Iron Isotopes Constrain the Metal Sources of Skarn Deposits: A Case Study from the Han-Xing Fe Deposit, China
Abstract
:1. Introduction
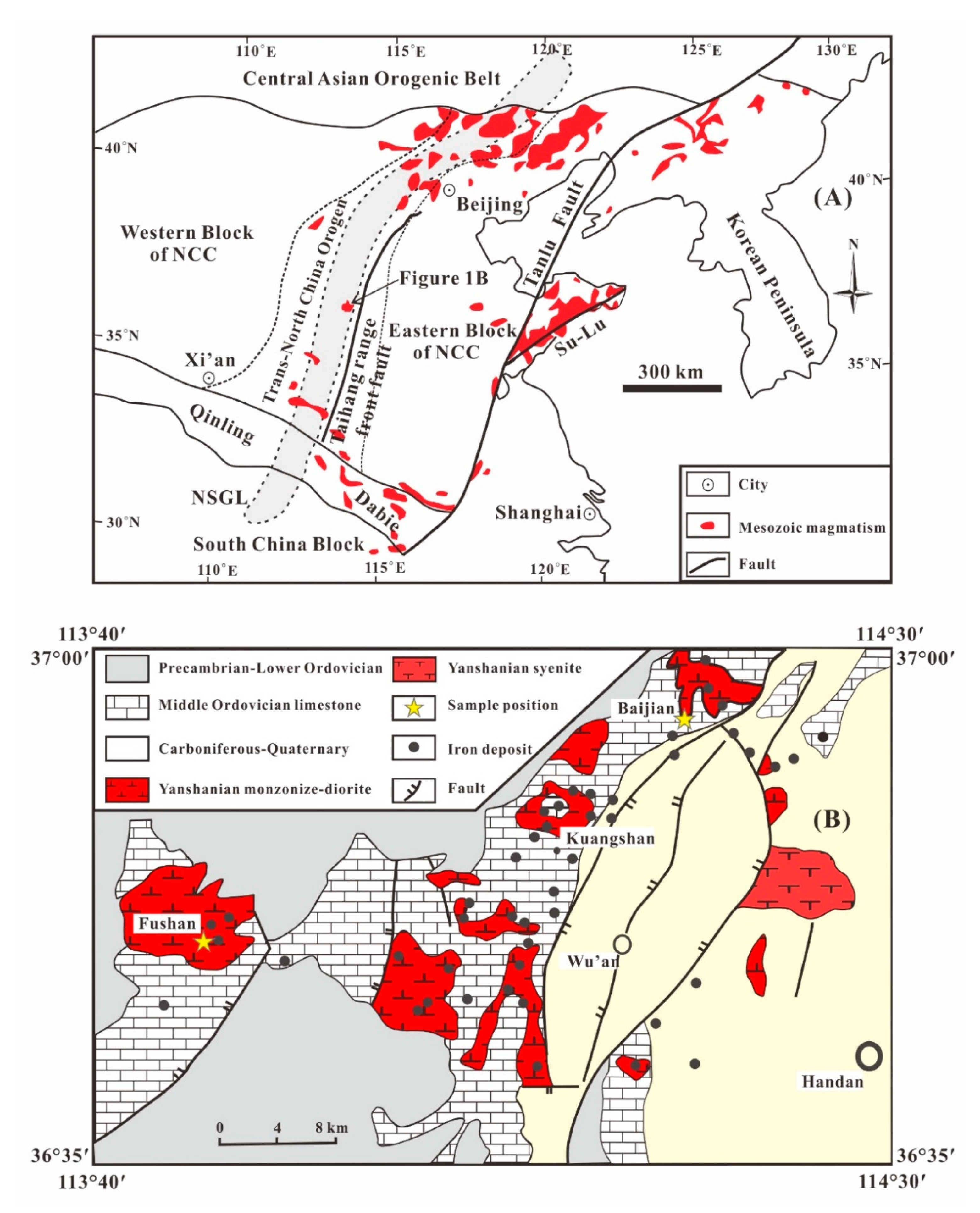
2. Geological Setting
3. Samples and Methods
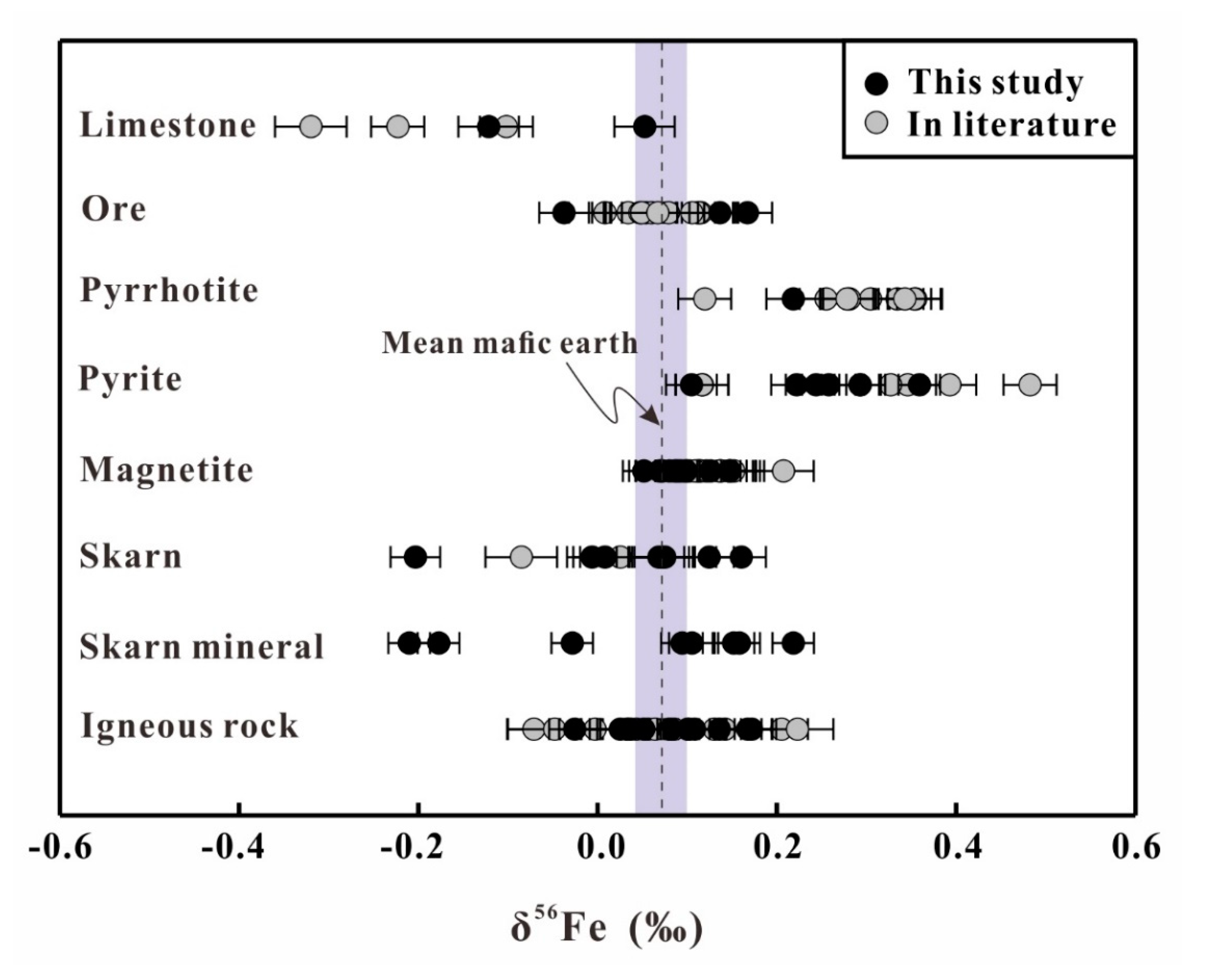
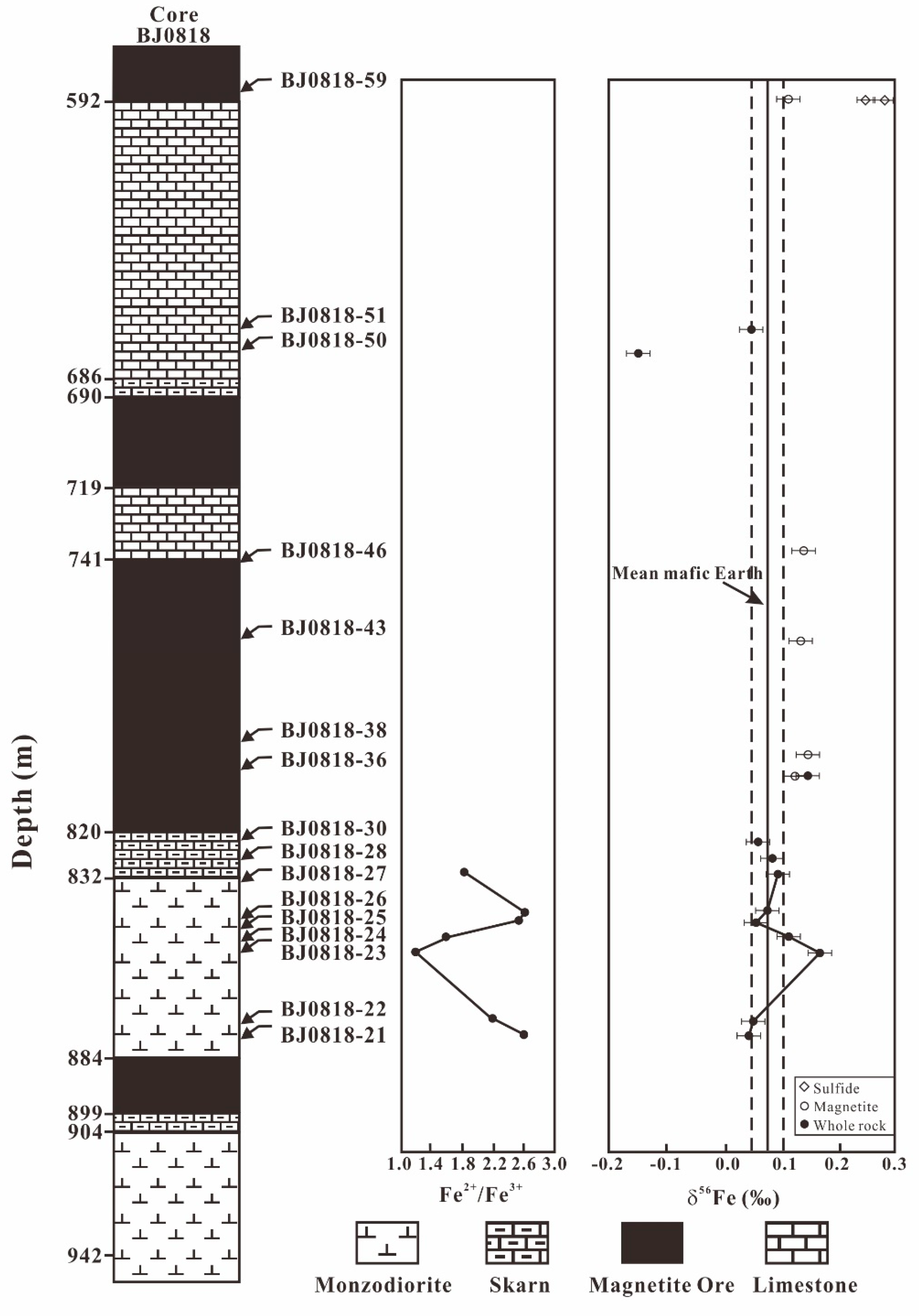
4. Result
5. Discussion
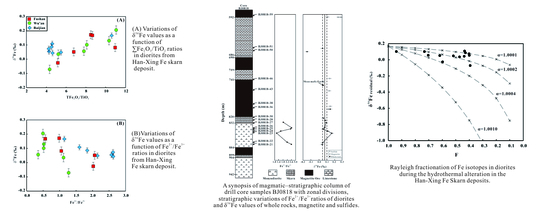
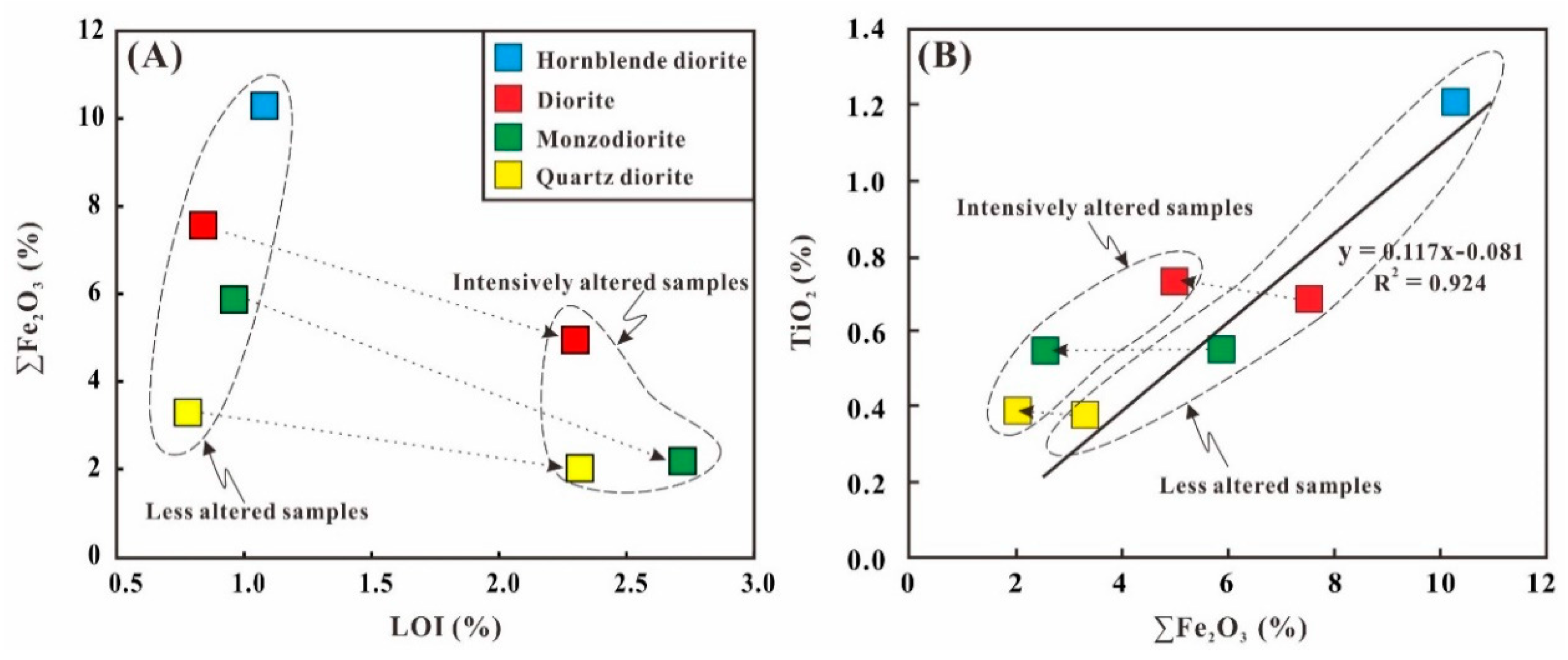
5.1. Fe Leached from the Diorites during the Hydrothermal Alteration
5.2. Fe Isotopic Variation of Whole Rocks and Mineral Separates
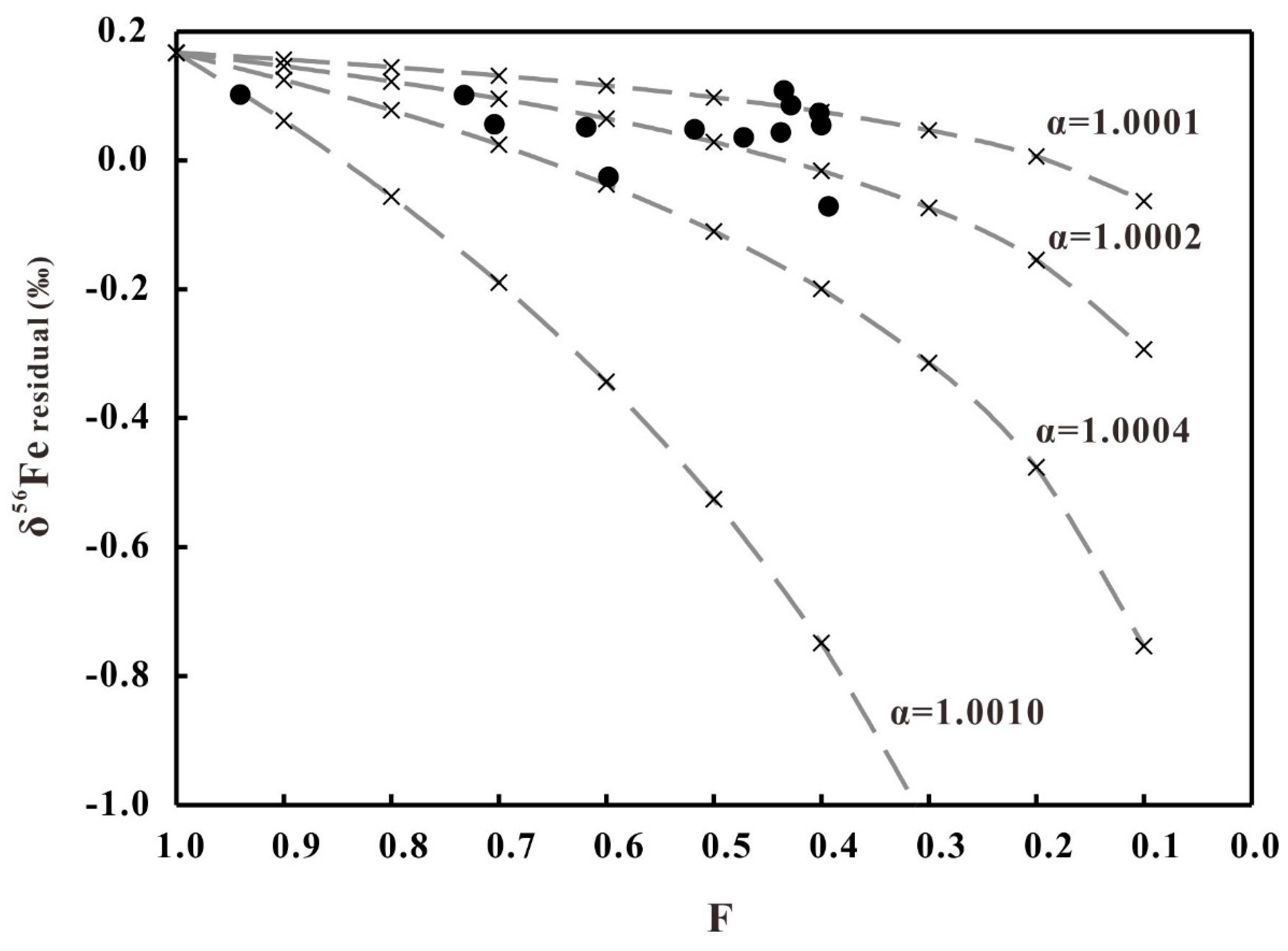
5.3. Fe Isotopes Fractionation of Diorites during the Hydrothermal Alteration
5.4. Iron Sources of the Han-Xing Fe Skarn Deposit
5.5. Estimation of the Relative Proportions of Iron Sources for the Han-Xing Fe Skarn Deposit
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilkinson, J.J. Triggers for the formation of porphyry ore deposits in magmatic arcs. Nat. Geosci. 2013, 6, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Candela, P.A.; Holland, H.D. A mass transfer model for copper and molybdenum in magmatic hydrothermal systems: The origin of porphyry-type ore deposits. Econ. Geol. 1986, 81, 1–19. [Google Scholar] [CrossRef]
- Rye, R.O. The carbon, hydrogen, and oxygen isotopic composition of the hydrothermal fluids responsible for the lead-zinc deposits at Providencia, Zacatecas, Mexico. Econ. Geol. 1966, 61, 1399–1427. [Google Scholar] [CrossRef]
