PGE-Cu-Ni Mineralization of Mafic-Ultramafic Massifs of the Khangai Upland, Western Mongolia
Abstract
:1. Introduction
2. Geological Setting
3. Geology, Petrology, and Age of Massifs
4. Sampling and Analytical Methods
5. Results
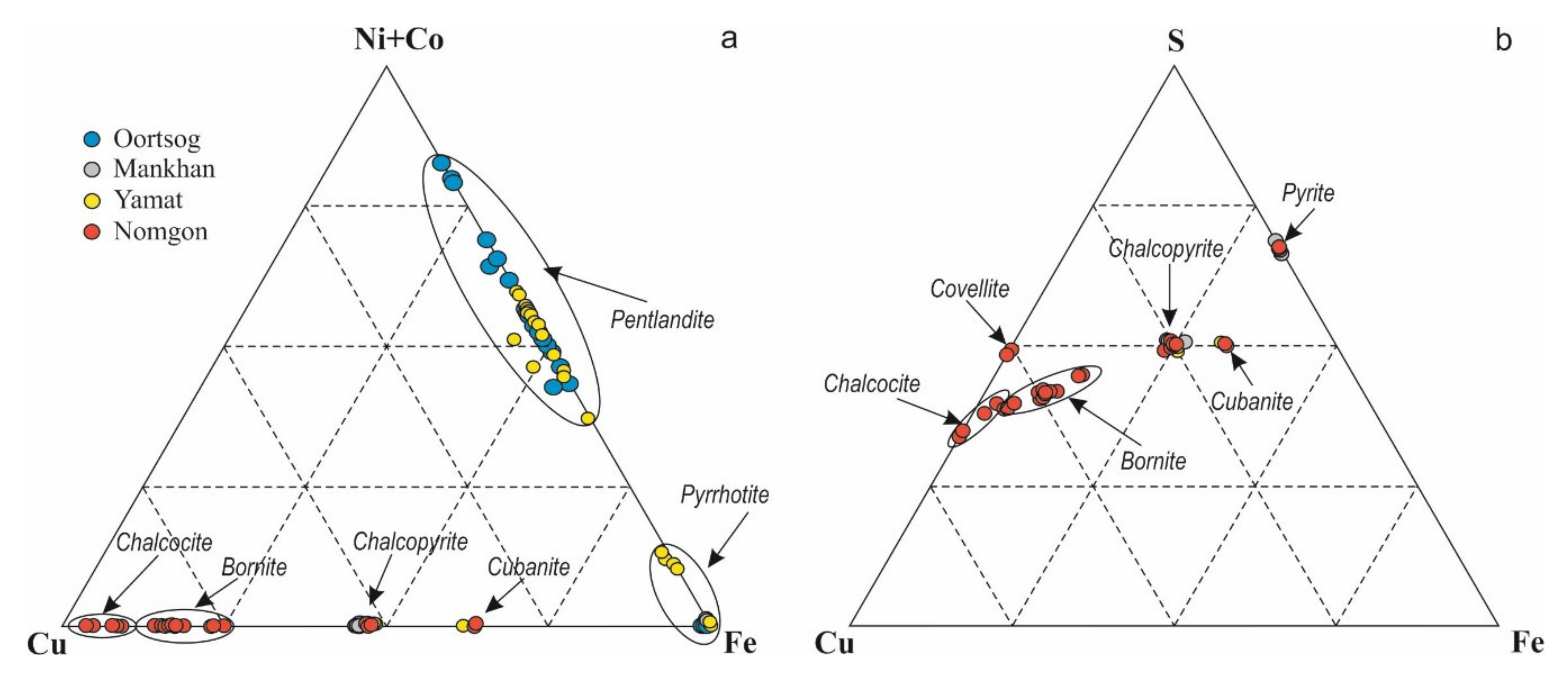
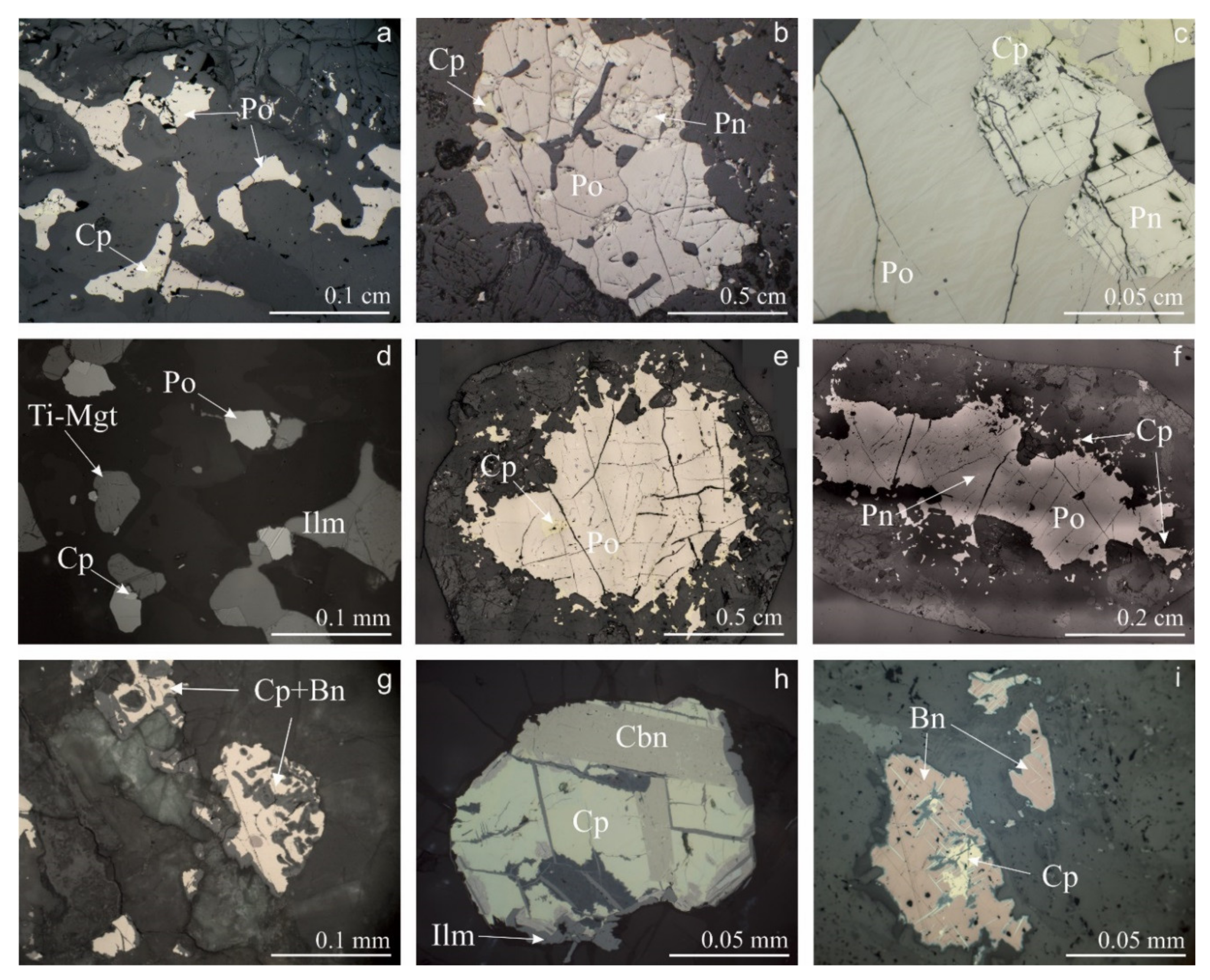
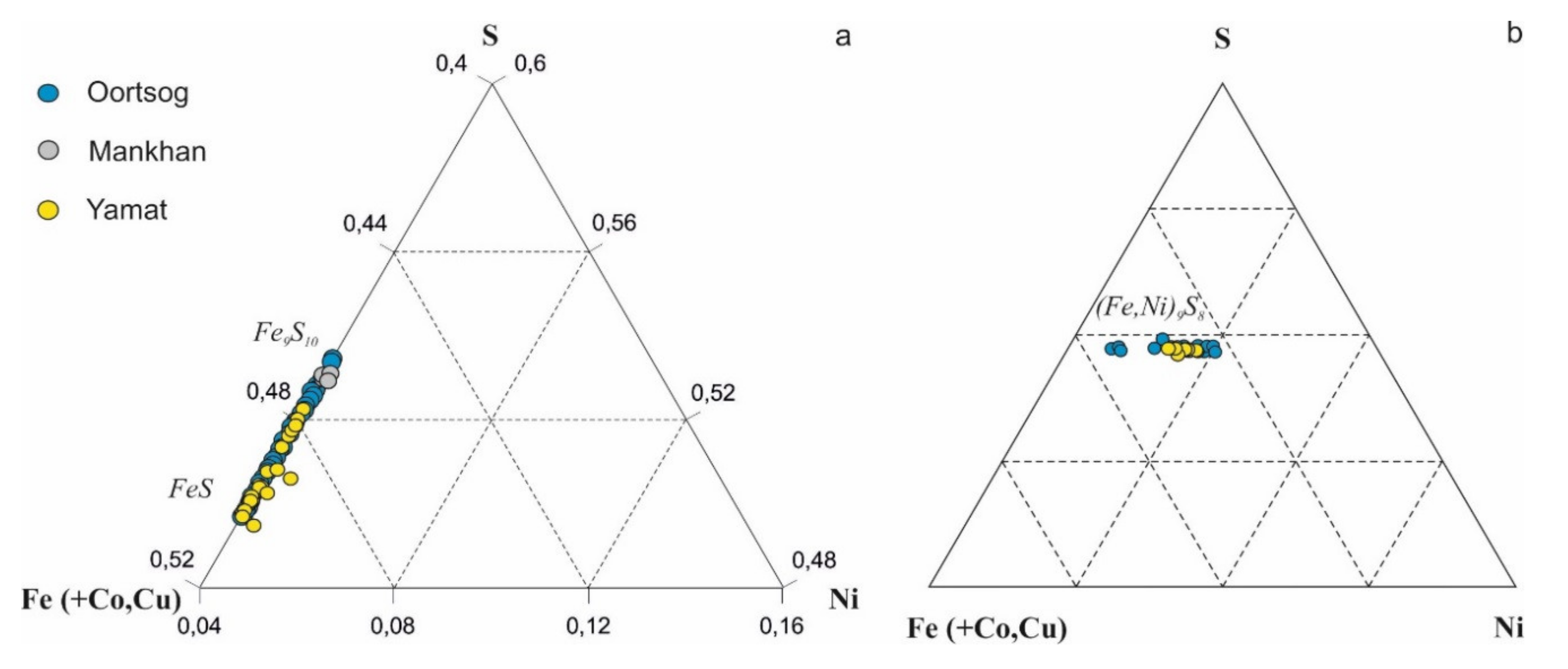
5.1. Sulfide Mineralization
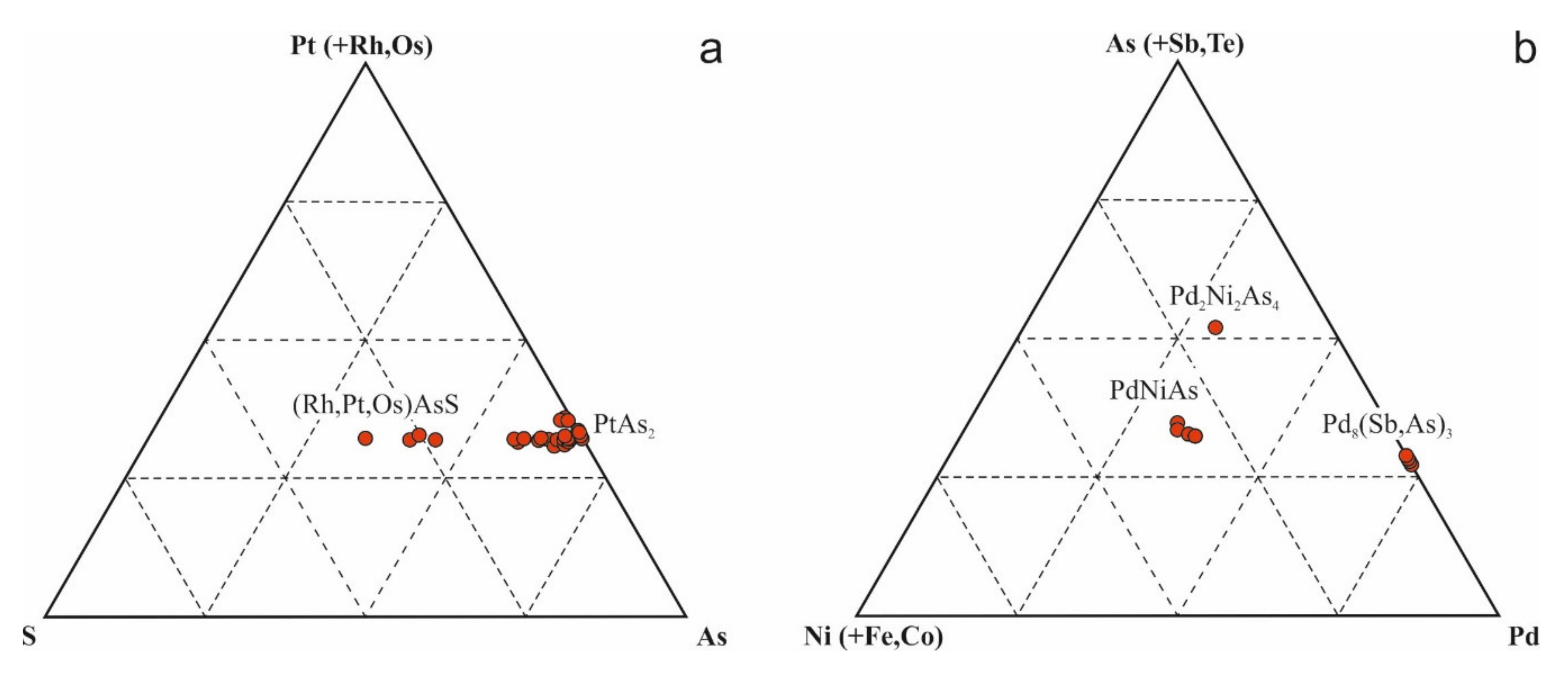
5.2. Noble Metal Mineralization
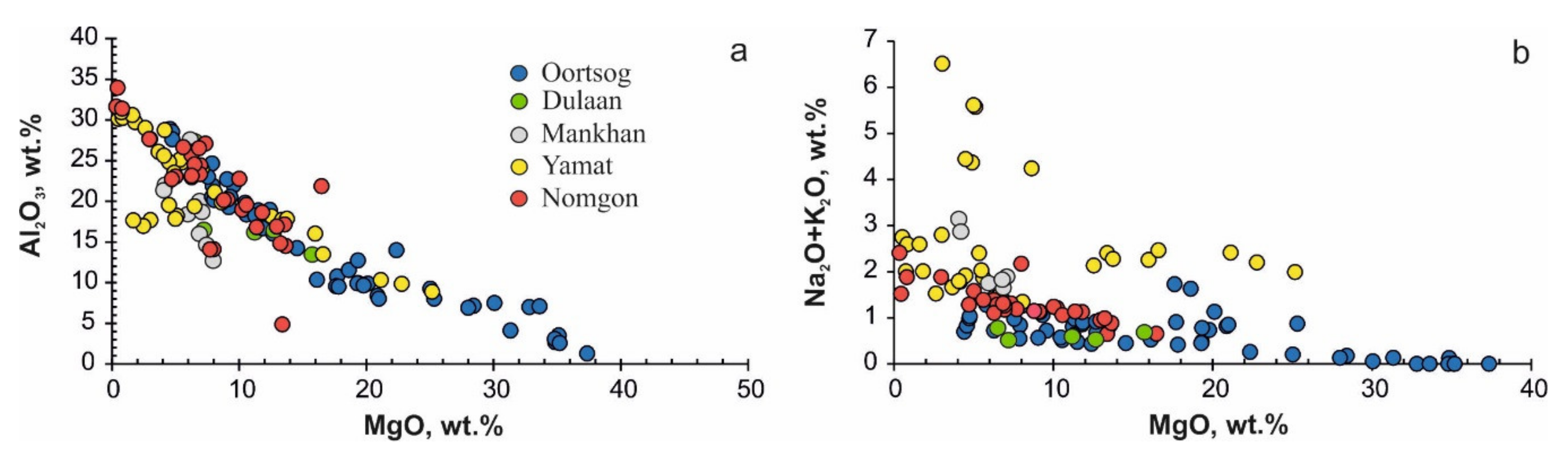
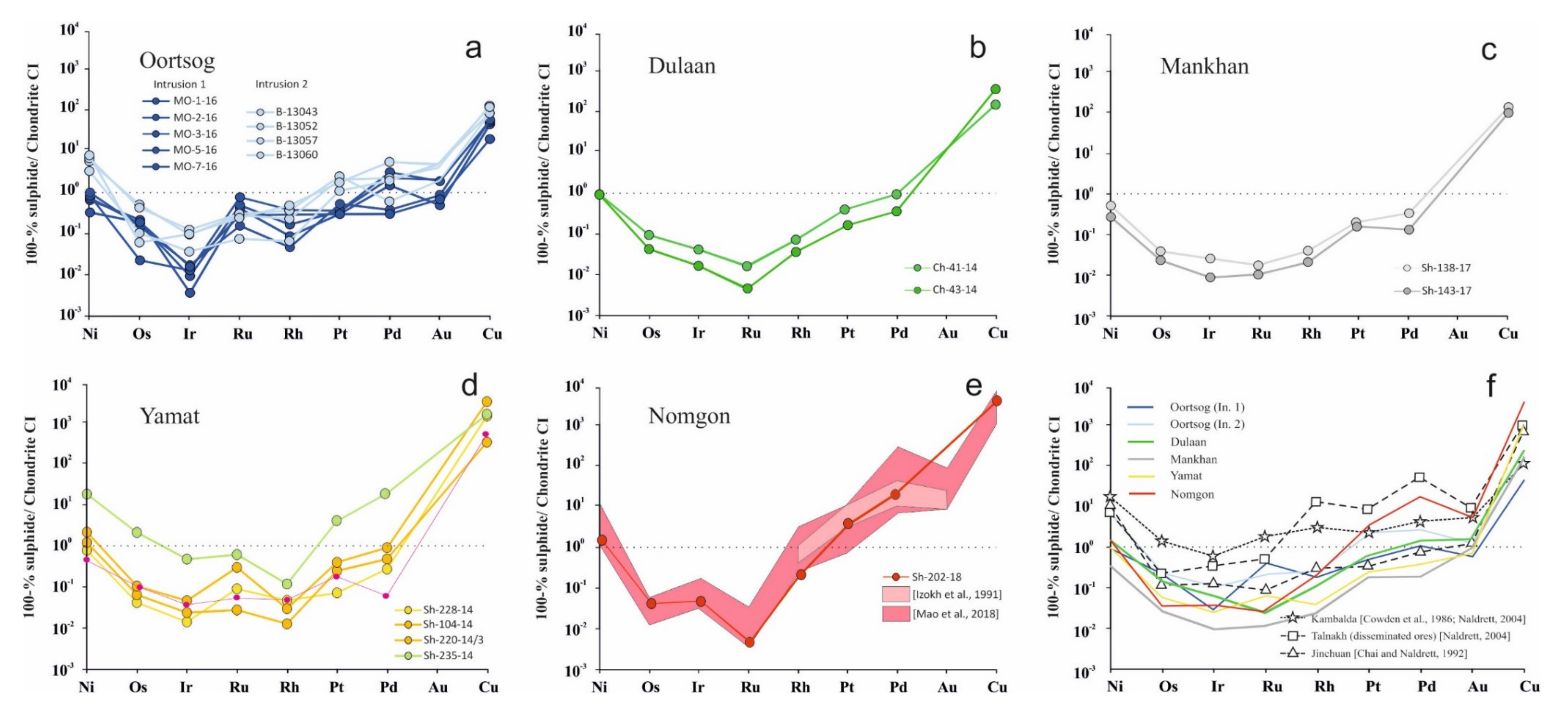
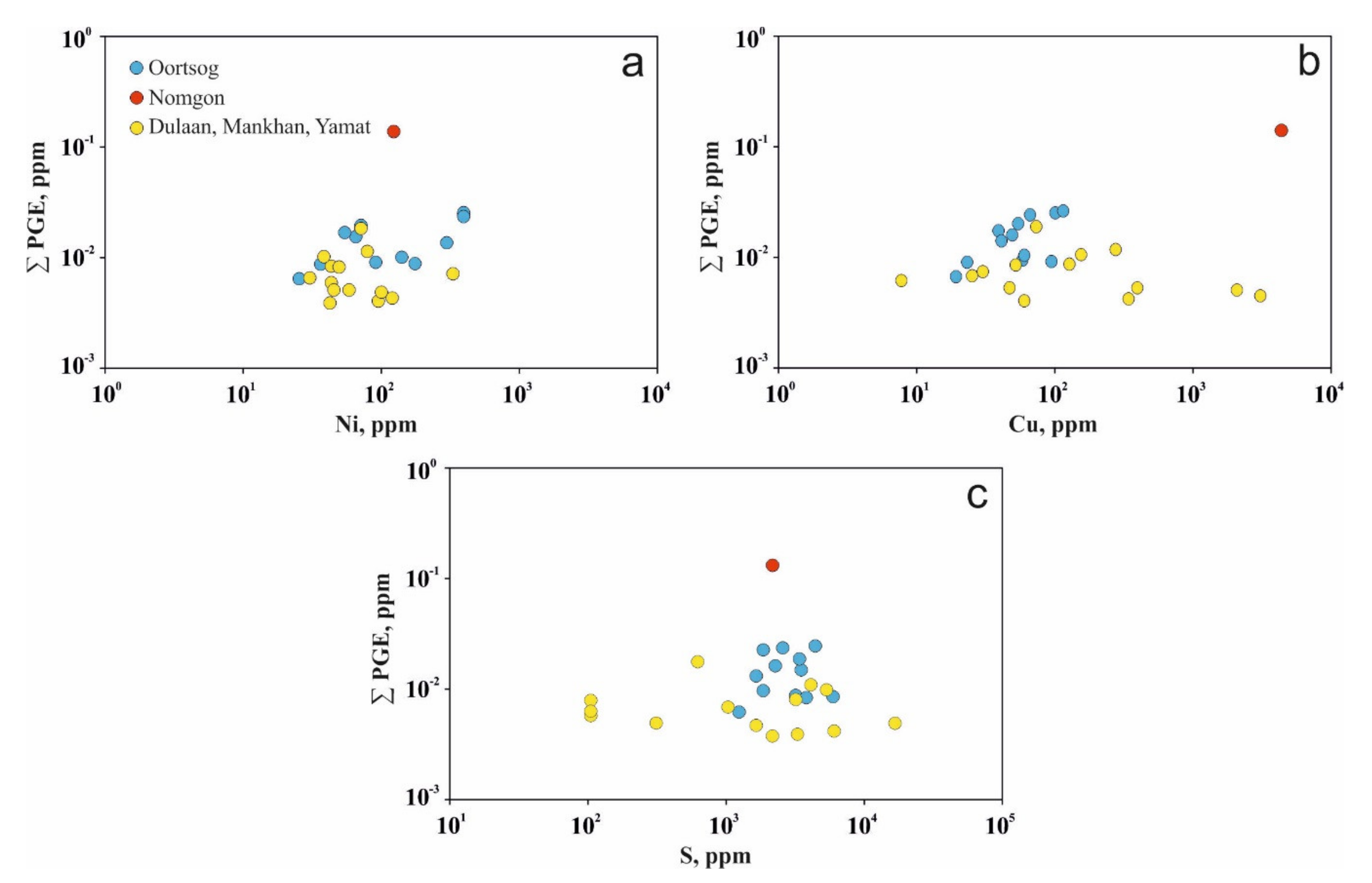
5.3. Geochemistry of Ore Elements
6. Discussion
6.1. Sulfides
6.2. PGE Minerals
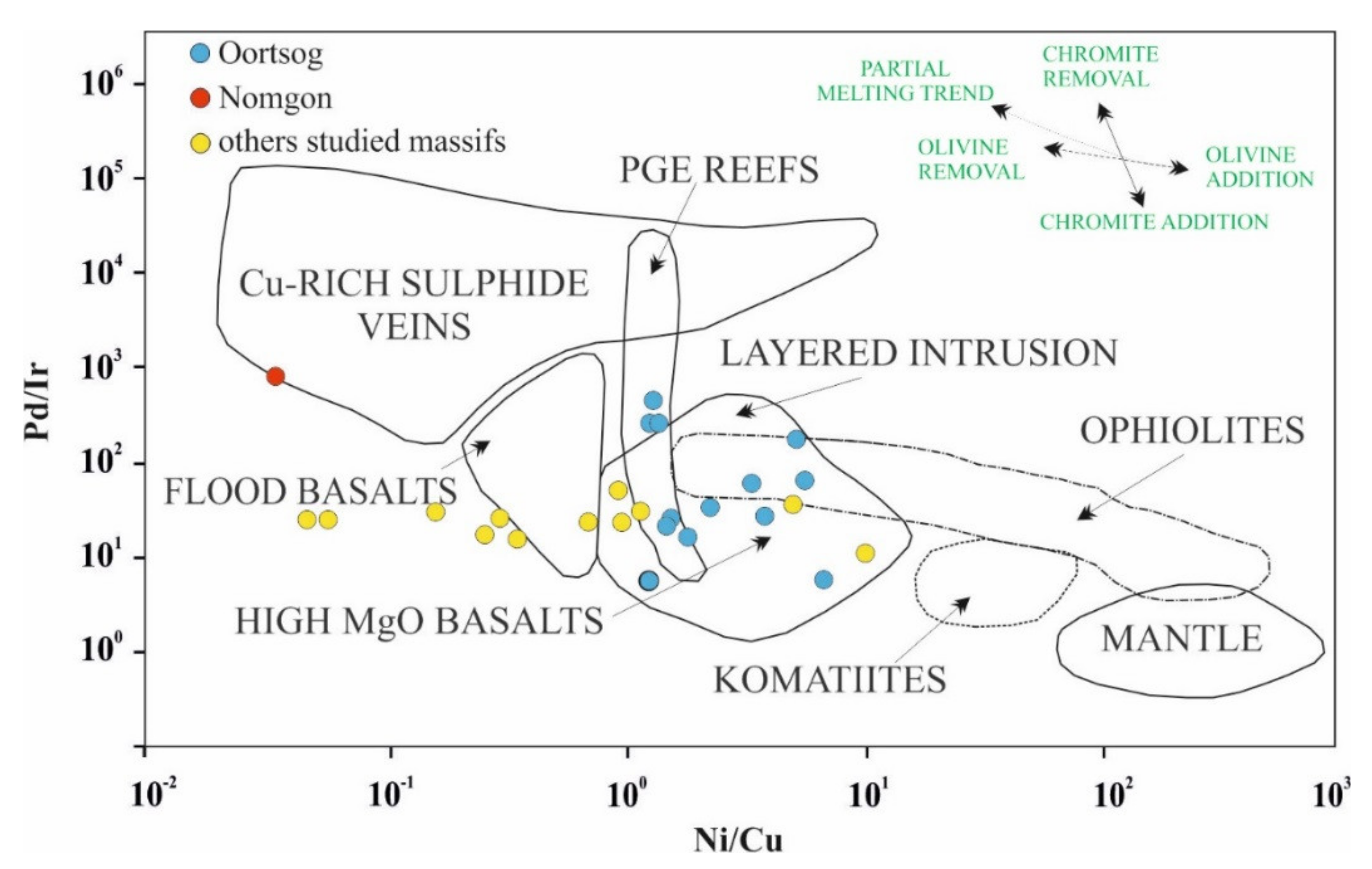
6.3. Ore Geochemisrtry
6.4. Parental Magmas
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbott, D.H.; Isley, A.E. The intensity, occurrence, and duration of superplume events and eras over geological time. J. Geodyn. 2002, 34, 265–307. [Google Scholar] [CrossRef]
- Maier, W.D. Platinum-group element (PGE) deposits and occurrences: Mineralization styles, genetic concepts, and exploration criteria. J. Afr. Earth Sci. 2005, 41, 165–191. [Google Scholar] [CrossRef]
- Dobretsov, N.L.; Borisenko, A.S.; Izokh, A.E.; Zhmodik, S.M. A thermochemical model of Eurasian Permo-Triassic mantle plumes as a basis for prediction and exploration for Cu-Ni-PGE and rare-metal ore deposits. Russ. Geol. Geophys. 2010, 51, 903–924. [Google Scholar] [CrossRef]
- Polyakov, G.V.; Tolstykh, N.D.; Mekhonoshin, A.S.; Izokh, A.E.; Podlipskii, M.Y.; Orsoev, D.A.; Kolotilina, T.B. Ultramafic-mafic igneous complexes of the Precambrian East Siberian metallogenic province (southern framing of the Siberian craton): Age, composition, origin, and ore potential. Russ. Geol. Geophys. 2013, 54, 1319–1331. [Google Scholar] [CrossRef]
- Ernst, R.E.; Jowitt, S.M. Large Igneous Provinces (LIPs) and metallogeny. Soc. Econ. Geol. Spec. Publ. 2013, 17, 17–51. [Google Scholar]
- Mekhonoshin, A.S.; Tolstykh, N.D.; Podlipsky, M.Y.; Kolotilina, T.B.; Vishnevsky, A.V.; Benedyuk, Y.P. PGE mineralization of dunite-wehrlite massifs at the Gutara-Uda interfluve, Eastern Sayan. Geol. Ore Depos. 2013, 55, 162–175. [Google Scholar] [CrossRef]
- Tolstykh, N. PGE mineralization in marginal sulfide ores of the Chineisky layered intrusion, Russia. Mineral. Petrol. 2008, 92, 283–306. [Google Scholar] [CrossRef]
- Borisenko, A.S.; Sotnikov, V.I.; Izokh, A.E.; Polyakov, G.V.; Obolensky, A.A. Permo-Triassic mineralization in Asia and its relation to plume magmatism. Russ. Geol. Geophys. 2006, 47, 170–186. [Google Scholar]
- Izokh, A.E.; Polyakov, G.V.; Hoa, T.T.; Balykin, P.A.; Phuong, N.T. Permian-triassic ultramafic-mafic magmatism of Northern Vietnam and Southern China as expression of plume magmatism. Russ. Geol. Geophys. 2005, 46, 922–932. [Google Scholar]
- Shellnutt, J.G. The Emeishan large igneous province: A synthesis. Geosci. Front. 2014, 5, 369–394. [Google Scholar] [CrossRef] [Green Version]
- Yarmolyuk, V.V.; Kozlovsky, A.M.; Savatenkov, V.M.; Kovach, V.P.; Kozakov, I.K.; Kotov, A.B.; Lebedev, V.I.; Eenjin, G. Composition, sources, and geodynamic nature of giant batholiths in Central Asia: Evidence from the geochemistry and Nd isotopic characteristics of granitoids in the Khangai zonal magmatic area. Petrology 2016, 24, 433–461. [Google Scholar] [CrossRef]
- Izokh, A.E.; Vishnevskii, A.V.; Polyakov, G.V.; Shelepaev, R.A. Age of picrite and picrodolerite magmatism in western Mongolia. Russ. Geol. Geophys. 2011, 52, 7–23. [Google Scholar] [CrossRef]
- Shelepaev, R.A.; Polyakov, G.V.; Izokh, A.E.; Vishnevsky, A.V.; Egorova, V.V.; Shelepov, Y.Y. The Perm Intraplate Mafic-Ultramafic Associations of Asia. Materials of Conference. Correlation of Altaides and Uralides: Magmatism, Metamorphism, Stratigraphy, Geochronology, Geodynamics and Metallogeny; Publishing House SB RAS: Novosibirsk, Russia, 2016; pp. 214–216. (In Russian) [Google Scholar]
- Yarmolyuk, V.V.; Kuzmin, M.I.; Ernst, R.E. Intraplate geodynamics and magmatism in the evolution of the Central Asian Orogenic Belt. Journal of Asian Earth Sciences. J. Asian Earth Sci. 2014, 93, 158–179. [Google Scholar] [CrossRef] [Green Version]
- Pirajno, F. Ore Deposits and Mantle Plumes; Kluwer Academic: Dordrecht, The Netherlands; Boston, MA, USA, 2000; p. 556. [Google Scholar]
- Naldrett, A.J. Magmatic Sulfide Deposits: Geology, Geochemistry and Exploration; Springer: New York, NY, USA, 2004; p. 730. [Google Scholar]
- Begg, G.C.; Hronsky, J.A.M.; Arndt, N.T.; Griffin, W.L.; O’Reilly, S.Y.; Hayward, N. Lithospheric, Cratonic, and Geodynamic Setting of Ni-Cu-PGE Sulfide Deposits. Econ. Geol. 2010, 105, 1057–1070. [Google Scholar] [CrossRef]
- Mao, Y.J.; Dash, B.; Qin, K.Z.; Bujinlkham, B.; Tang, D.M. Comparisons among the Oortsog, Dulaan, and Nomgon mafic-ultramafic intrusions in central Mongolia and Ni-Cu deposits in NW China: Implications for economic Ni-Cu-PGE ore exploration in central Mongolia. Russ. Geol. Geophys. 2018, 59, 1–18. [Google Scholar] [CrossRef]
- Tolstykh, N.D.; Podlipsky, M.Y. Heavy concentrate halos as prospecting guides for PGE mineralization. Geol. Ore Depos. 2010, 52, 196–214. [Google Scholar] [CrossRef]
