Derivation of Flotation Kinetic Model for Activated and Depressed Copper Sulfide Minerals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Mineral Specimens for Contact Angle Measurements and Single Mineral Flotation Tests
2.1.2. Mixed Copper Sulfide Minerals Ore Sample for Mixture Mineral Flotation Tests
2.2. NaHS Treatments Before Each Measurement and Test
2.2.1. Contact Angle Measurements
2.2.2. Single Mineral Flotation Tests
2.2.3. Mixture Mineral Flotation Tests
2.3. Contact Angle Measurement
2.4. Single Mineral Flotation Tests
2.5. Mixture Mineral Flotation Tests
3. Results and Discussion
3.1. Contact Angle Measurements
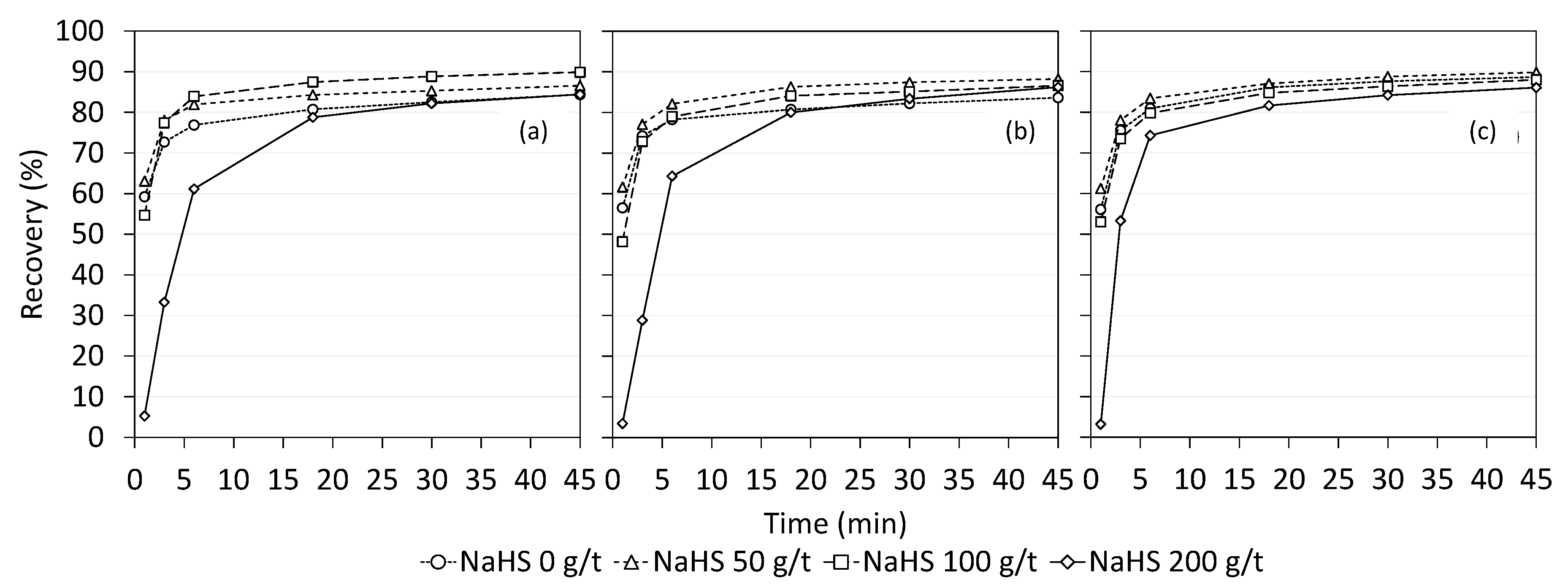
3.2. Single Mineral Flotation Tests with the Small Column Cell
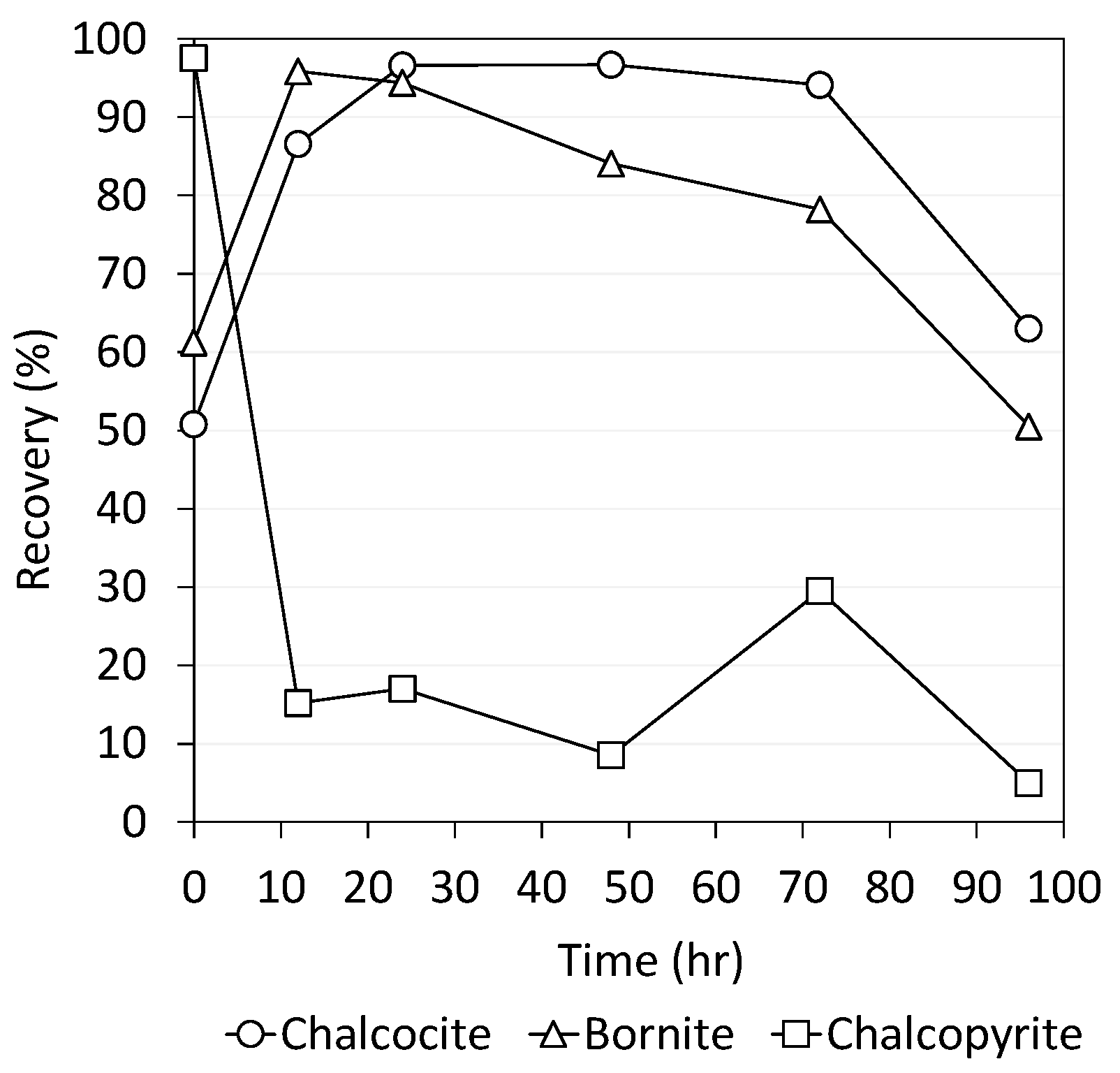
3.3. Mixture Mineral Flotation Tests
3.4. Derivation of Flotation Kinetic Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Law, K.-Y.; Zhao, H. Surface Wetting: Characterization, Contact Angle, and Fundamentals, 1st ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Young, T. An essay on the cohesion of flui ds. Phil. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Wills, B.A. Will’s Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Hirajima, T.; Miki, H.; Suyantara, G.P.W.; Matsuoka, H.; Elmahdy, A.M.; Sasaki, K.; Kuroiwa, S. Selective flotation of chalcopyrite and molybdenite with H2O2 oxidation. Miner. Eng. 2017, 100, 83–92. [Google Scholar] [CrossRef]
- Gardner, J.R.; Woods, R. An electrochemical investigation of the natural flotability of chalcopyrite. Int. J. Miner. Process. 1979, 6, 1–16. [Google Scholar] [CrossRef]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Adsorption characteristics and mechanisms of O-Carboxymethyl chitosan on chalcopyrite and molybdenite. J. Colloid Interface Sci. 2019, 552, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.N.; Woods, R. An X-ray photoelectron spectroscopic study of the oxidation of chalcopyrite. Aust. J. Chem. 1984, 37, 2403–2413. [Google Scholar] [CrossRef]
- Pratesi, G.; Cipriani, C. Selective depth analyses of the alteration products of bornite, chalcopyrite and pyrite performed by XPS, AES and RBS. Eur. J. Mineralol. 2000, 12, 397–409. [Google Scholar] [CrossRef]
- Yin, Q.; Kelsall, G.H.; Vaughan, D.J.; England, K. Atmospheric and electrochemical oxidation of the surface of chalcopyrite (CuFeS2). Geochim. Cosmochim. Acta 1995, 59, 1091–1100. [Google Scholar] [CrossRef]