- Ohmoto, H. Systematics of sulfur and carbon isotopes in hydrothermal ore deposit. Econ. Geol. 1972, 67, 551–578. [Google Scholar] [CrossRef]
- Taylor, H.P., Jr. The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposition. Econ. Geol. 1974, 69, 843–883. [Google Scholar] [CrossRef]
- Jia, Y.; Kerrich, R. Nitrogen isotope systematics of mesothermal lode gold deposits: Metamorphic, granitic, meteoric water, or mantle origin? Geology 1999, 27, 1051–1054. [Google Scholar] [CrossRef]
- Meinert, L.D.; Hedenquist, J.W.; Satoh, H.; Matsuhisa, Y. Formation of anhydrous and hydrous skarn in Cu-Au ore deposits by magmatic fluids. Econ. Geol. 2003, 98, 147–156. [Google Scholar] [CrossRef]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, T. World skarn deposit. Econ. Geol. 2005, 100, 299–336. [Google Scholar]
- Graham, S.; Pearson, N.; Jackson, S.; Griffin, W.; O’Reilly, S.Y. Tracing Cu and Fe from source to porphyry: In situ determination of Cu and Fe isotope ratios in sulfides from the grasberg Cu-Au deposit. Chem. Geol. 2004, 207, 147–169. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.K.; Mao, J.W.; Li, Z.H.; Cheng, Y.B. Iron isotope fractionation during skarn-type metallogeny: A case study of Xinqiao Cu-S-Fe-Au deposit in the Middle-Lower Yangtze valley. Ore Geol. Rev. 2011, 43, 194–202. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, H.F.; Zhao, X.M.; He, Y.S. Iron isotope fractionation during skarn-type alteration: Implications for metal source in the Han-Xing iron skarn deposit. Ore Geol. Rev. 2016, 74, 139–150. [Google Scholar] [CrossRef]
