Thermal Beneficiation of Sra Ouertane (Tunisia) Low-Grade Phosphate Rock
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USGS. Phosphate Rock; United States Geological Survey: Reston, VA, USA, 2015.
- Boujlel, H.; Daldoul, G.; Tlil, H.; Souissi, R.; Chebbi, N.; Fattah, N.; Souissi, F. The Beneficiation Processes of Low-Grade Sedimentary Phosphates of Tozeur-Nefta Deposit. Minerals 2019, 9, 2. [Google Scholar] [CrossRef]
- Khleifia, N.; Hannachi, A.; Abbes, N. Studies of uranium recovery from tunisian wet process phosphoric acid. Int. J. Innov. Appl. Stud. 2013, 3, 1066–1071. [Google Scholar]
- Scholz, R.W.; Wellmer, F.-W. Although there is no Physical Short-Term Scarcity of Phosphorus, its Resource Efficiency Should be Improved. J. Ind. Ecol. 2018, 23, 313–318. [Google Scholar] [CrossRef]
- Geissler, B.; Hermann, L.; Mew, M.C.; Steiner, G. Striving Toward a Circular Economy for Phosphorus: The Role of Phosphate Rock Mining. Minerals 2018, 8, 395. [Google Scholar] [CrossRef]
- Steiner, G.; Geissler, B.; Haneklaus, N. Making Uranium Recovery from Phosphates Great Again? Environ. Sci. Technol. 2020, 54, 1287–1289. [Google Scholar] [CrossRef]
- Geissler, B.; Mew, M.C.; Matschullat, J.; Steiner, G. Innovation potential along the phosphorus supply chain: A micro and macro perspective on the mining phase. Sci. Total Environ. 2020, 714, 136701. [Google Scholar] [CrossRef]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; du Bray, E.A.; Koenig, A.E. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Chen, M.; Graedel, T.E. The potential for mining trace elements from phosphate rock. J. Clean. Prod. 2015, 91, 337–346. [Google Scholar] [CrossRef]
- Hermann, L.; Kraus, F.; Hermann, R. Phosphorus processing—Potentials for higher efficiency. Sustainability 2018, 10, 1482. [Google Scholar] [CrossRef]
- Haneklaus, N.; Sun, Y.; Bol, R.; Lottermoser, B.; Schnug, E. To extract, or not to extract uranium from phosphate rock, that is the question. Environ. Sci. Technol. 2017, 51, 753–754. [Google Scholar] [CrossRef]
- Da Silva, E.; Mlayah, A.; Gomes, C.; Noronha, F.; Charef, A.; Sequeira, C.; Esteves, V.; Raquel, A.; Marques, F. Heavy elements in the phosphorite from Kalaat Khasba mine (North-western Tunisia): Potential implications on the environment and human health. J. Hazard. Mater. 2010, 182, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Tlig, S.; Sassi, A.; Belayouni, H.; Michel, D. Distribution de l’Uranium, du Thorium, du Zironium, du Hafnium et des Terres Rares (TR) dans des Grains de Phosphates Sedimentaires. Chem. Geol. 1987, 62, 209–221. [Google Scholar] [CrossRef]
- Sassi, A.B.; Zaier, A.; Joron, J.L.; Treuil, M.; Mao, J.; Bierlein, F.P. Rare earth elements distribution of Tertiary phosphorites in Tunisia. Miner. Depos. Res. Meet. Glob. Chall. 2005, 1161–1164. [Google Scholar] [CrossRef]
- Galfati, I.; Beji Sassi, A.; Zaier, A.; Bouchardon, J.L.; Bilal, E.; Joron, J.L.; Sassi, S. Geochemistry and mineralogy of Paleocene—Eocene Oum El Khecheb phosphorites (Gafsa—Metlaoui Basin) Tunisia. Geochem. J. 2010, 44, 189–210. [Google Scholar] [CrossRef][Green Version]
- Gallala, W.; Saïdi, M.; Zayani, K. Characterization and Valorization of Tozeur-Nefta Phosphate Ore Deposit (Southwestern Tunisia). Procedia Eng. 2016, 138, 8–18. [Google Scholar] [CrossRef]