- Sal’nikova, E.B.; Yakovleva, S.Z.; Kotov, A.B.; Tolmacheva, E.V.; Plotkina, Y.V.; Fedoseenko, A.M.; Kozlovskii, A.M.; Yarmolyuk, V.V. Crystallogenesis of zircon in alkaline granites and specifics of zircon U-Pb dating: A case study of the Khangai magmatic area. Petrology 2014, 22, 450–461. [Google Scholar] [CrossRef]
- Izokh, A.E.; Polyakov, G.V.; Krivenko, A.P.; Bognibov, V.I.; Bayarbileg, L. The Gabbro Formation of Western Mongolia; Nauka: Novosibirsk, Russia, 1990; p. 269. (In Russian) [Google Scholar]
- Izokh, A.E.; Polyakov, G.V.; Anoshin, G.N.; Golovanova, N.P. Geochemistry Of Platinum Group-Metals, Gold And Silver In Nomgonsky Troctolite-Anorthozite-Gabbro Massif (Mongolia). Geochemistry 1991, 10, 1398–1405. [Google Scholar]
- Izokh, A.E.; Mayorova, O.N.; Lavrentiev, Y.G. Minerals of the platinum metals in the Nomgon troctolite–anorthozite–gabbro intrusive massif (Mongolia). Russ. Geol. Geophys. 1992, 33, 104–110. [Google Scholar]
- Sengor, A.M.C.; Natalin, B.A.; Burtman, V.S. Evolution of the Altaid Tectonic Collage and Paleozoic Crustal Growth in Eurasia. Nature 1993, 364, 299–307. [Google Scholar] [CrossRef]
- Sengor, A.M.C.; Natal’in, B.A. Palaeotectonics of Asia: Fragments of a synthesis. In Tectonic Evolution of Asia; Yin, A., Harrison, M., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 486–640. [Google Scholar]
- Xiao, W.J.; Zhang, L.C.; Qin, K.Z.; Sun, S.; Li, J.L. Paleozoic accretionary and collisional tectonics of the Eastern Tianshan (China): Implications for the continental growth of Central Asia. Am. J. Sci. 2004, 304, 370–395. [Google Scholar] [CrossRef] [Green Version]
- Windley, B.F.; Alexeiev, D.; Xiao, W.J.; Kroner, A.; Badarch, G. Tectonic models for accretion of the Central Asian Orogenic Belt. J. Geol. Soc. 2007, 164, 31–47. [Google Scholar] [CrossRef] [Green Version]
- Kruk, N.N.; Rudnev, S.N.; Vladimirov, A.G.; Shokalsky, S.P.; Kovach, V.P.; Serov, P.A.; Volkova, N.I. Early-Middle Paleozoic granitoids in Gorny Altai, Russia: Implications for continental crust history and magma sources. J. Asian Earth Sci. 2011, 42, 928–948. [Google Scholar] [CrossRef]
- Safonova, I.; Seltmann, R.; Kroner, A.; Gladkochub, D.; Schulmann, K.; Xiao, W.J.; Kim, J.; Komiya, T.; Sun, M. A new concept of continental construction in the Central Asian Orogenic Belt (compared to actualistic examples from the Western Pacific). Episodes 2011, 34, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Kuzmin, M.I.; Yarmolyuk, V.V. Mantle plumes of Central Asia (Northeast Asia) and their role in forming endogenous deposits. Russ. Geol. Geophys. 2014, 55, 120–143. [Google Scholar] [CrossRef]
- Koval, P.V.; Antipin, V.S.; Tsypukov, Y.P.; Smirnov, V.N. Geological structure and material composition of the Baga-Khenteiskiy batholith (MPR). Russ. Geol. Geophys. 1978, 5, 68–78. (In Russian) [Google Scholar]
- Litvinovsky, B.A.; Zanvilevich, A.N.; Alakshin, A.M.; Podladchikov, Y.Y. Angara-Vitim Batholith is the Largest Granitoid Pluton; Science: Novosibirsk, Russia, 1992; p. 141. (In Russian) [Google Scholar]
- Yarmolyuk, V.V.; Kovalenko, V.I.; Kozakov, I.K.; Sal’nikova, E.B.; Bibikova, E.V.; Kovach, V.P.; Kozlovsky, A.M.; Kotov, A.B.; Lebedev, V.I.; Eenjin, G.; et al. The age of the Khangai batholith and the problem of batholith formation in Central Asia. Dokl. Earth Sci. 2008, 423, 1223–1228. [Google Scholar] [CrossRef]
- Donskaya, T.V.; Gladkochub, D.P.; Mazukabzov, A.M.; Ivanov, A.V. Late Paleozoic-Mesozoic subduction-related magmatism at the southern margin of the Siberian continent and the 150 million-year history of the Mongol-Okhotsk Ocean. J. Asian Earth Sci. 2013, 62, 79–97. [Google Scholar] [CrossRef]
- Kovalenko, V.I.; Yarmolyuk, V.V.; Kovach, V.P.; Kotov, A.B.; Kozakov, I.K.; Salnikova, E.B.; Larin, A.M. Isotope provinces, mechanisms of generation and sources of the continental crust in the Central Asian Mobile Belt: Geological and isotopic evidence. J. Asian Earth Sci. 2004, 23, 605–627. [Google Scholar] [CrossRef]
- Yarmolyuk, V.V.; Kozlovsky, A.M.; Sal’nikova, E.B.; Kozakov, I.K.; Kotov, A.B.; Lebedev, V.I.; Eenjin, G. Age of the Khangai batholith and challenge of polychronic batholith formation in Central Asia. Dokl. Earth Sci. 2013, 452, 1001–1007. [Google Scholar] [CrossRef]
- Tomurtogoo, O.; Windley, B.F.; Kroner, A.; Badarch, G.; Liu, D.Y. Zircon age and occurrence of the Adaatsag ophiolite and Muron shear zone, central Mongolia: Constraints on the evolution of the Mongol-Okhotsk ocean, suture and orogen. J. Geol. Soc. 2005, 162, 125–134. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Wilde, S.A.; Tong, Y. Evolution, source and tectonic significance of Early Mesozoic granitoid magmatism in the Central Asian Orogenic Belt (central segment). Earth-Sci. Rev. 2013, 126, 206–234. [Google Scholar] [CrossRef]
- Ernst, R.E. Large Igneous Provinces; Cambridge University Press: Cambridge, UK, 2014; pp. 1–653. [Google Scholar]
- Tang, G.J.; Chung, S.L.; Hawkesworth, C.J.; Cawood, P.A.; Wang, Q.; Wyman, D.A.; Xu, Y.G.; Zhao, Z.H. Short episodes of crust generation during protracted accretionary processes: Evidence from Central Asian Orogenic Belt, NW China. Earth Planet. Sci. Lett. 2017, 464, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Tsukada, K.; Nuramkhaan, M.; Purevsuren, N.; Kabashima, T.; Kondo, T.; Gantumur, O.; Hasegawa, H.; Yamamoto, K. Permian adakitic magmatism in the Khanui Group, Northern Mongolia—Late Paleozoic slab-melting of subducted oceanic plate beneath the “Siberian continent”. J. Geodyn. 2018, 121, 49–63. [Google Scholar] [CrossRef]