- Ohmoto, H. Stable isotope geochemistry of ore deposits. Rev. Mineral. 1986, 16, 491–559. [Google Scholar]
- Kodera, P.; Rankinm, A.H.; Lexa, J. Evolution of fluids responsible for iron skarn mineralisation: An example from the Vyhne-Klokoc deposit, Western Carpathians, Slovakia. Mineral. Petrol. 1998, 64, 119–147. [Google Scholar] [CrossRef]
- Zheng, J.M. The Ore-Forming Fluid and Mineralization of Skarn Fe Deposits in Handan-Xingtai Area, South Hebei. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2007. (In Chinese). [Google Scholar]
- Einaudi, M.T.; Meinert, L.D.; Newberry, R.J. Skarn deposits. In Economic Geology 75th Anniversary Volume; The Economic Geology Publishing Company Library of Congress: New Haven, CT, USA, 1981; pp. 317–391. [Google Scholar]
- Feng, Z.Y. Comparison of iron skarn generating intrusions with barren intrusions in southern taihang mountain, China. Geosciences 1998, 1, 467–476. (In Chinese) [Google Scholar]
- Shen, J.-F.; Santosh, M.; Li, S.-R.; Zhang, H.-F.; Yin, N.; Dong, G.-C.; Wang, Y.-J.; Ma, G.-G.; Yu, H.-J. The Beiminghe skarn iron deposit, eastern China: Geochronology, isotope geochemistry and implications for the destruction of the North China Craton. Lithos 2013, 156–159, 218–229. [Google Scholar] [CrossRef]
- Zhai, M.G.; Santosh, M. The early Precambrian odyssey of the North China Craton: A synoptic overview. Gondwana Res. 2011, 20, 6–25. [Google Scholar] [CrossRef]