- Salem, M.; Souissi, R.; Souissi, F.; Abbes, N.; Moutte, J. Phosphoric acid purification sludge: Potential in heavy metals and rare earth elements. Waste Manag. 2019, 83, 46–56. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Daghbouj, N.; Abda, H.; Castet, S.; Josse, C.; Van Beek, P.; Souhaut, M.; Michel, S.; Bejaoui, N.; et al. Characterization of phosphate rock and phosphogypsum from Gabes phosphate fertilizer factories (SE Tunisia): High mining potential and implications for environmental protection. Environ. Sci. Pollut. Res. 2018, 25, 14690–14702. [Google Scholar] [CrossRef]
- Abbes, N. Uranium extraction from phosphoric acid: Challenges and opportunities. In Proceedings of the Workshop on “Application of UNFC-2009” for Uranium Resources, Johannesburg, South Africa, 10–14 November 2014. [Google Scholar]
- Abbes, N. Processing Low Grade Phosphate Rock from Tunisia using HTGR: Opportunities and challenges. In Proceedings of the CRP-Meeting, Vienna, Austria, 2–5 November 2015. [Google Scholar]
- Gharabaghi, M.; Irannajad, M.; Noaparast, M. A review of the beneficiation of calcareous phosphate ores using organic acid leaching. Hydrometallurgy 2010, 103, 96–107. [Google Scholar] [CrossRef]
- Al-Fariss, T.F.; Ozbelge, H.O.; Abdulrazik, A.M. Flotation of a carbonate rich sedimentary phosphate rock. Nutr. Cycl. Agroecosyst. 1991, 10, 203–208. [Google Scholar] [CrossRef]
- Guo, F.; Li, J. Separation strategies for Jordanian phosphate rock with siliceous and calcareous gangues. Int. J. Miner. Process. 2010, 97, 74–78. [Google Scholar] [CrossRef]
- Khaddor, M.; Ziyad, M.; Joffre, J.; Amblès, A. Pyrolysis and characterization of the kerogen from the Moroccan Youssoufia rock phosphate. Chem. Geol. 2002, 186, 17–30. [Google Scholar] [CrossRef]
- Watti, A.; Alnjjar, M.; Hammal, A. Improving the specifications of Syrian raw phosphate by thermal treatment. Arab. J. Chem. 2016, 9, S637–S642. [Google Scholar] [CrossRef]
- Bezzi, N.; Aïfa, T.; Hamoudi, S.; Merabet, D. Trace Elements of Kef Es Sennoun Natural Phosphate (Djebel Onk, Algeria) and how they Affect the Various Mineralurgic Modes of Treatment. Procedia Eng. 2012, 42, 1915–1927. [Google Scholar] [CrossRef]
- Abouzeid, A.Z.M. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Haneklaus, N.; Schröders, S.; Zheng, Y.; Allelein, H.-J. Economic evaluation of flameless phosphate rock calcination with concentrated solar power and high temperature reactors. Energy 2017, 140, 1148–1157. [Google Scholar] [CrossRef]
- Haneklaus, N.; Zheng, Y.; Allelein, H.-J. Stop Smoking—Tube-In-Tube Helical System for Flameless Calcination of Minerals. Processes 2017, 5, 67. [Google Scholar] [CrossRef]
- Bojinova, D. Thermal treatment of mixtures of Tunisian phosphorite and additives of aluminum silicate. Thermochim. Acta 2003, 404, 155–162. [Google Scholar] [CrossRef]
- Pelovski, Y.; Petkova, V.; Dombalov, I. Thermal analysis of mechanoactivated mixtures of tunisia phosphorite and ammonium sulfate. J. Therm. Anal. Calorim. 2003, 72, 967–980. [Google Scholar] [CrossRef]
- Galai, H.; Sliman, F. Mineral characterization of the Oum El Khacheb phosphorites (Gafsa-Metlaoui basin; S Tunisia). Arab. J. Chem. 2013. [Google Scholar] [CrossRef]
- Elgharbi, S.; Horchani-Naifer, K.; Férid, M. Investigation of the structural and mineralogical changes of Tunisian phosphorite during calcinations. J. Therm. Anal. Calorim. 2015, 119, 265–271. [Google Scholar] [CrossRef]
- Bachouâ, H.; Othmani, M.; Coppel, Y.; Fatteh, N.; Debbabi, M.; Badraoui, B. Structural and thermal investigations of a Tunisian natural phosphate rock. J. Mater. Environ. Sci. 2014, 5, 1152–1159. [Google Scholar]