- Shapovalova, M.O.; Tolstykh, N.D.; Shelepaev, R.A.; Tsibizov, L.V. The Oortsog Peridotite-Troctolite-Gabbro Intrusion, Western Mongolia: New Petrological and Geochronological Constraints. Russ. Geol. Geophys. 2019, 60, 845–861. [Google Scholar] [CrossRef]
- Shelepaev, R.A.; Egorova, V.V.; Izokh, A.E.; Vishnevsky, A.V.; Shelepov, Y.Y.; Rudnev, S.N. Permian gabbroid intrusions of the Khangai highlands (Western Mongolia). Isotope dating of geological processes: New results, approaches and prospects. In Proceedings of the VI Russian Conference on Isotope Geochronology; Sprinter: St. Petersburg, Russia, 2015; pp. 337–338. (In Russian) [Google Scholar]
- Izokh, A.E.; Polyakov, G.V.; Gibsher, A.S.; Balykin, P.A.; Zhuravlev, D.Z.; Parkhomenko, V.A. High-alumina layered gabbroids of the Central-Asian fold belt: Geochemical composition, Sm-Nd isotopic age, and geodynamic conditions of formation. Russ. Geol. Geophys. 1998, 39, 1565–1577. [Google Scholar]
- Shapovalova, M.O.; Shelepaev, R.A.; Tolstykh, N.D.; Izokh, A.E. Gabbroid massifs of the Khangai Upland as a result of the interaction of the mantle plume with the lithospheric mantle. Petrology of magmatic and metamorphic complexes. In Proceedings of the X Russian Petrographic Conference with International Participation; Tomsk Center for Science and Technology: Tomsk, Russia, 2018; Volume 10, pp. 428–432. (In Russian) [Google Scholar]
- Shapovalova, M.; Shelepaev, R.; Tolstykh, N. Petrological characteristics of mafic-ultramafic intrusions of the Khangay upland (Mongolia). In Proceedings of the 15th SGA Biennial Meeting, Glasgow, Scotland, 27–30 August 2019; Volume 2, pp. 561–564. [Google Scholar]
- Shapovalova, M.; Tolstykh, N.; Shelepaev, R.; Safonova, I. Petrologo-geochemical features of the mafic-ultramafic massifs of the Khangai upland, Western Mongolia. J. Asia Earth Sci. 2021. under review. [Google Scholar]
- Lavrent’ev, Y.G.; Karmanov, N.S.; Usova, L.V. Electron-probe determination of the composition of minerals: Microanalyzer or scanning electron microscope. Russ. Geol. Geophys. 2015, 56, 1473–1482. (In Russian) [Google Scholar] [CrossRef]
- Lavrent’ev, Y.G.; Usova, L.V. The choice of the optimal method for calculating correction factors in X-ray microanalysis of rock-forming minerals. J. Anal. Chem. 1996, 51, 323–331. (In Russian) [Google Scholar]
- Shapovalova, M.O.; Tolstykh, N.D.; Shelepaev, R.A. Cu-Ni-PGE mineralization of the peridotite-gabbro massif Oortsog, Western Mongolia. Ore-magmatic systems. Magmatism, metallogeny and tectonics of North Asia. Collection of scientific papers on fundamental research of the Institute of Geology and Mineralogy SB RAS. Novosibirsk: IGM SB RAS 2018, 1, 44–55. (In Russian) [Google Scholar]
- Kuova, O.; Huhma, M.; Vuorelainen, Y. A natural cobalt analog of pentlandite. Am. Mineral. 1959, 44, 897–900. [Google Scholar]
- Kretz, R. SYMBOLS FOR ROCK-FORMING MINERALS. Am. Mineral. 1983, 68, 277–279. [Google Scholar]
- Likhachev, A.P. Platinum-Copper-Nickel and Platinum Deposits; Eslan: Moscow, Russia, 2006; p. 496. (In Russian) [Google Scholar]
- Krivenko, A.P.; Lopukhov, A.S.; Glotov, A.I. Geochemical Associations of Rare and Radioactive Elements in Ore and Magmatic Complexes; Nauka: Novosibirsk, Russia, 1990; p. 55. (In Russian) [Google Scholar]
- Tolstykh, N.; Krivolutskaya, N.; Safonova, I.; Shapovalova, M.; Zhitova, L.; Abersteiner, A. Unique Cu-rich sulphide ores of the Southern-2 orebody in the Talnakh Intrusion, Noril’sk area (Russia): Geochemistry, mineralogy and conditions of crystallization. Ore Geol. Rev. 2020, 122. [Google Scholar] [CrossRef]
- Shapovalova, M.; Shelepaev, R.; Tolstykh, N.; Kalugin, V.; Safonova, I. Petrology of the Ortsog-Uul Gabbro-Peridotite PGE-Bearing Complex, Western Mongolia. Min. Resour. Sustain. World 2015, 1–5, 983–985. [Google Scholar]
- Barnes, S.-J.; Lightfoot, P.C. Formation of magmatic nickel-sulphide ore deposits and processes affecting their copper and platinum-group element contents. Econ. Geol. 2005, 100, 179–213. [Google Scholar]
- Vinogradov, A.P. The average content of chemical elements in the main types of eruptions genus of the earth’s crust. Geochemical 1962, 7, 555–571. (In Russian) [Google Scholar]
- Taylor, S.R. Abundance of chemical elements in the continental crust: A new table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Anders, E.; Grevesse, N. Abundances of the elements: Meteoric and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, J.; Chai, F.; Yan, S.; Chen, B.; Pirajno, F. Geochemistry of the Permian Kalatongke mafic intrusions, Northern Xinjiang, Northwest China: Implications for the genesis of magmatic Ni-Cu sulfide deposits. Econ. Geol. 2009, 104, 185–203. [Google Scholar] [CrossRef]
- Radomskaya, T.A.; Glazunov, O.M.; Vlasova, V.N.; Suvorova, L.F. Geochemistry and mineralogy of platinum group element in ores of the Kingash deposit, Eastern Sayan, Russia. Geol. Ore Depos. 2017, 59, 354–374. [Google Scholar] [CrossRef]