- Chen, B.; Tian, W.; Jahn, B.M.; Chen, Z.C. Zircon SHRIMP U-Pb ages and in-situ Hf isotopic analysis for the Mesozoic intrusions in South Taihang, North China Craton: Evidence for hybridization between mantle-derived magmas and crustal components. Lithos 2008, 102, 118–137. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, T.; Santosh, M.; Li, H.; Li, J.; Zhang, Z.; Song, X.; Wang, M. Spatio-temporal distribution and tectonic settings of the major iron deposits in China: An overview. Ore Geol. Rev. 2014, 57, 247–263. [Google Scholar] [CrossRef]
- Zhao, G.C.; Sun, M.; Wilde, S.A.; Li, S.Z. Late Archean to Paleoproterozoic evolution of the North China Craton: Key issues revisited. Precambrian Res. 2005, 136, 177–202. [Google Scholar] [CrossRef]
- Qian, Q.; Hermann, J. Formation of high-Mg diorites through assimilation of peridotite by monzodiorite magma at crustal depths. J. Petrol. 2010, 51, 1381–1416. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Ke, S.; Teng, F.-Z.; Wang, T.; Wu, H.; Lu, Y.; Li, S. High precision iron isotope analysis of geological standards by high resolution MC-ICPMS. Geostand. Geoanal. Res. 2015, 39, 341–356. [Google Scholar] [CrossRef]
- Dauphas, N.; Pourmand, A.; Teng, F.Z. Routine isotopic analysis of iron by HR-MC-ICPMS: How precise and how accurate? Chem. Geol. 2009, 267, 175–184. [Google Scholar] [CrossRef]