- Jaballi, F.; Felhi, M.; Khelifi, M.; Fattah, N.; Zayani, K.; Abbes, N.; Elouadi, B.; Tlili, A. Mineralogical and geochemical behavior of heated natural carbonate-apatite of the Ypresian series, Maknassy-Mezzouna basin, central Tunisia. Carbonates Evaporites 2019, 34, 1689–1702. [Google Scholar] [CrossRef]
- Daik, R.; Lajnef, M.; Amor, S.B.; Ezzaouia, H. Results in Physics Effect of the temperature and the porosity of the gettering process on the removal of heavy metals from Tunisian phosphate rock. Res. Phys. 2017, 7, 4189–4194. [Google Scholar]
- Dabbebi, R.; De Aguiar, J.L.B.; Samet, B.; Baklouti, S. Mineralogical and chemical investigation of Tunisian phosphate washing waste during calcination. J. Therm. Anal. Calorim. 2019, 3. [Google Scholar] [CrossRef]
- Qualité du Sol—Préparation d’un Échantillon—NF X 31-101; Office International de l’Eau (OIEau): Paris, France, 1992.
- International Centre for Diffraction Data (ICDD). Available online: https://www.icdd.com/ (accessed on 4 April 2020).
- Komar Kawatra, S.; Carlson, J.T. Beneficiation of Phosphate Ore; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2013. [Google Scholar]
- Tonsuaadu, K.; Gross, K.A.; Pluduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim. 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Chaney, R.L. Chapter Two—Food Safety Issues for Mineral and Organic Fertilizers; Elsevier Science & Technology: Amsterdam, The Netherlands, 2012; Volume 117, ISBN 9780123942784. [Google Scholar]
- Ulrich, A.E. Cadmium governance in Europe’s phosphate fertilizers: Not so fast? Sci. Total Environ. 2019, 650, 541–545. [Google Scholar] [CrossRef]
- Tulsidas, H.; Gabriel, S.; Kiegiel, K.; Haneklaus, N. Uranium resources in EU phosphate rock imports. Resour. Policy 2019, 61, 151–156. [Google Scholar] [CrossRef]
- Haneklaus, N.; Bayok, A.; Fedchenko, V. Phosphate Rocks and Nuclear Proliferation. Sci. Glob. Secur. 2017, 25, 143–158. [Google Scholar] [CrossRef]
- López, L.; Castro, L.N.; Scasso, R.A.; Grancea, L.; Tulsidas, H.; Haneklaus, N. Uranium supply potential from phosphate rocks for Argentina’s nuclear power fleet. Resour. Policy 2019, 62, 397–404. [Google Scholar] [CrossRef]
- Ye, Y.; Al-Khaledi, N.; Barker, L.; Darwish, M.S.; El Naggar, A.M.A.; El-Yahyaoui, A.; Hussein, A.; Hussein, E.-S.; Shang, D.; Taha, M.; et al. Uranium resources in China’s phosphate rocks—Identifying low-hanging fruits Uranium resources in China ’ s phosphate rocks—Identifying low-hanging fruits. IOP Conf. Ser. Earth Environ. Sci. 2019, 227. [Google Scholar] [CrossRef]
- Garnit, H.; Bouhlel, S.; Barca, D.; Chtara, C. Application of LA-ICP-MS to sedimentary phosphatic particles from Tunisian phosphorite deposits: Insights from trace elements and REE into paleo-depositional environments. Chemie Erde 2012, 72, 127–139. [Google Scholar] [CrossRef]
- Khater, A.E.M.; Galmed, M.A.; Nasr, M.M.; El-Taher, A. Uranium and rare earth elements in Hazm El-Jalamid phosphate, Saudi Arabia: Concentrations and geochemical patterns comparison. Environ. Earth Sci. 2016, 75, 1261. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhang, L. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes—A critical review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar] [CrossRef]
- Al Khaledi, N.; Taha, M.; Hussein, A.; Hussein, E.; El Yahyaoui, A.; Haneklaus, N. Direct leaching of rare earth elements and uranium from phosphate rocks. IOP Conf. Ser. Mater. Sci. Eng. 2019, 479, 012065. [Google Scholar] [CrossRef]
- Jefferson Lab The Periodic Table of Elements. Available online: https://education.jlab.org/itselemental/index.html (accessed on 4 August 2020).
