 590 | 64 |
| Zr | PPM | 156 | 113 |
| Pb | PPM | 3775 | 145 |
| Oxide | Refractory Material | Slag | Contact Zone | ||
|---|---|---|---|---|---|
| qz | crn | spl | qz | alhem | |
| SiO2 | 98.71 | 0.02 | 0.42 | 97.72 | bd |
| TiO2 | bd | bd | 0.69 | bd | 0.28 |
| Al2O3 | 0.81 | 99.68 | 43.98 | 0.19 | 85.63 |
| FeOcalc | - | - | 13.74 | 0.28 | na |
| Fe2O3calc | 0.77 | - | - | na | 13.07 |
| MnO | na | na | 0.18 | bd | bd |
| ZnO | na | na | 36.79 | 0.22 | na |
| MgO | na | na | 2.94 | bd | bd |
| CaO | 0.29 | na | bd | bd | 0.11 |
| K2O | 0.11 | na | na | na | bd |
| Total | 100.69 | 99.7 | 98.74 | 98.41 | 99.09 |
| Element | Atoms per formula unit | ||||
| qz | crn | spl | qz | alhem | |
| Si | 0.99 | - | 0.01 | 0.99 | - |
| Ti4+ | - | - | 0.02 | - | - |
| Al | 0.01 | 2 | 1.69 | - | 1.81 |
| Fe2+ | - | - | 0.37 | - | - |
| Fe3+ | 0.01 | - | - | - | 0.18 |
| Mn | - | - | - | - | - |
| Zn | - | - | 0.88 | - | - |
| Mg | - | - | 0.14 | - | - |
| Ca | - | - | - | - | - |
| K | - | - | - | - | - |
| O2- | 2.00 | 3.00 | 4.00 | 2.00 | 3.00 |
| Oxide | Refractory Material | Slag | Contact Zone | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mulcore | mulrim | cpx 1 | cpx 2 | mll | mc 1 | an 1 | cpx 3 | mc 3 | mc 4 | an2 | mul | |
| SiO2 | 25.55 | 26.23 | 44.23 | 48.74 | 37.91 | 62.31 | 44.94 | 48.96 | 60.29 | 42.40 | 45.97 | 25.89 |
| TiO2 | 0.90 | 0.74 | 0.22 | 0.72 | bd | 1.02 | bd | 0.15 | 0.36 | 0.11 | bd | 0.36 |
| Al2O3 | 72.52 | 64.84 | 8.44 | 3.73 | 5.75 | 18.56 | 33.77 | 1.44 | 20.12 | 19.39 | 33.42 | 72.81 |
| FeOcalc | - | - | 8.96 | 5.83 | 5.86 | - | - | 24.12 | - | 0.76 | - | na |
| Fe2O3calc | 0.87 | 7.03 | - | - | - | 0.66 | 1.62 | - | 0.41 | 1.45 | 0.40 | |
| MnO | na | na | 0.60 | 0.48 | 0.87 | bd | bd | 1.23 | bd | bd | bd | bd |
| ZnO | na | na | 0.34 | 5.16 | 2.13 | bd | 0.29 | 5.67 | bd | 1.93 | 0.59 | na |
| BaO | na | na | na | na | na | 4.67 | 0.11 | bd | 4.83 | 0.43 | 0.19 | na |
| MgO | na | na | 10.60 | 12.55 | 8.05 | bd | bd | 9.61 | bd | 0.10 | 0.13 | na |
| CaO | bd | bd | 25.52 | 21.69 | 36.36 | 0.36 | 19.70 | 6.88 | 0.31 | 1.39 | 18.98 | na |
| Na2O | na | na | bd | 0.21 | 0.67 | 0.79 | 0.51 | 0.12 | 0.86 | 0.27 | 0.17 | na |
| K2O | bd | bd | bd | bd | 0.20 | 11.65 | 0.32 | na | 13.26 | 4.80 | 0.38 | na |
| V2O3 | na | na | bd | 0.12 | bd | na | na | na | na | na | na | na |
| Cr2O3 | na | na | 0.19 | bd | 0.20 | na | na | na | na | na | na | na |
| PbO | na | na | na | na | na | bd | bd | bd | 0.14 | 29.48 | bd | na |
| Total | 99.84 | 98.84 | 99.10 | 99.23 | 98.00 | 100.02 | 101.26 | 98.18 | 100.58 | 101.06 | 101.28 | 99.46 |
| Element | Atoms per Formula Unit | |||||||||||
| mulcore | mulrim | cpx 1 | cpx 2 | mll 10 | mc 1 | an 1 | cpx 3 | mc 3 | mc 4 | an 2 | mul | |
| Si | 1.83 | 1.94 | 1.71 | 1.87 | 1.83 | 2.93 | 2.07 | 1.99 | 2.87 | 2.52 | 2.11 | 1.85 |
| Ti4+ | 0.05 | 0.04 | 0.01 | 0.02 | - | 0.04 | - | - | - | - | - | 0.02 |
| Al | 6.12 | 5.64 | 0.38 | 0.17 | 0.33 | 1.03 | 1.84 | 0.07 | 1.13 | 1.36 | 1.81 | 6.15 |
| Fe2+calc | - | - | 0.29 | 0.19 | 0.24 | - | - | 0.82 | - | 0.04 | - | - |
| Fe3+calc | 0.05 | 0.39 | - | - | - | 0.02 | 0.06 | - | - | - | 0.05 | 0.02 |
| Mn | - | - | 0.02 | 0.01 | 0.03 | - | - | 0.04 | 0.02 | - | - | - |
| Zn | - | - | 0.01 | 0.15 | 0.08 | - | 0.01 | 0.17 | - | 0.08 | 0.02 | - |