- Craddock, P.R.; Dauphas, N. Iron isotopic compositions of geological reference materials and chondrites. Geostand. Geoanal. Res. 2011, 35, 101–123. [Google Scholar] [CrossRef]
- Irvine, T.N.; Baragar, W.R.A. A guide to the chemical classification of the common volcanic rocks. Can. J. Earth Sci. 1971, 8, 523–547. [Google Scholar] [CrossRef]
- Rouxel, O.; Dobbek, N.; Ludden, J.; Fouquet, Y. Iron isotope fractionation during oceanic crust alteration. Chem. Geol. 2003, 202, 155–182. [Google Scholar] [CrossRef]
- Dauphas, N.; van Zuilen, M.; Wadhwa, M.; Davis, A.M.; Marty, B.; Janney, P.E. Clues from Fe isotope variations on the origin of early Archean BIFs from Greenland. Science 2004, 306, 2077–2080. [Google Scholar] [CrossRef] [Green Version]
- Poitrasson, F.; Halliday, A.N.; Lee, D.C.; Levasseur, S.; Teutsch, N. Iron isotope differences between Earth, Moon, Mars and Vesta as possible records of contrasted accretion mechanisms. Earth Planet. Sci. Lett. 2004, 223, 253–266. [Google Scholar] [CrossRef]
- Foden, J.; Sossi, P.A.; Wawryk, C.M. Fe isotopes and the contrasting petrogenesis of A–, I– and S–type granite. Lithos 2015, 212–215, 32–44. [Google Scholar] [CrossRef]
- Polyakov, V.B.; Mineev, S.D. The use of Mössbauer spectroscopy in stable isotope geochemistry. Geochim. Cosmochim. Acta 2000, 64, 849–865. [Google Scholar] [CrossRef]
- Blanchard, M.; Poitrasson, F.; Meheut, M.; Lazzeri, M.; Mauri, F.; Balan, E. Iron isotope fractionation between pyrite (FeS2), hematite (Fe2O3) and siderite (FeCO3): A first-principles density functional theory study. Geochim. Cosmochim. Acta 2009, 73, 6565–6578. [Google Scholar] [CrossRef]