- Koleva, V.; Petkova, V. IR spectroscopic study of high energy activated Tunisian phosphorite. Vib. Spectrosc. 2012, 58, 125–132. [Google Scholar] [CrossRef]
- Reitsma, F.; Woods, P.; Fairclough, M.; Kim, Y.; Tulsidas, H.; Lopez, L.; Zheng, Y.; Hussein, A.; Brinkmann, G.; Haneklaus, N.; et al. On the sustainability and progress of energy neutral mineral processing. Sustainability 2018, 10, 235. [Google Scholar] [CrossRef]


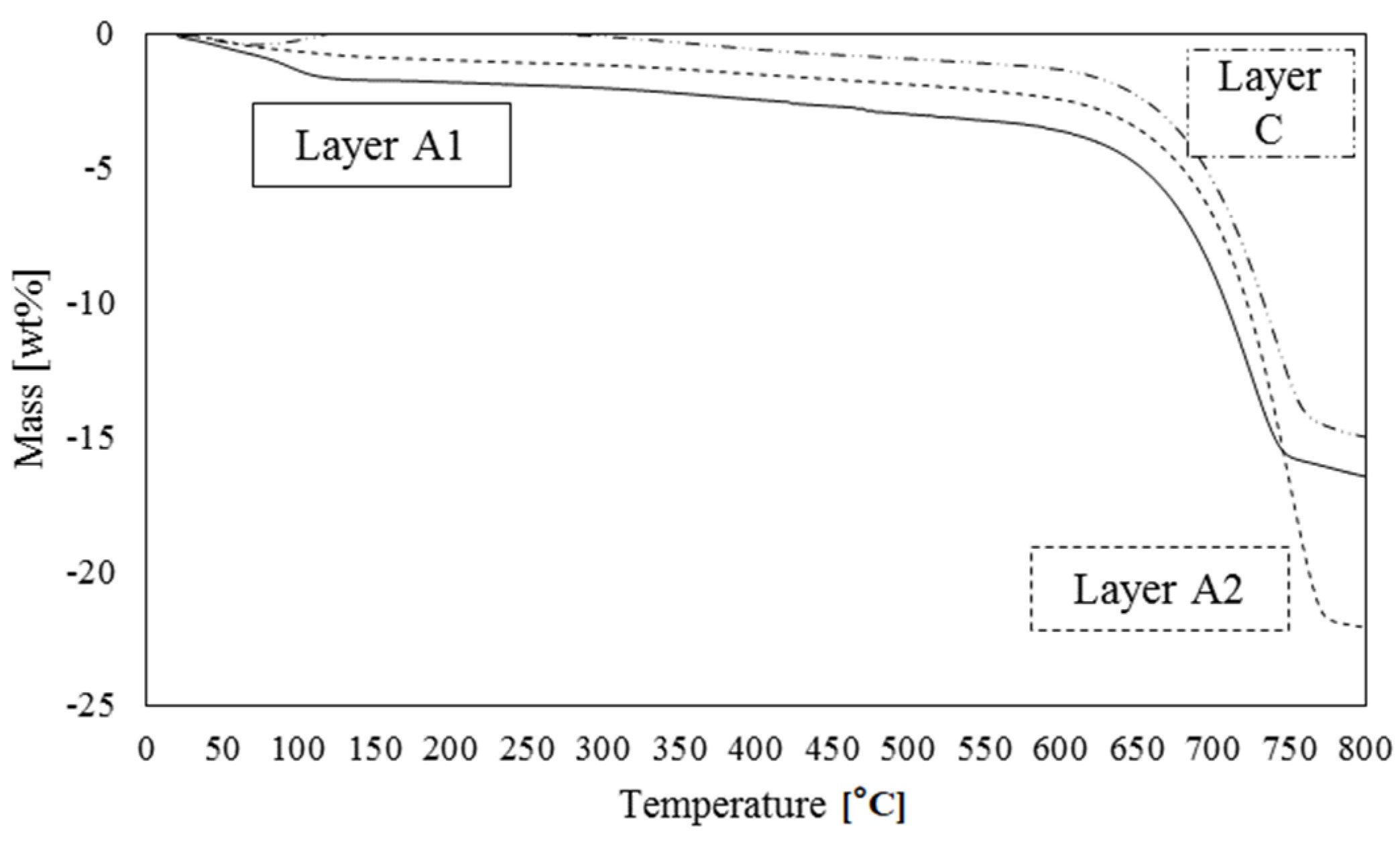


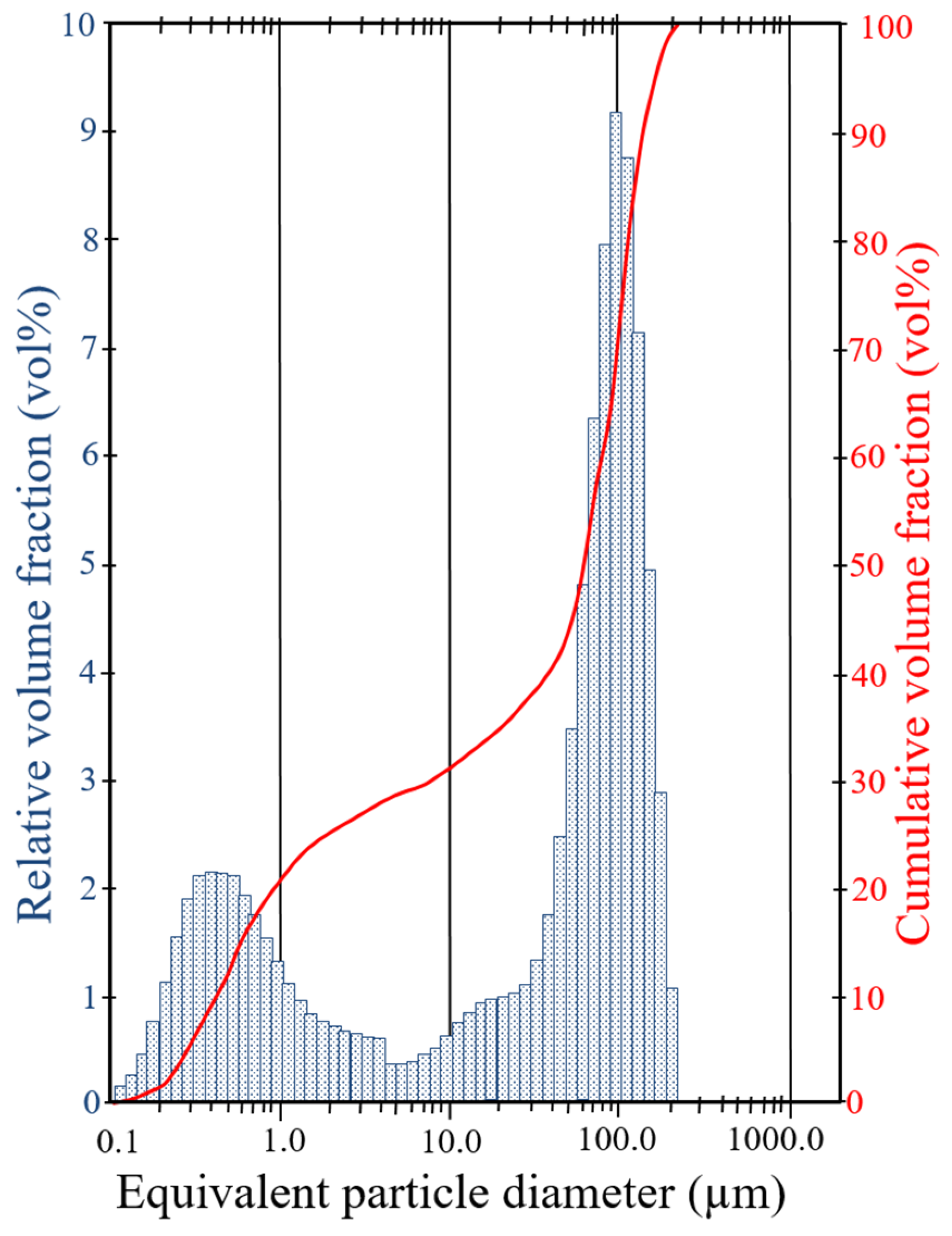
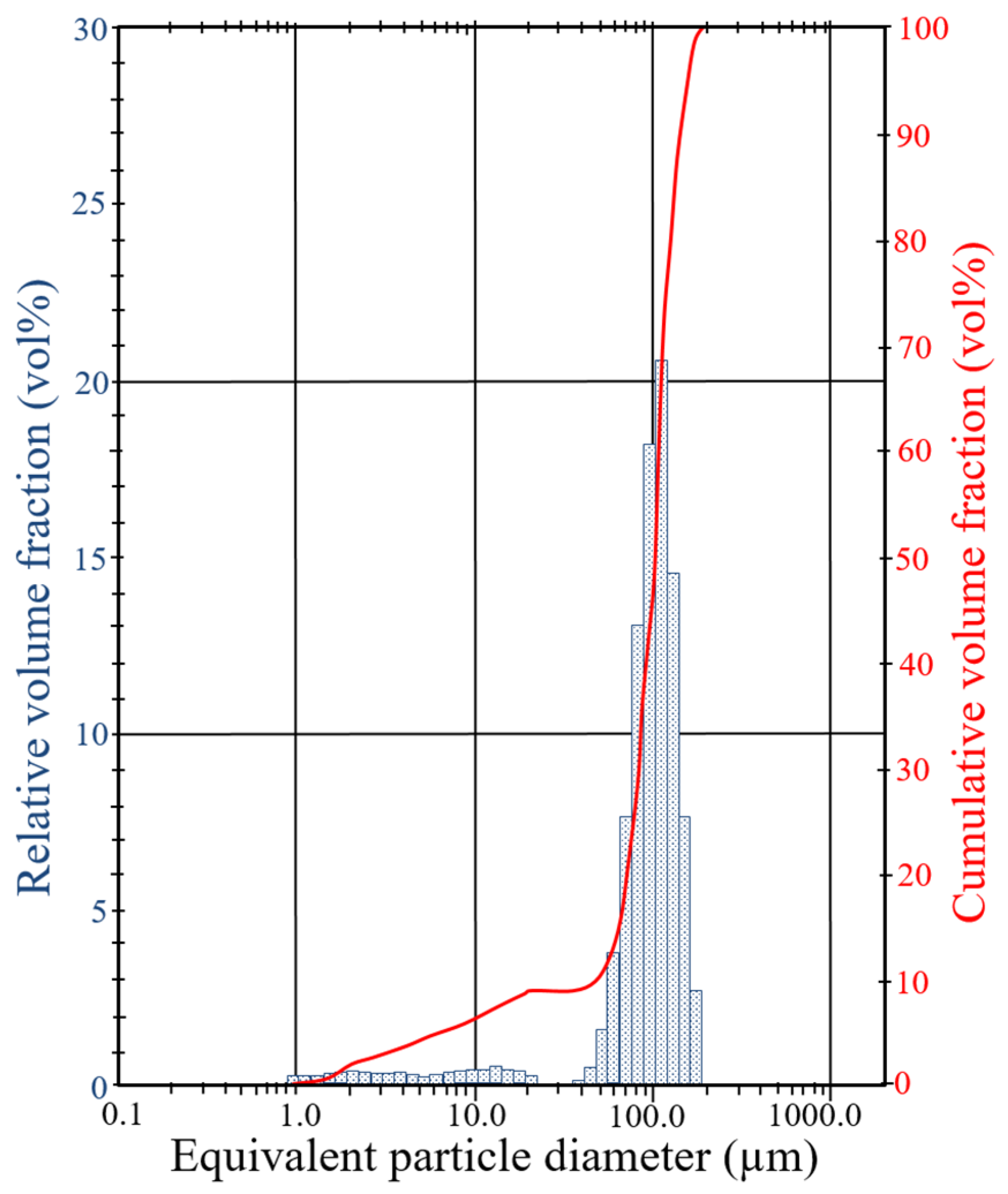
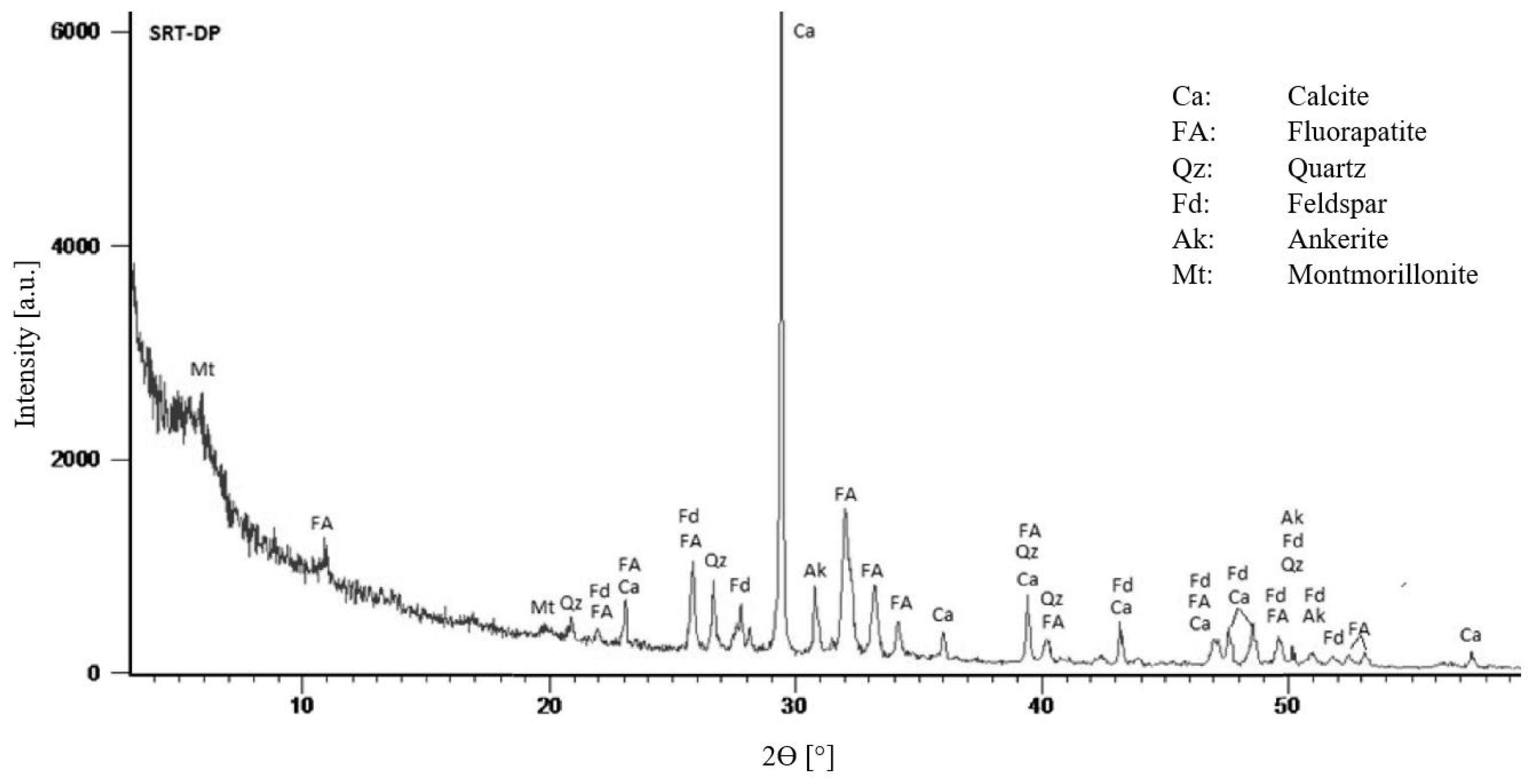