- Yin, Q.; Vaughan, D.J.; England, K.E.R.; Kelsall, G.H.; Brandon, N.P. Surface oxidation of chalcopyrite (CuFeS2) in alkaline solutions. J. Electrochem. Soc. 2000, 147, 2945–2951. [Google Scholar] [CrossRef]
- Rosso, K.M.; Vaughan, D.J. Reactivity of sulfide mineral surfaces. Rev. Mineral. Geochem. 2006, 61, 557–607. [Google Scholar] [CrossRef]
- Agar, G.E.; Chia, J.; Requis, C.L. Flotation rate measurements to optimize an operating circuit. Miner. Eng. 1998, 11, 347–360. [Google Scholar] [CrossRef]
- Zuniga, H.G. Flotation recovery is an exponential function of its rate. Min. Bull. Nat. Soc. Min. Santiago Chile 1935, 47, 83–86. [Google Scholar]
- Jameson, G.J.S.; Nam, S.; Young, M.M. Physical Factors Affecting Recovery Rates in Flotation. Miner. Sci. Eng. 1977, 9, 103–118. [Google Scholar]
- Niemi, A.J. Role of kinetics in modelling and control of flotation plants. Powder Technol. 1995, 82, 69–77. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Saraiva, S.M.; Pimenta, J.S.; Oliveira, A.P.A. Kinetics of pyrochlore flotation from Araxa mineral deposits. Miner. Eng. 2001, 14, 99–105. [Google Scholar] [CrossRef]
- Uçurum, M. Influences of Jameson flotation operation variables on the kinetics and recovery of unburned carbon. Powder Technol. 2009, 191, 240–246. [Google Scholar] [CrossRef]
- Ma, G.; Xia, W.; Xie, G. Effect of particle shape on the flotation kinetics of fine coking coal. J. Clean. Prod. 2018, 195, 470–475. [Google Scholar] [CrossRef]
- Sutherland, K.L. Physical chemistry of flotation. XI. Kinetics of the flotation process. J. Phys. Colloid Chem. 1948, 52, 394. [Google Scholar] [CrossRef]
- Bayat, O.; Ucurum, M.; Poole, C. Effects of size distribution on flotation kinetics of Turkish sphalerite. Miner. Process. Extr. Metall. 2013, 113, 53–59. [Google Scholar] [CrossRef]
- Dowling, E.C.; Klimpel, R.R.; Aplan, F.F. Model discrimination in the flotation of a porphyry copper ore. Miner. Metall. Process. 1985, 2, 87–101. [Google Scholar] [CrossRef]
- Welsby, S.D.D.; Vianna, S.M.S.M.; Franzidis, J.-P. Assigning physical significance to floatability components. Int. J. Miner. Process. 2010, 97, 59–67. [Google Scholar] [CrossRef]
- Runge, K.C.; Harris, M.C.; Frew, J.A.; Manlapig, E.V. Floatability of streams around the Cominco Red Dog lead cleaning circuit. In Sixth Mill Operators Conference, AusIMM, Madang, Papua New Guinea; Australasian Institute of Mining and Metallurgy: Carlton, Australia, 1997; pp. 157–163. [Google Scholar]