- Chen, Y.J.; Su, S.; He, Y.; Li, S.; Hou, J.G.; Feng, S.C.; Ke, G. Fe isotope compositions and implications on the mineralization of Xishimen iron deposit in Wuan, Hebei. Acta Petrol. Sin. 2014, 30, 3443–3454. (In Chinese) [Google Scholar]
- Schauble, E.A.; Rossman, G.R.; Taylor, H.P., Jr. Theoretical estimates of equilibrium Fe-isotope fractionations from vibrational spectroscopy. Geochim. Cosmochim. Acta 2001, 65, 2487–2497. [Google Scholar] [CrossRef]
- Heimann, A.; Beard, B.L.; Johnson, C.M. The role of volatile exsolution and sub-solidus fluid/rock interactions in producing high 56Fe/54Fe ratios in siliceous igneous rocks. Geochim. Cosmochim. Acta 2008, 72, 4379–4396. [Google Scholar] [CrossRef]
- Shahar, A.; Young, E.D.; Manning, C. Equilibrium high temperature Fe isotope fractionation between fayalite and magnetite: An experimental calibration. Earth Planet. Sci. Lett. 2008, 268, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Chou, I.M.; Eugster, H.P. Solubility of magnetite in supercritical chloride solutions. Am. J. Sci. 1977, 277, 1296–1314. [Google Scholar] [CrossRef]
- Fein, J.B.; Hemley, J.J.; D’Angelo, W.M.; Komninou, A.; Svergensky, D.A. Experimental study of iron-chloride complexing in hydrothermal fluids. Geochim. Cosmochim. Acta 1992, 56, 3179–3190. [Google Scholar] [CrossRef]