| Ba | - | - | - | - | - | 0.09 | - | - | 0.09 | 0.01 | - | - |
| Mg | - | - | 0.61 | 0.72 | 0.58 | - | - | 0.58 | - | 0.01 | 0.01 | - |
| Ca | - | - | 1.06 | 0.89 | 1.88 | 0.02 | 0.97 | 0.30 | 0.02 | 0.09 | 0.93 | - |
| Na | - | - | - | 0.02 | 0.06 | 0.07 | 0.05 | 0.01 | 0.08 | 0.03 | 0.02 | - |
| K | - | - | - | - | 0.01 | 0.70 | 0.02 | - | 0.80 | 0.36 | 0.02 | - |
| V3+ | - | - | - | - | - | - | - | - | - | - | - | - |
| Cr3+ | - | - | 0.01 | - | 0.01 | - | - | - | - | - | - | - |
| Pb | - | - | - | - | - | - | - | - | - | 0.47 | - | - |
| O2- | 13.00 | 13.00 | 6.00 | 6.00 | 7.00 | 8.00 | 8.00 | 6.00 | 8.00 | 8.00 | 8.00 | 13.00 |
| Oxide | gl 1 | gl 2 | gl 3 | gl 4 * | gl 5 * |
|---|---|---|---|---|---|
| SiO2 | 65.92 | 75.12 | 44.02 | 1.64 | 36.05 |
| TiO2 | 1.02 | 1.27 | 1.43 | 0.11 | 0.70 |
| Al2O3 | 15.98 | 11.94 | 16.99 | 0.24 | 4.09 |
| Fe2O3calc | 1.51 | 0.39 | 14.34 | 3.25 | 5.92 |
| MnO | bd | bd | bd | 0.22 | 0.56 |
| ZnO | bd | 1.23 | na | 2.68 | 6.60 |
| BaO | 2.22 | 0.23 | 13.43 | bd | bd |
| MgO | 0.16 | bd | 0.21 | bd | bd |
| CaO | 1.60 | 0.78 | 1.24 | 1.50 | 2.80 |
| Na2O | 0.89 | 0.54 | 0.12 | 0.16 | 0.13 |
| K2O | 9.41 | 5.92 | 6.27 | 0.42 | 0.21 |
| PbO | bd | 3.18 | 0.53 | 69.40 | 28.39 |
| Sb2O5 | bd | na | na | bd | bd |
| As2O3 | bd | na | na | 15.52 | 15.37 |
| SO3 | bd | bd | 0.26 | 1.98 | bd |
| P2O5 | na | 0.14 | na | na | na |
| Cl | bd | na | na | 1.58 | bd |
| Total | 98.71 | 100.74 | 98.84 | 98.70 | 100.82 |
| Component | Density [g/cm3] | Melting Point [°C] | Boiling Point [°C] |
|---|---|---|---|
| Pb | 11.34 | 327.4 | 1740 |
| Fe | 7.87 | 1538 | 2861 |
| Zn | 7.13 | 419 | 907 |
| As | 5.78 | 817 1 | 614 |
| Mg | 1.74 | 649 | 1100 |
| Ca | 1.54 | 842 | 1484 |
| Na | 0.97 | 97.82 | 881.4 |
| K | 0.86 | 63.5 | 758.8 |
| PbO | 9.5 | 888 | 1470 |
| PbO2 | 9.38 | 290 | 2 |
| FeO | 5.7 | 1360 | 3414 |
| Fe2O3 | 5.24 | 1565 | 3 |
| ZnO | 5.6 | 1975 | 2 360 |
| As2O3 | 3.74 | 312.8 * | 457.2 * |
| As2O5 | 4.3 | 315 | 4 |
| MgO | 3.58 | 2800 | 3600 |
| CaO | 3.34 | 2570 | 2850 |
| Na2O | 2.27 | 1275 | 1275 5 |
| K2O | 2.3 | 6 | |
| Al2O3 | 3.97 | 2054 | 3000 |
| SiO2 | 2.6 | 1713 | 2230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupczak, K.; Warchulski, R.; Dulski, M.; Środek, D. Chemical and Phase Reactions on the Contact between Refractory Materials and Slags, a Case from the 19th Century Zn-Pb Smelter in Ruda Śląska, Poland. Minerals 2020, 10, 1006. https://doi.org/10.3390/min10111006
Kupczak K, Warchulski R, Dulski M, Środek D. Chemical and Phase Reactions on the Contact between Refractory Materials and Slags, a Case from the 19th Century Zn-Pb Smelter in Ruda Śląska, Poland. Minerals. 2020; 10(11):1006. https://doi.org/10.3390/min10111006
Chicago/Turabian StyleKupczak, Krzysztof, Rafał Warchulski, Mateusz Dulski, and Dorota Środek. 2020. "Chemical and Phase Reactions on the Contact between Refractory Materials and Slags, a Case from the 19th Century Zn-Pb Smelter in Ruda Śląska, Poland" Minerals 10, no. 11: 1006. https://doi.org/10.3390/min10111006
APA StyleKupczak, K., Warchulski, R., Dulski, M., & Środek, D. (2020). Chemical and Phase Reactions on the Contact between Refractory Materials and Slags, a Case from the 19th Century Zn-Pb Smelter in Ruda Śląska, Poland. Minerals, 10(11), 1006. https://doi.org/10.3390/min10111006