- Zhang, H.; Liu, J.; Cao, Y.; Wang, Y. Effects of particle size on lignite reverse flotation kinetics in the presence of sodium chloride. Powder Technol. 2013, 246, 658–663. [Google Scholar] [CrossRef]
- Chaves, A.P.; Ruiz, A.S. Considerations on the kinetics of froth flotation of ultrafine coal contained in tailings. Coal Prep. 2009, 29, 289–297. [Google Scholar] [CrossRef]
- Saleh, A.M. A Study on the Performance of Second Order Models and Two Phase Models in Iron Ore Flotation. Physicochem. Probl. Miner. Process. 2010, 44, 215. [Google Scholar]
- Yuan, X.M.; Palsson, B.I.; Forssberg, K.S.E. Statistical interpretation of flotation kinetics for a complex sulphide ore. Miner. Eng. 1996, 9, 429–442. [Google Scholar] [CrossRef]
- Finkelstein, N.P. The activation of sulphide minerals for flotation: A review. Int. J. Miner. Process. 1997, 52, 81–120. [Google Scholar] [CrossRef]
- Allison, S.A.; O’Connor, C.T. An investigation into the flotation behaviour of pyrrhotite. Int. J. Miner. Process. 2011, 98, 202–207. [Google Scholar] [CrossRef]
- Reddy, G.S.; Reddy, C.K. The chemistry of activation of sphalerite—A review. Miner. Proc. Extract. Metall. Rev. 1988, 4, 1–38. [Google Scholar] [CrossRef]
- Gerson, A.R.; Lange, A.G.; Prince, K.E.; Smart, R.St.C. The mechanism of copper activation of sphalerite. Appl. Surf. Sci. 1999, 137, 207–223. [Google Scholar] [CrossRef]
- Zhou, R.; Chander, S. Kinetics of sulfidization of malachite in hydrosulfide and tetrasulfide solutions. Int. J. Miner. Process. 1993, 37, 257–272. [Google Scholar] [CrossRef]
- Ma, R.; Stegemeier, J.; Levard, C.; Dale, J.G.; Noack, C.W.; Yang, T.; Brown, G.E., Jr.; Lowry, G.V. Sulfidation of copper oxide nanoparticles and properties of resulting copper sulfide. Environ. Sci. Nano 2014, 1, 347–357. [Google Scholar] [CrossRef]
- Bulatovic, S. Handbook of Flotation Reagents: Chemistry, Theory and Practice: Volume 1: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ansar, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Matsuoka, H.; Mitsuhashi, K.; Kawata, M.; Kato, T.; Tokoro, C.; Haga, K.; Shibayama, A. Surface properties of copper-sulfide minerals with sodium-hydrosulfide activation. Miner. Eng. 2020, 156, 106530. [Google Scholar] [CrossRef]
- Fairthorne, G.; Fornasiero, D.; Ralston, J. Effect of oxidation on the collectorless flotation of chalcopyrite. Int. J. Miner. Process. 1997, 49, 31–48. [Google Scholar] [CrossRef]