| Basin | Deposit | P2O5 (wt%) | Uranium (mg/kg) | ∑ REE (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Avg. | Min. | Max. | Avg. | Min. | Max. | Avg. | ||
| Northern Basin | Sekerna | 19.7 | 23.8 | 21.8 | 36.2 | 55.1 | 45.6 | 750 | 800 | 775 |
| Sra Ourtane | 21.9 | 26.1 | 24.0 | 73.9 | 112.2 | 93.6 | 401 | 690 | 546 | |
| Eastern Basin | Jebel Jebs | 27.9 | 29.5 | 28.7 | 40.8 | 46.7 | 44.1 | 935 | 1020 | 974 |
| Gafsa Basin | Jellabia | 16.4 | 30.3 | 26.0 | 19.9 | 53.7 | 32.7 | 155 | 617 | 432 |
| Kef Eddour | 25.4 | 28.8 | 26.5 | 23.9 | 41.1 | 31.9 | 171 | 478 | 320 | |
| Metlaoui | 24.1 | 28.3 | 26.4 | 20.9 | 36.2 | 29.9 | 175 | 550 | 326 | |
| Naguess | 23.9 | 29 | 27.2 | 25.4 | 44.2 | 33.6 | 204 | 508 | 345 | |
| Component | Untreated Phosphate Rock (wt%) | Calcined Phosphate Rock (wt%) | Calcined and Treated Phosphate Rock (wt%) |
|---|---|---|---|
| P2O5 | 20.01 | 24.24 | 27.24 |
| CaO | 42.93 | 47.01 | 45.65 |
| SO3 | 1.88 | 2.03 | 2.25 |
| MgO | 1.29 | 1.18 | 0.92 |
| Fe2O3 | 1.52 | 1.26 | 1.16 |
| Al2O3 | 2.56 | 2.14 | 1.55 |
| SiO2 | 3.08 | 12.4 | 9.81 |
| CO2 | 14.78 | 1.95 | 1.95 |
| F | 2.50 | 2.45 | 2.20 |
| Element | Untreated Phosphate Rock (mg/kg) | Calcined Phosphate Rock (mg/kg) | Calcined and Treated Phosphate Rock (mg/kg) |
|---|---|---|---|
| Cd | 30 | 26 | 14 |
| Cr | 160 | 93 | 74 |
| Mn | 67 | 48 | 42 |
| V | 66 | 45 | 43 |