- Krivolutskaya, N.; Tolstykh, N.; Kedrovskaya, T.; Naumov, K.; Kubrakova, I.; Tyutyunnik, O.; Gongalsky, B.; Kovalchuk, E.; Magazina, L.; Bychkova, Y.; et al. World-Class PGE-Cu-Ni Talnakh Deposit: New Data on the Structure and Unique Mineralization of the South-Western Branch. Minerals 2018, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Yarmolyuk, V.V.; Kuzmin, M.I.; Kozlovsky, A.M. Late paleozoic-Early Mesozoic within-plate magmatism in North Asia: Traps, rifts, giant batholiths, and the geodynamics of their origin. Petrology 2013, 21, 101–126. [Google Scholar] [CrossRef]
- Kissin, S.A.; Scott, S.D. PHASE-RELATIONS INVOLVING PYRRHOTITE BELOW 350-DEGREES-C. Econ. Geol. 1982, 77, 1739–1754. [Google Scholar] [CrossRef]
- Lygin, A.V. Features of the Composition of the Ores of the Verkhnekingash Platinoid-Cobalt-Copper-Nickel Deposit (Krasnoyarsk Region); Moscow University: Moscow, Russia, 2010; Volume 2, pp. 69–72. (In Russian) [Google Scholar]
- Svetlitskaya, T.V.; Tolstykh, N.D.; Izokh, A.E.; Thi, P.N. PGE geochemical constraints on the origin of the Ni-Cu-PGE sulfide mineralization in the Suoi Cun intrusion, Cao Bang province, Northeastern Vietnam. Mineral. Petrol. 2015, 109, 161–180. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Craig, J.R. Mineral Chemistry of Sulfides; Cambridge University Press: Cambridge, UK, 1978; p. 512. [Google Scholar]
- Kolonin, G.R.; Orsoev, D.A.; Sinyakova, E.F.; Kislov, E.V. The use of Ni:Fe ratio in pentlandite for estimation of sulfur fugacity during the formation of PGE-bearing sulfide mineralization of Yoko-Dovyren massif. Dokl. Akad. Nauk 2000, 370, 87–91. [Google Scholar]
- Kaneda, H.; Takenouchi, S.; Shoji, T. Stability of pentlandite in the fe-ni-co-s system. Mineral. Depos. 1986, 21, 169–180. [Google Scholar] [CrossRef]
- Craig, J.R.; Kullerud, G. Phase relations in the Cu–Fe–Ni–S system and their application to magmatic ore deposits. Econ. Geol. 1969, 4, 344–358. [Google Scholar]
- Kullerud, G.; Yund, R.A.; Moh, G.H. Phase relations in the Cu-Fe-S, Cu-Ni-S and Fe-Ni-S systems. In Magmatic Ore Deposits; Wilson, H.D.B., Ed.; Economic Geology Publishing Co.: Lancaster, PA, USA, 1969; pp. 323–343. [Google Scholar]
- Fleet, M.E.; Pan, Y.M. Fractional crystallization of anhydrous sulfide liquid in the system Fe-Ni-Cu-S, with application to magmatic sulfide deposits. Geochim. Cosmochim. Acta 1994, 58, 3369–3377. [Google Scholar] [CrossRef]
- Sinyakova, E.; Kosyakov, V.; Nenashev, B.; Tsirkina, N.L. Single-crystal growth of (FeyNi1-y)S1-delta solid solution. J. Cryst. Growth 2005, 275, E2055–E2060. [Google Scholar] [CrossRef]
- Cabri, L.J. NEW DATA ON PHASE RELATIONS IN CU-FE-S SYSTEM. Econ. Geol. 1973, 68, 443–454. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Danyushevsky, L.V.; Gilbert, S. Minor and trace elements in bornite and associated Cu–(Fe)-sulfides: A LA-ICP-MS study Bornite mineral chemistry. Geochim. Cosmochim. Acta 2011, 75, 6473–6496. [Google Scholar] [CrossRef]
- Ramdohr, P. The Ore Minerals and Their Intergrowths, 2nd ed.; International series in earth science; Pergamon Press: London, UK, 1980; Volume 35, p. 1207. [Google Scholar]
- Robb, L. Introduction to Ore-Forming Processes; Blackwell Publishing: Oxford, UK, 2005; 373p. [Google Scholar]
- Mirsa, K.; Fleet, M.E. The chemical compositions of synthetic and natural pentlandite assemblages. Econ. Geol. 1973, 68, 518–539. [Google Scholar]
- Distler, V.V.; Genkin, A.D.; Filimonova, A.A.; Hitrov, V.G.; Laputina, I.P. The zoning of copper-nickel ores of Talnakh and Oktyabr’sky deposits. Geol. Ore Depos. 1975, 2, 16–27. [Google Scholar]
- Makovicky, E. Ternary and quaternary phase systems with PGE. The geology, geochemistry, mineralogy and mineral beneficiation of platinum-group elements. In CBM Special Canadian Institute of Mining, Metallurgy and Petroleum; Cabri, L.J., Ed.; Marc Veilleux Imprimeur Inc.: Boucherville, QC, Canada, 2002; Volume 54, pp. 131–175. [Google Scholar]
- Izokh, A.E.; Maiorova, O.N. Rhodium sperrylite from the nomgon massif (mongolia). Dokl. Akad. Nauk 1990, 313, 1212–1215. [Google Scholar]
- Stumpel, E.F.; Clark, A.M. Hollingworthite, a new rhodium mineral, identified by electron probe microanalysis. Am. Mineral. 1965, 50, 1068–1074. [Google Scholar]
- Lorand, J.P. Sur l’origine mantellaire de l’arsenic dans les roches du manteaux: Exemple des pyroxénites à grenat du massif lherzolitique des beni bousera (Rif, maroc). CR Acad. Sci. Paris 1987, 305, 383–386. [Google Scholar]
- Leblanc, M.; Fischer, W. Gold and platinum group elements in cobalt-arsenide ores—Hydrothermal concentration from a serpentinite source-rock (Bou-Azzer, Morocco). Mineral. Petrol. 1990, 42, 197–209. [Google Scholar] [CrossRef]
- Gervilla, F.; Leblanc, M.; TorresRuiz, J.; HachAli, P.F. Immiscibility between arsenide and sulfide melts: A mechanism for the concentration of noble metals. Can. Mineral. 1996, 34, 485–502. [Google Scholar]
- Gervilla, F.; Sanchez-Anguita, A.; Acevedo, R.D.; Hach-Ali, P.F. Platinum-group element sulpharsenides and Pd bismuthotellurides in the metamorphosed Ni-Cu deposit at Las Aguilas (Province of San Luis, Argentina). Mineral. Mag. 1997, 61, 861–877. [Google Scholar] [CrossRef]