- Hirajima, T.; Mori, M.; Ichikawa, O.; Sasaki, K.; Miki, H.; Farahat, M.; Sawada, M. Selective flotation of chalcopyrite and molybdenite with plasma pretreatment. Miner. Eng. 2014, 66–68, 102–111. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Wei, D.; Wang, W.; Cui, B.; Liu, W. Effect of copper ions on the flotation separation of chalcopyrite and molybdenite using sodium sulfide as a depressant. Miner. Eng. 2018, 115, 44–52. [Google Scholar] [CrossRef]










| Name of the Model | Formula |
|---|---|
| Classical first-order model [19] | |
| First-order model with rectangular distribution of floatability [20,21] | |
| Floatability component model [22,23] | |
| Fully mixed reactor model [24] | |
| Improved gas and solid adsorption model [25] | |
| Second-order model with rectangular distribution of floatability [26,27] |
| Element | Cu | Fe | S | Si | As | Ag | Pb |
|---|---|---|---|---|---|---|---|
| (Mass%) | |||||||
| Chalcocite, Cu2S | 83.9 | 1.64 | 14.3 | 0.04 | 0.03 | - | - |
| Bornite, Cu5FeS4 | 71.5 | 8.78 | 19.3 | 0.12 | - | 0.19 | - |
| Chalcopyrite, CuFeS2 | 38.2 | 31.6 | 28.4 | 0.05 | - | - | 1.64 |
| Minerals | Content Ratio (Mass %) |
|---|---|
| Calcite | 69.3 |
| Orthoclase | 11.2 |
| Albite | 6.4 |
| Quartz | 5.7 |
| Copper sulfides | 2.0 |
| Others | 5.4 |
| Copper-Sulfide Minerals | Content Ratio (Mass %) |
|---|---|
| Bornite | 53.9 |
| Chalcopyrite | 27.7 |
| Chalcocite | 18.4 |
| Minerals | pH | NaHS | |
|---|---|---|---|
| Chalcocite | Activation | + | + |
| Depression | + | + | |
| Bornite | Activation | − | + |
| Depression | + | + | |
| Chalcopyrite | Activation | + | + |
| Depression | + | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka, H.; Mitsuhashi, K.; Kawata, M.; Tokoro, C. Derivation of Flotation Kinetic Model for Activated and Depressed Copper Sulfide Minerals. Minerals 2020, 10, 1027. https://doi.org/10.3390/min10111027
Matsuoka H, Mitsuhashi K, Kawata M, Tokoro C. Derivation of Flotation Kinetic Model for Activated and Depressed Copper Sulfide Minerals. Minerals. 2020; 10(11):1027. https://doi.org/10.3390/min10111027
Chicago/Turabian StyleMatsuoka, Hidekazu, Kohei Mitsuhashi, Masanobu Kawata, and Chiharu Tokoro. 2020. "Derivation of Flotation Kinetic Model for Activated and Depressed Copper Sulfide Minerals" Minerals 10, no. 11: 1027. https://doi.org/10.3390/min10111027
APA StyleMatsuoka, H., Mitsuhashi, K., Kawata, M., & Tokoro, C. (2020). Derivation of Flotation Kinetic Model for Activated and Depressed Copper Sulfide Minerals. Minerals, 10(11), 1027. https://doi.org/10.3390/min10111027