| Zn | 214 | 207 | 142 |
| U | 60 | 68 | 79 |
| Th | 25 | 29 | 34 |
| Element | Untreated Phosphate Rock (mg/kg) | Calcined Phosphate Rock (mg/kg) | Calcined and Treated Phosphate Rock (mg/kg) | Crustal Abundance (mg/kg) [52] |
|---|---|---|---|---|
| Ce | 220 | 240 | 285 | 67 |
| La | 161 | 175 | 192 | 39 |
| Nd | 67 | 76 | 89 | 42 |
| Pr | 29 | 37 | 49 | 9 |
| Sm | 28 | 34 | 47 | 7 |
| Y | 66 | 76 | 88 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbes, N.; Bilal, E.; Hermann, L.; Steiner, G.; Haneklaus, N. Thermal Beneficiation of Sra Ouertane (Tunisia) Low-Grade Phosphate Rock. Minerals 2020, 10, 937. https://doi.org/10.3390/min10110937
Abbes N, Bilal E, Hermann L, Steiner G, Haneklaus N. Thermal Beneficiation of Sra Ouertane (Tunisia) Low-Grade Phosphate Rock. Minerals. 2020; 10(11):937. https://doi.org/10.3390/min10110937
Chicago/Turabian StyleAbbes, Noureddine, Essaid Bilal, Ludwig Hermann, Gerald Steiner, and Nils Haneklaus. 2020. "Thermal Beneficiation of Sra Ouertane (Tunisia) Low-Grade Phosphate Rock" Minerals 10, no. 11: 937. https://doi.org/10.3390/min10110937
APA StyleAbbes, N., Bilal, E., Hermann, L., Steiner, G., & Haneklaus, N. (2020). Thermal Beneficiation of Sra Ouertane (Tunisia) Low-Grade Phosphate Rock. Minerals, 10(11), 937. https://doi.org/10.3390/min10110937