- Gervilla, F.; Papunen, H.; Kojonen, K.; Johanson, B. Platinum-, palladium- and gold-rich arsenide ores from the Kylmakoski Ni-Cu deposit (Vammala Nickel Belt, SW Finland). Mineral. Petrol. 1998, 64, 163–185. [Google Scholar] [CrossRef]
- Hanley, J.J. The role of arsenic-rich melts and mineral phases in the development of high-grade Pt-Pd mineralization within komatiite-associated magmatic Ni-Cu sulfide horizons at dundonald beach south, Abitibi subprovince, Ontario, Canada. Econ. Geol. 2007, 102, 305–317. [Google Scholar] [CrossRef]
- Tolstykh, N.D.; Sidorov, E.G.; Kozlov, A.P. Platinum-group minerals in lode and placer deposits associated with the Ural-Alaskan-type Gal’moenan complex, Koryak-Kamchatka Platinum Belt, Russia. Can. Mineral. 2004, 42, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Tolstykh, N.D.; Lapukhov, A.S.; Krivenko, A.P.; Lazareva, E.V. Platinum-group minerals in gold placers in northwestern Salair. Russ. Geol. Geophys. 1999, 40, 916–925. (In Russian) [Google Scholar]
- Helmy, H.M.; Ballhaus, C.; Fonseca, R.O.C.; Wirth, R.; Nagel, T.; Tredoux, M. Noble metal nanoclusters and nanoparticles precede mineral formation in magmatic sulphide melts. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Helmy, H.M.; Ballhaus, C.; Wohlgemuth-Ueberwasser, C.; Fonseca, R.O.C.; Laurenz, V. Partitioning of Se, As, Sb, Te and Bi between monosulfide solid solution and sulfide melt—Application to magmatic sulfide deposits. Geochim. Cosmochim. Acta 2010, 74, 6174–6179. [Google Scholar] [CrossRef]
- Cowden, A.; Donaldson, M.J.; Naldrett, A.J.; Campbell, I.H. Platinum-group elements and gold in the komatiite-hosted Fe-Ni-Cu sulfide deposits at Kambalda, Western-Australia. Econ. Geol. 1986, 81, 1226–1235. [Google Scholar] [CrossRef]
- Chai, G.; Naldrett, A.J. Characteristics of Ni-Cu-Pge Mineralization and Genesis of the Jinchuan Deposit, Northwest China. Econ. Geol. Bullet. Soc. Econ. Geol. 1992, 87, 1475–1495. [Google Scholar] [CrossRef]
- Qin, K.-Z.; Tang, D.-M.; Su, B.-X.; Mao, Y.-J.; Xue, S.-C. The tectonic setting, stytle, basic feature, relative erosion degree, ore-bearing evaluation sign, potential analysis of mineralization of Cu-Ni bearing Permian mafic–ultramafic complexes, Northern Xinjiang. Northwest Geol. 2012, 45, 83–116. [Google Scholar]
- Naldrett, A.J. Secular Variation of Magmatic Sulfide Deposits and Their Source Magmas. Econ. Geol. 2010, 105, 669–688. [Google Scholar] [CrossRef]
- Barnes, S.J.; Naldrett, A.J.; Gorton, M.P. The origin of the fractionation of platinum-group elements in terrestrial magmas. Chem. Geol. 1985, 53, 303–323. [Google Scholar] [CrossRef]
- Wei, B.; Wang, C.Y.; Li, C.; Sun, Y. Origin of PGE-depleted Ni-Cu sulfide mineralization in the Triassic Hongqiling No. 7 orthopyroxenite intrusion, Central Asian orogenic belt, northeastern China. Econ. Geol. 2013, 108, 1813–1831. [Google Scholar] [CrossRef] [Green Version]




| Intrusion | Oortsog | Dulaan | Mankhan | Yamat | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 3 | |||||||||
| SiO2 | 45.28 | 45.47 | 45.56 | 45.02 | 43.85 | 42.82 | 38.53 | 41.18 | 39.47 | 44.23 | 43.81 | 44.98 | 51.50 |
| TiO2 | 0.18 | 0.20 | 0.52 | 0.38 | 0.28 | 0.84 | 1.97 | 1.69 | 1.74 | 0.53 | 0.36 | 0.48 | 1.18 |
| Al2O3 | 17.43 | 17.30 | 9.89 | 8.40 | 8.02 | 16.36 | 16.55 | 15.86 | 14.54 | 26.83 | 15.86 | 24.61 | 17.77 |
| Fe2O3 | 7.99 | 7.29 | 12.12 | 12.59 | 14.61 | 14.67 | 19.54 | 19.32 | 21.78 | 5.40 | 12.57 | 7.11 | 9.96 |
| MnO | 0.11 | 0.11 | 0.17 | 0.17 | 0.20 | 0.17 | 0.15 | 0.23 | 0.23 | 0.08 | 0.16 | 0.08 | 0.15 |
| MgO | 12.77 | 12.61 | 20.11 | 20.93 | 25.27 | 11.35 | 7.23 | 6.81 | 7.37 | 2.91 | 15.83 | 5.24 | 4.98 |
| CaO | 14.31 | 14.63 | 9.78 | 11.12 | 6.01 | 14.14 | 15.60 | 12.13 | 12.54 | 13.64 | 7.63 | 12.68 | 7.82 |
| Na2O | 0.66 | 0.65 | 0.78 | 0.58 | 0.56 | 0.54 | 0.47 | 1.51 | 1.24 | 2.40 | 1.67 | 1.94 | 3.73 |
| K2O | 0.07 | 0.10 | 0.35 | 0.24 | 0.32 | 0.08 | 0.05 | 0.13 | 0.09 | 0.31 | 0.56 | 0.42 | 1.87 |
| LOI | 0.52 | 0.78 | 0.69 | 0.55 | 0.58 | 0.04 | 0.02 | 0.49 | 0.60 | 2.13 | 1.27 | 1.88 | −0.25 |
| Total | 99.75 | 99.76 | 100.66 | 100.74 | 100.35 | 101.19 | 100.33 | 99.55 | 99.82 | 99.11 | 100.31 | 99.84 | 99.32 |
| Rocks | Ol mezogabbro | Ol mezogabbro | Melanogabbro | Mezogabbro | Mezogabbro | Ol mezogabbro | Ol mezogabbro | Leucogabbro | Mezogabbro | Mezogabbro | Hbl mezogabbro | Bt-Hbl mezogabbro | Bt-Hbl mezzo- gabbronorite |
| Sample | MO1-16 | MO3-16 | B13043 | B13052 | B13060 | Ch41-14 | Ch43-14 | Sh138-17 | Sh143-17 | Sh228-14 | Sh104-14 | Sh220-14/3 | Sh235-14 |
| № | Mineral | Fe | Co | S | Ni | Cu | Total | Formula |
|---|---|---|---|---|---|---|---|---|
| Oortsog | ||||||||
| 1 | Po | 61.04 | 0.07 | 38.66 | 0.21 | - | 99.98 | (Fe8.99Ni0.04)9.03S9.97 |
| 2 | 61.20 | 0.16 | 38.65 | - | - | 100.07 | (Fe9.02Co0.02)9.04S9.96 | |
| 3 | 62.71 | 0.07 | 36.66 | - | - | 99.49 | Fe0.99S1.01 | |
| 4 | 63.09 | 0.08 | 35.99 | - | - | 99.16 | Fe1.00S1.00 | |
| 5 | 60.87 | 0.09 | 38.99 | 0.23 | - | 100.18 | (Fe8.94Ni0.03Co0.01)8.98S10.02 | |
| 6 | 61.11 | 0.08 | 38.98 | 0.24 | - | 100.41 | (Fe8.96Ni0.03Co0.01)9.00S10.00 | |
| 7 | 60.63 | 0.13 | 38.65 | 0.33 | - | 99.74 | (Fe8.95Ni0.05Co0.01)9.01S9.99 | |
| 8 | 63.36 | 0.07 | 35.89 | - | - | 99.33 | Fe1.00S1.00 | |
| 9 | Pn | 31.05 | 7.23 | 33.05 | 29.48 | - | 100.82 | (Fe4.27Ni3.84Co0.94)9.05S7.95 |