- Richards, J.P. Tectono-magmatic precursors for porphyry Cu-(Mo-Au) deposit formation. Econ. Geol. 2003, 98, 1515–1533. [Google Scholar] [CrossRef]
- Yardley, B.W.D. Metal concentrations in crustal fluids and their relationship to ore formation. Econ. Geol. 2005, 100, 613–632. [Google Scholar] [CrossRef]
- Poitrasson, F.; Freydier, R. Heavy iron isotope composition of granites determined by high resolution MC-ICP-MS. Chem. Geol. 2005, 222, 132–147. [Google Scholar] [CrossRef]
- Zhang, B.M.; Zhao, G.L.; Ma, G.X. The Metallogenic Series and Models of the Main Metallogenic Zones in Hebei Province; Petroleum Industry Press: Beijing, China, 1996; pp. 1–273. (In Chinese) [Google Scholar]
- Frierdich, A.J.; Beard, B.L.; Scherer, M.M.; Johnson, C.L. Determination of the Fe (II) aq–magnetite equilibrium iron isotope fractionation factor using the three-isotope method and a multi-direction approach to equilibrium. Earth Planet. Sci. Lett. 2014, 391, 77–86. [Google Scholar] [CrossRef]
- Telus, M.; Dauphas, N.; Moynier, F.; Tissot, F.L.H.; Teng, F.-Z.; Nabelek, P.I.; Graddock, P.R.; Groat, L.A. Iron, zinc, magnesium and uranium isotopic fractionation during continental crust differentiation: The tale from migmatites, granitoids and pegmatites. Geochim. Cosmochim. Acta 2012, 97, 247–265. [Google Scholar] [CrossRef]
- Baker, T.; van Achterberg, E.; Ryan, C.G.; Lang, J.R. Composition and evolution of ore fluids in a magmatic-hydrothermal skarn deposit. Geology 2004, 32, 117–120. [Google Scholar] [CrossRef]