| 10 | 30.01 | 8.76 | 32.90 | 28.63 | - | 100.31 | (Fe4.14Ni3.75Co1.15)9.05S7.95 | |
| 11 | 29.89 | 8.08 | 32.99 | 29.49 | 0.11 | 100.55 | (Fe4.12Ni3.86Co1.06)9.05S7.95 | |
| 12 | 29.15 | 8.31 | 33.18 | 29.89 | - | 100.56 | (Fe4.01Ni3.91Co1.09)9.01S7.99 | |
| 13 | 32.14 | 4.60 | 33.28 | 30.40 | - | 100.47 | (Fe4.42Ni3.97Co0.60)8.99S8.01 | |
| 14 | 25.30 | 15.28 | 32.97 | 25.47 | - | 99.06 | (Fe3.53Ni3.38Co2.03)8.94S8.06 | |
| 15 | 31.59 | 4.77 | 32.85 | 29.58 | - | 98.80 | (Fe4.41Ni3.92Co0.63)8.97S8.03 | |
| 16 | 37.19 | 3.38 | 32.66 | 25.03 | - | 98.28 | (Fe5.21Ni3.33Co0.45)8.99S8.01 | |
| 17 | Co-pn | 11.84 | 45.99 | 32.73 | 10.01 | - | 100.58 | (Co6.07Fe1.65Ni1.32)9.04S7.96 |
| 18 | 13.53 | 42.15 | 32.82 | 11.29 | - | 99.79 | (Co5.59Fe1.89Ni1.50)8.98S8.02 | |
| 19 | Cp | 30.36 | 0.11 | 34.40 | 0.09 | 34.09 | 99.04 | Cu0.99Fe1.01S2.00 |
| 20 | 29.78 | 0.06 | 34.24 | - | 34.47 | 98.56 | Cu1.01Fe0.99S2.00 | |
| 21 | 30.45 | 0.03 | 34.59 | - | 34.07 | 99.14 | Cu0.99Fe1.01S2.00 | |
| Mankhan | ||||||||
| 22 | Po | 59.72 | 0.32 | 39.09 | 0.05 | - | 99.19 | (Fe6.97Co0.04)7.01S7.99 |
| 23 | 60.04 | 0.08 | 38.85 | 0.33 | - | 99.31 | (Fe7.01Ni0.04)7.05S7.95 | |
| 24 | 60.48 | 0.08 | 38.63 | 0.27 | - | 99.47 | (Fe8.95Ni0.04)8.99S10.01 | |
| 25 | 60.74 | 0.08 | 38.55 | 0.13 | - | 99.51 | (Fe8.99Ni0.02Co0.01)9.02S9.98 | |
| 26 | 60.67 | 0.10 | 38.48 | 0.18 | - | 99.42 | (Fe8.99Ni0.03)9.02S9.98 | |
| 27 | Cp | 30.47 | 0.04 | 34.67 | - | 33.86 | 99.05 | Cu0.98Fe1.01S2.01 |
| 28 | 29.87 | 0.03 | 34.63 | - | 33.94 | 98.47 | Cu0.99Fe0.99S2.02 | |
| 29 | 30.21 | 0.03 | 34.60 | - | 34.68 | 99.52 | Cu1.00Fe1.00S2.00 | |
| 30 | Py | 46.00 | 0.03 | 53.13 | - | - | 99.16 | Fe0.99S2.01 |
| 31 | 47.22 | 0.06 | 53.79 | - | - | 101.07 | Fe1.00S2.00 | |
| 32 | 47.37 | 0.07 | 53.40 | - | - | 100.87 | Fe1.01S1.99 | |
| Yamat | ||||||||
| 33 | Po | 61.23 | - | 38.43 | 0.20 | - | 99.88 | (Fe9.04Ni0.03)9.07S9.93 |
| 34 | 63.11 | 0.05 | 37.50 | 0.14 | 0.22 | 101.04 | Fe0.98S1.02 | |
| 35 | 63.10 | - | 36.55 | - | - | 99.72 | Fe0.99S1.01 | |
| 36 | 62.85 | - | 35.84 | - | - | 98.75 | Fe1.00S1.00 | |
| 37 | 63.80 | - | 36.33 | - | - | 100.17 | Fe1.00S1.00 | |
| 38 | 63.23 | - | 35.81 | - | - | 99.07 | Fe1.00S1.00 | |
| 39 | Pn | 27.14 | 11.13 | 32.75 | 27.79 | - | 98.81 | (Fe3.80Ni3.69Co1.48)8.97S8.03 |
| 40 | 28.16 | 14.49 | 33.12 | 23.91 | 0.07 | 99.77 | (Fe3.90Ni3.15Co1.91)8.96S8.04 | |
| 41 | 30.28 | 12.21 | 33.01 | 24.32 | - | 99.88 | (Fe4.19Ni3.20Co1.60Cu0.01)9.00S8.00 | |
| 42 | 30.74 | 9.95 | 32.92 | 26.15 | 0.26 | 100.05 | (Fe4.25Ni3.43Co1.31Cu0.03)9.03S7.97 | |
| 42 | 31.36 | 10.25 | 33.18 | 25.84 | - | 100.66 | (Fe4.31Ni3.37Co1.34)9.02S7.98 | |
| 43 | 55.86 | 2.10 | 36.21 | 4.88 | - | 99.05 | (Fe7.95Ni0.63Co0.27)8.85S8.15 | |
| 44 | Cp | 30.95 | 0.08 | 34.79 | 0.09 | 34.19 | 100.10 | Cu0.98Fe1.02S2.00 |
| 45 | 30.59 | - | 34.52 | - | 34.23 | 99.35 | Cu0.99Fe1.01S2.00 | |
| 46 | 30.86 | - | 34.70 | 0.06 | 34.29 | 99.95 | Cu0.99Fe1.01S2.01 | |
| 47 | Cbn | 40.63 | - | 35.16 | - | 23.32 | 99.11 | Cu1.00Fe1.99S3.01 |
| 48 | 39.53 | - | 35.11 | - | 24.32 | 98.95 | Cu1.04Fe1.94S3.02 | |
| Nomgon | ||||||||
| 49 | Cp | 30.63 | - | 34.60 | - | 34.76 | 100.00 | Cu1.00Fe1.01S1.99 |
| 50 | 30.39 | - | 34.76 | - | 34.90 | 100.05 | Cu1.00Fe1.00S2.00 | |
| 51 | 30.16 | - | 34.40 | - | 33.95 | 98.52 | Cu0.99Fe1.00S2.01 | |
| 52 | Cbn | 40.62 | - | 35.11 | - | 23.28 | 99.02 | Cu1.00Fe1.99S3.01 |
| 53 | 40.70 | - | 34.80 | - | 23.38 | 98.88 | Cu1.01Fe2.00S2.99 | |
| 54 | Bn | 10.97 | - | 25.82 | - | 63.18 | 100.02 | Cu4.97Fe0.98S4.05 |
| 55 | 10.50 | - | 25.15 | - | 63.05 | 98.70 | Cu5.03Fe0.96S4.01 | |
| 56 | 10.91 | - | 25.30 | - | 62.58 | 98.80 | Cu4.98Fe0.99S4.03 | |
| 57 | Cv | - | - | 30.62 | - | 65.16 | 95.78 | Cu1.03S0.97 |
| 58 | Cc | - | - | 20.57 | - | 77.41 | 97.98 | Cu1.96S1.04 |
| 59 | - | - | 20.40 | - | 79.47 | 99.87 | Cu1.98S1.02 | |
| 60 | Py | 46.19 | - | 53.01 | - | - | 99.20 | Fe1.00S2.00 |
| 61 | 46.30 | - | 53.02 | - | - | 99.32 | Fe1.00S2.01 | |
| № | Mineral | Fe | Co | Ni | Cu | Au | Ag | Pt | Pd | Os | Rh | Sb | As | S | Total | Formula |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oortsog | ||||||||||||||||
| 1 | Sperrylite | 0.61 | 0.32 | 0.40 | - | - | - | 56.68 | - | - | - | - | 41.12 | 0.23 | 99.37 | (Pt1.00Fe0.04Ni0.02Co0.02)1.08(As1.89S0.03)1.92 |
| 2 | 0.90 | 0.50 | 0.41 | - | - | - | 53.9 | - | - | - | - | 39.03 | 0.24 | 94.97 | (Pt1.00Fe0.05Ni0.03Co0.02)1.10(As1.87S0.03)1.90 | |
| 3 | - | - | - | - | - | - | 56.02 | - | - | - | - | 42.88 | - | 98.90 | Pt1.10As1.90 | |
| 4 | - | - | - | - | - | - | 57.13 | - | - | - | - | 42.66 | - | 99.79 | Pt1.09As1.91 | |
| 5 | - | - | - | - | - | - | 56.91 | - | - | - | - | 42.08 | - | 98.99 | Pt1.11As1.89 | |
| 6 | Braggite | - | - | 1.41 | - | - | - | 75.44 | 9.64 | - | - | - | - | 16.24 | 102.74 | (Pt0.77Pd0.18Ni0.05)1.00S1.00 |
| 7 | - | - | 1.21 | - | - | - | 76.98 | 8.16 | - | - | - | - | 15.76 | 102.11 | (Pt0.80Pd0.16Ni0.04)1.00S1.00 | |
| 8 | Electrum | 0.48 | - | - | 2.69 | 58.61 | 35.85 | - | - | - | - | - | - | - | 97.63 | Ag0.49Au0.44Cu0.06Fe0.01 |
| 9 | 0.96 | - | 0.82 | 2.75 | 58.76 | 36.9 | - | - | - | - | - | - | - | 100.18 | Ag0.48Au0.42Cu0.06Fe0.02Ni0.02 | |
| Nomgon | ||||||||||||||||
| 10 | Sperrylite | - | - | - | - | - | - | 50.21 | - | - | 4.08 | - | 42.55 | 1.92 | 98.77 | (Pt0.78Rh0.18)0.96(As1.76S0.28)2.04 |
| 11 | - | - | - | - | - | - | 51.04 | - | - | 4.35 | - | 43.5 | 2.09 | 101.91 | (Pt0.83Rh0.13)0.96(As1.84S0.18)2.04 | |
| 12 | - | - | - | - | - | - | 52.98 | - | - | - | - | 42.39 | 1.40 | 96.77 | (Pt0.91Rh0.05)0.96(As1.95S0.09)2.04 | |
| 13 | - | - | - | - | - | - | 53.37 | - | - | 1.88 | - | 43.72 | 0.98 | 99.94 | Pt0.93(As1.98S0.09)2.07 | |
| 14 | - | - | - | - | - | - | 54.74 | - | - | - | - | 42.86 | 0.82 | 98.42 | Pt0.97As2.03 | |
| 15 | - | - | - | - | - | - | 55.58 | - | - | - | - | 43.41 | 0.59 | 99.58 | Pt0.98As2.02 | |
| 16 | - | - | - | - | - | - | 55.79 | - | - | - | 0.89 | 43.04 | - | 99.72 | Pt0.99As2.01 | |
| 17 | Hollingworthite | - | - | - | - | - | - | 26.59 | - | 2.39 | 24.21 | - | 37.08 | 10.36 | 100.62 | (Rh0.59Pt0.34Os0.03)0.96(As1.24S0.80)2.04 |
| 18 | - | - | - | - | - | - | 28.75 | - | 2.12 | 22.60 | - | 36.55 | 9.39 | 99.40 | (Rh0.57Pt0.38Os0.03)0.98(As1.26S0.76)2.02 | |
| 19 | - | - | - | - | - | - | 31.03 | - | - | 20.15 | - | 37.51 | 8.17 | 97.91 | (Rh0.50Pt0.41)0.91(As1.28S0.81)2.09 | |
| 20 | Majakite | - | 0.54 | 23.95 | - | - | - | - | 43.64 | - | - | 1.27 | 31.72 | - | 101.12 | Pd0.99(Ni0.97Co0.02)0.99(As1.00Sb0.02)1.02 |
| 21 | - | 0.40 | 24.03 | - | - | - | - | 43.92 | - | - | 1.16 | 31.4 | - | 100.9 | Pd0.98(Ni0.98Co0.02)1.00(As1.00Sb0.02)1.02 | |
| 22 | 1.35 | - | 19.82 | - | - | - | - | 44.12 | - | - | 2.78 | 26.16 | - | 94.51 | Pd1.07(Ni0.88Fe0.08)0.96(As0.91Sb0.06)0.97 | |
| 23 | Mertieite -II | - | - | - | 0.96 | - | - | - | 65.69 | - | - | 25.9 | 3.03 | - | 95.59 | (Pd7.67Cu0.19)7.86(Sb2.64As0.50)3.14 |
| 24 | - | - | - | 1.35 | - | - | - | 68.45 | - | - | 27.76 | 2.73 | - | 100.29 | (Pd7.62Cu0.25)7.87(Sb2.70As0.43)3.13 | |
| 25 | - | - | - | 1.22 | - | - | - | 68.47 | - | - | 31.89 | - | - | 101.58 | (Pd7.66Cu0.23)7.89Sb3.11 | |
| 26 | - | - | - | 1.5 | - | - | - | 68.85 | - | - | 28.05 | 2.63 | - | 101.03 | (Pd7.60Cu0.28)7.88(Sb2.71As0.41)3.12 | |
| 27 | - | - | - | 1.25 | - | - | - | 68.96 | - | - | 27.86 | 3.15 | - | 101.22 | (Pd7.60Cu0.23)7.83(Sb2.68As0.49)3.17 | |
| 28 | - | - | - | 1.05 | - | - | - | 69.07 | - | - | 27.39 | 3.15 | - | 100.66 | (Pd7.66Cu0.19)7.85(Sb2.65As0.50)3.15 | |
| 29 | - | - | - | - | - | 1.35 | - | 69.32 | - | - | 26.97 | 3.13 | - | 100.77 | (Pd7.73Ag0.15)7.88(Sb2.62As0.50)3.12 | |
| 30 | Electrum | - | - | - | - | 67.44 | 32.78 | - | - | - | - | - | - | - | 100.22 | Au0.53Ag0.47 |
| 31 | - | - | - | - | 63.87 | 35.04 | - | - | - | - | - | - | - | 98.91 | Au0.50Ag0.50 | |
| 32 | - | - | - | - | 55.5 | 41.77 | - | - | - | - | - | - | - | 97.27 | Au0.58Ag0.42 | |
| № | Intrusion | Name of Rock | Sample | S wt% | Ni wt% | Cu wt% | Co wt% | Cr wt% | Pt ppb | Pd ppb | Ir ppb | Os ppb | Rh ppb | Ru ppb | Au ppb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oortsog | |||||||||||||||
| 1 | 1 | Ol mezogabbro | M01-16 | 0.340 | 0.007 | 0.005 | - | 0.060 | 2.72 | 7.55 | 0.02 | 0.96 | 0.11 | 2.64 | 0.62 |
| 2 | Ol mezogabbro | M03-16 | 0.220 | 0.005 | 0.004 | - | 0.060 | 2.12 | 10.10 | 0.04 | 0.07 | 0.30 | 3.22 | 1.53 | |
| 3 | Ol melanogabbro | M05-16 | 0.310 | 0.009 | 0.006 | - | 0.070 | 4.36 | 1.78 | 0.07 | 0.61 | 0.05 | 0.92 | 0.99 | |
| 4 | 2 | melanogabbro | B-13043 | 0.250 | 0.040 | 0.010 | - | 0.110 | 13.00 | 8.00 | 0.30 | 1.60 | 0.20 | 1.30 | 0.00 |
| 5 | mezogabbro | B-13052 | 0.430 | 0.040 | 0.012 | - | 0.140 | 12.00 | 12.00 | 0.20 | 0.57 | 0.10 | 0.60 | 0.00 | |
| 6 | mezogabbro | B-13060 | 0.180 | 0.040 | 0.007 | - | 0.150 | 8.00 | 14.00 | 0.22 | 0.14 | 0.30 | 0.80 | 0.00 | |
| Dulaan | |||||||||||||||
| 7 | Ol mezogabbro | Ch41-14 | 0.210 | 0.004 | 0.006 | 0.005 | 0.007 | 1.37 | 2.12 | 0.09 | 0.21 | 0.04 | 0.06 | - | |
| 8 | Ol mezogabbro | Ch43-14 | 0.400 | 0.008 | 0.027 | 0.006 | 0.005 | 1.38 | 1.62 | 0.07 | 0.18 | 0.04 | 0.04 | - | |
| Mankhan | |||||||||||||||
| 9 | leucogabbro | Sh138-17 | 0.310 | 0.004 | 0.013 | 0.005 | 0.004 | 1.54 | 1.43 | 0.09 | 0.14 | 0.04 | < 1 | - | |
| 10 | mezogabbro | Sh143-17 | 0.520 | 0.004 | 0.015 | 0.005 | 0.002 | 1.92 | 0.94 | 0.06 | 0.15 | 0.03 | < 1 | - | |
| Yamat | |||||||||||||||
| 11 | 1 | mezogabbro | Sh228-14 | 0.590 | 0.012 | 0.287 | 0.005 | 0.010 | 0.94 | 2.08 | 0.09 | 0.27 | 0.08 | 0.85 | - |
| 12 | 2 | Hbl mezogabbro | Sh104-14 | 0.320 | 0.010 | 0.033 | 0.006 | 0.017 | 1.71 | 1.90 | 0.08 | 0.21 | 0.11 | 0.13 | - |
| 13 | Bt-Hbl mezog | Sh220-14/3 | 0.160 | 0.010 | 0.196 | 0.004 | 0.012 | 1.61 | 2.09 | 0.09 | 0.20 | 0.15 | 0.86 | - | |
| 14 | 3 | Bt-Hbl mezog-n | Sh235-14 | < 0.1 | 0.005 | 0.005 | 0.003 | 0.006 | 1.19 | 2.62 | 0.05 | 0.24 | 0.03 | 0.10 | - |
| Nomgon | |||||||||||||||
| 15 | leucogabbro | Sh202-18 | 0.210 | 0.012 | 0.404 | 0.004 | 0.013 | 35.64 | 132.83 | 0.16 | 0.14 | 1.85 | 0.23 | - | |
| 16 | Sulfide-bearing zone I | 0.600 | 0.022 | 0.341 | 0.005 | - | 114.00 | 388.00 | - | - | 2.40 | - | - | ||
| 17 | Sulfide-bearing zone II | 0.500 | 0.014 | 0.175 | 0.006 | - | 69.00 | 132.00 | - | - | 1.20 | - | - | ||
| 18 | Sulfide-bearing zone III | 0.700 | 0.022 | 0.276 | 0.004 | - | 142.00 | 300.00 | - | - | 2.60 | - | - | ||
| 19 | Sulfide-bearing zone IV | 0.130 | 0.028 | 0.857 | 0.007 | - | 190.00 | 540.00 | - | - | 3.20 | - | - | ||
| Contents of Ore Elements Calculated on 100% Sulfide 1 | |||||||||||||||
| Oortsog | |||||||||||||||
| 1 | 1 | Ol mezogabbro | M01-16 | 39.96 | 0.76 | 0.59 | - | - | 0.320 | 0.888 | 0.002 | 0.113 | 0.013 | 0.310 | 0.073 |
| 2 | Ol mezogabbro | M03-16 | 39.95 | 0.98 | 0.73 | - | - | 0.385 | 1.834 | 0.007 | 0.012 | 0.054 | 0.585 | 0.278 | |
| 3 | Ol melanogabbro | M05-16 | 39.96 | 1.17 | 0.76 | - | - | 0.562 | 0.229 | 0.009 | 0.078 | 0.007 | 0.119 | 0.127 | |
| 4 | 2 | melanogabbro | B-13043 | 40.16 | 6.43 | 1.62 | - | - | 2.088 | 1.285 | 0.048 | 0.257 | 0.032 | 0.209 | - |
| 5 | mezogabbro | B-13052 | 40.08 | 3.73 | 1.07 | - | - | 1.118 | 1.118 | 0.019 | 0.053 | 0.009 | 0.056 | - | |
| 6 | mezogabbro | B-13060 | 40.34 | 8.96 | 1.50 | - | - | 1.793 | 3.138 | 0.049 | 0.031 | 0.067 | 0.179 | - | |
| Dulaan | |||||||||||||||
| 7 | Ol mezogabbro | Ch41-14 | 39.88 | 0.80 | 1.16 | 0.99 | - | 0.261 | 0.403 | 0.018 | 0.039 | 0.008 | 0.011 | - | |
| 8 | Ol mezogabbro | Ch43-14 | 39.66 | 0.78 | 2.68 | 0.56 | - | 0.138 | 0.161 | 0.007 | 0.018 | 0.004 | 0.04 | - | |
| Mankhan | |||||||||||||||
| 9 | leucogabbro | Sh138-17 | 39.79 | 0.55 | 1.63 | 0.60 | - | 0.198 | 0.183 | 0.012 | 0.019 | 0.005 | 0.013 | - | |
| 10 | mezogabbro | Sh143-17 | 39.84 | 0.29 | 1.18 | 0.41 | - | 0.147 | 0.072 | 0.004 | 0.011 | 0.003 | 0.008 | - | |
| Yamat | |||||||||||||||
| 11 | 1 | mezogabbro | Sh228-14 | 37.38 | 0.76 | 18.15 | 0.29 | - | 0.060 | 0.132 | 0.006 | 0.017 | 0.005 | 0.054 | - |
| 12 | 2 | Hbl mezogabbro | Sh104-14 | 39.47 | 1.17 | 4.11 | 0.78 | - | 0.211 | 0.235 | 0.009 | 0.026 | 0.001 | 0.016 | - |
| 13 | Bt-Hbl mezog. | Sh220-14/3 | 34.02 | 2.13 | 41.57 | 0.85 | - | 0.343 | 0.444 | 0.019 | 0.043 | 0.003 | 0.184 | - | |
| 14 | 3 | Bt-Hbl mezog-n | Sh235-14 | 38.19 | 18.72 | 20.24 | 11.46 | - | 5.557 | 10.005 | 0.198 | 0.923 | 0.013 | 0.384 | - |
| Nomgon | |||||||||||||||
| 15 | leucogabbro | Sh202-18 | 31.28 | 1.83 | 60.10 | 0.60 | - | 5.213 | 19.785 | 0.024 | 0.022 | 0.028 | 0.004 | - | |
| 16 | Sulfide-bearing zone I | 36.99 | 1.33 | 21.05 | 0.30 | - | 7.028 | 23.920 | - | - | 0.148 | - | - | ||
| 17 | Sulfide-bearing zone II | 38.11 | 1.05 | 13.32 | 0.44 | - | 5.259 | 10.060 | - | - | 0.091 | - | - | ||
| 18 | Sulfide-bearing zone III | 37.88 | 1.17 | 14.93 | 0.23 | - | 7.684 | 16.233 | - | - | 0.141 | - | - | ||
| 19 | Sulfide-bearing zone IV | 36.51 | 0.79 | 24.07 | 0.20 | - | 5.336 | 15.166 | - | - | 0.090 | - | - | ||
| № | In. | Rock | Samples | ∑PGE, ppb | Pt/Pd | PPGE/ IPGE | Pd/Ir | Cu/Pd (×1000) | Ni/Cu | Cu/S | Ni /Co |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oortsog | |||||||||||
| 1 | 1 | Ol mezogabbro | MO1-16 | 15.42 | 0.36 | 2.84 | 450.79 | 6.62 | 1.30 | 68.00 | - |
| 2 | Ol mezogabbro | MO3-16 | 16.82 | 0.21 | 3.68 | 266.10 | 3.96 | 1.35 | 55.00 | - | |
| 3 | Ol melanogabbro | MO5-16 | 9.07 | 2.45 | 3.83 | 25.15 | 33.15 | 1.54 | 52.54 | - | |
| 4 | 2 | melanogabbro | B13043 | 24.40 | 1.63 | 6.56 | 26.67 | 12.63 | 3.9 | 24.75 | - |
| 5 | mezogabbro | B13052 | 25.47 | 1.00 | 17.52 | 60.00 | 9.58 | 3.48 | 37.39 | - | |
| 6 | mezogabbro | B13060 | 23.46 | 0.57 | 18.97 | 63.64 | 4.79 | 5.97 | 26.87 | - | |
| Dulaan | |||||||||||
| 7 | Ol mezogabbro | Ch41-14 | 3.90 | 0.64 | 8.77 | 22.82 | 28.72 | 0.69 | 34.43 | 0.81 | |
| 8 | Ol mezogabbro | Ch43-14 | 3.35 | 0.85 | 8.85 | 22.03 | 166.30 | 0.29 | 14.81 | 1.41 | |
| Mankhan | |||||||||||
| 9 | leucogabbro | Sh138-17 | 3.35 | 1.08 | 7.82 | 15.07 | 89.03 | 0.34 | 24.41 | 0.91 | |
| 10 | mezogabbro | Sh143-17 | 3.20 | 2.03 | 8.49 | 17.10 | 163.03 | 0.25 | 33.77 | 0.70 | |
| Yamat | |||||||||||
| 11 | 1 | mezogabbro | Sh228-14 | 4.32 | 0.45 | 2.34 | 23.80 | 1375.73 | 0.04 | 2.06 | 2.61 |
| 12 | 2 | Hbl mezogabbro | Sh104-14 | 4.05 | 0.89 | 8.36 | 24.75 | 175.01 | 0.29 | 9.61 | 1.51 |
| 13 | Bt-Hbl mezog. | Sh220-14/3 | 4.87 | 0.77 | 3.17 | 23.99 | 935.35 | 0.05 | 0.82 | 2.50 | |
| 14 | 3 | Bt-Hbl mezog-n. | Sh235-14 | 8.21 | 0.45 | 9.59 | 50.48 | 20.23 | 0.92 | 1.89 | 1.63 |
| Nomgon | |||||||||||
| 15 | leucogabbro | Sh202-18 | 186.63 | 0.26 | 8.93 | 822.15 | 30.38 | 0.03 | 0.52 | 3.08 | |
| 16 | Sulfide-bearing zone I | 504.40 | 0.29 | - | - | 8.80 | 0.06 | 1.76 | 4.48 | ||
| 17 | Sulfide-bearing zone II | 202.20 | 0.52 | - | - | 13.24 | 0.08 | 2.86 | 2.38 | ||
| 18 | Sulfide-bearing zone III | 444.60 | 0.47 | - | - | 9.20 | 0.08 | 2.54 | 5.02 | ||
| 19 | Sulfide-bearing zone IV | 733.20 | 0.35 | - | - | 15.87 | 0.03 | 1.52 | 4.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapovalova, M.; Tolstykh, N.; Shelepaev, R.; Kalugin, V. PGE-Cu-Ni Mineralization of Mafic-Ultramafic Massifs of the Khangai Upland, Western Mongolia. Minerals 2020, 10, 942. https://doi.org/10.3390/min10110942
Shapovalova M, Tolstykh N, Shelepaev R, Kalugin V. PGE-Cu-Ni Mineralization of Mafic-Ultramafic Massifs of the Khangai Upland, Western Mongolia. Minerals. 2020; 10(11):942. https://doi.org/10.3390/min10110942
Chicago/Turabian StyleShapovalova, Maria, Nadezhda Tolstykh, Roman Shelepaev, and Valery Kalugin. 2020. "PGE-Cu-Ni Mineralization of Mafic-Ultramafic Massifs of the Khangai Upland, Western Mongolia" Minerals 10, no. 11: 942. https://doi.org/10.3390/min10110942