- Severmann, S.; Johnson, C.M.; Beard, B.L.; German, C.R.; Edmonds, H.N.; Chiba, H.; Green, D.R.H. The effect of plume processes on the Fe isotope composition of hydrothermally derived Fe in the deep ocean as inferred from the Rainbow vent site, Mid–Atlantic Ridge, 36°14’N. Earth Planet. Sci. Lett. 2004, 225, 63–76. [Google Scholar] [CrossRef]
- Bennett, S.A.; Rouxel, O.; Schmidt, K.; Schönberg, D.G.; Statham, P.J.; German, C.R. Iron isotope fractionation in a buoyant hydrothermal plume, 5°S Mid–Atlantic Ridge. Geochim. Cosmochim. Acta 2009, 73, 5619–5634. [Google Scholar] [CrossRef]
- Chilton, J. Water Quality Assessments—A Guide to Use of Biota, Sediments and Water in Environmental Monitoring; Chapman, D., Ed.; Great Britain at the University Press: Cambridge, UK, 1996; pp. 394–481. [Google Scholar]
- Wawryk, C.M.; Foden, J.D. Fe-isotope fractionation in magmatic-hydrothermal mineral deposits: A case study from the Renison Sn–W deposit, Tasmania. Geochim. Cosmochim. Acta 2014, 150, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, X.K.; Cheng, Y.B. Fe isotope behaviors during sulfide-dominated skarn type mineralization. J. Asian Earth Sci. 2015, 103, 374–392. [Google Scholar] [CrossRef]

| Type | Sample | Description |
|---|---|---|
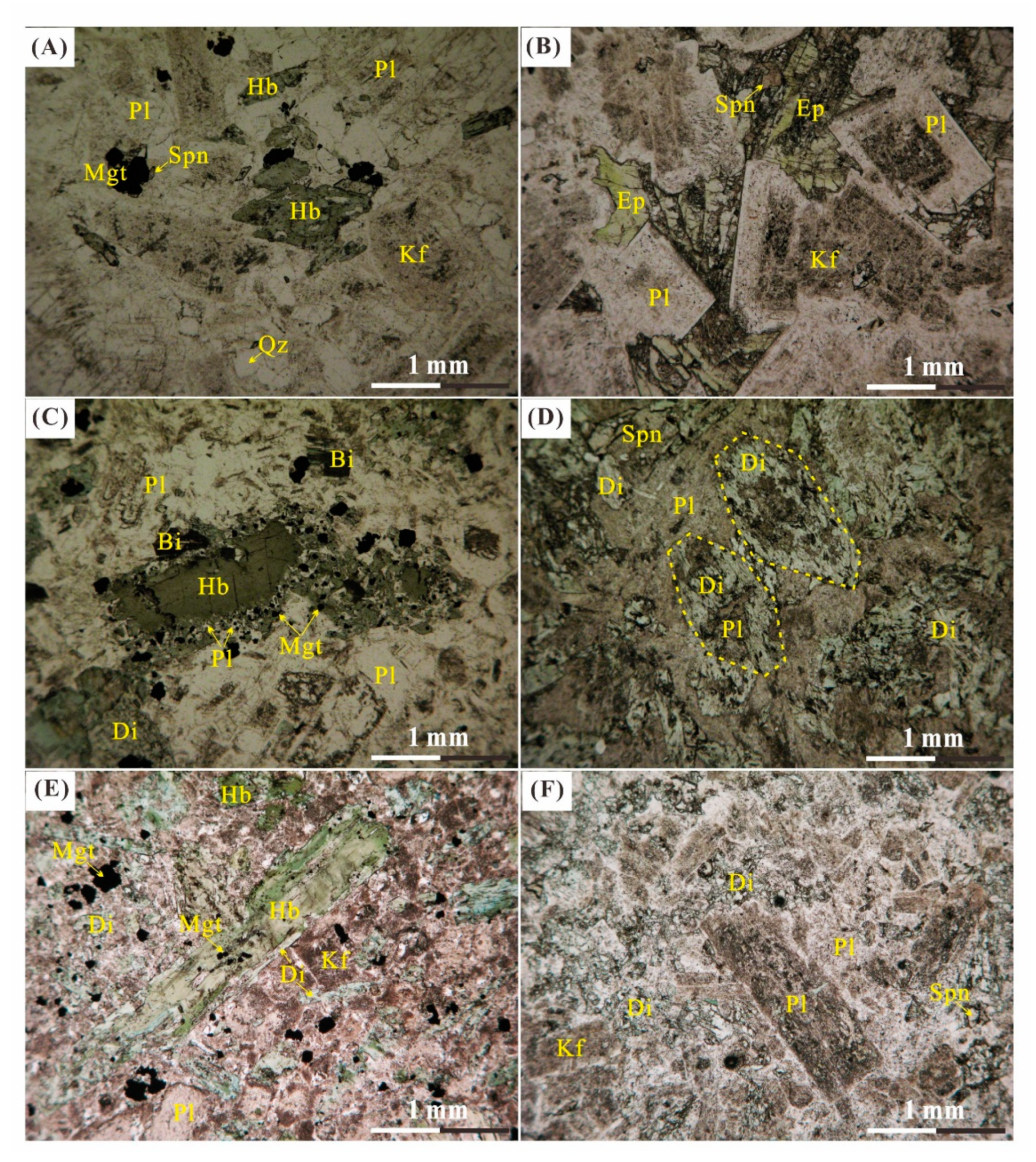
| Igneous Rock | FS12-01 | Fresh to less altered quartz diorite consists of medium-grained, black-green amphibole (10%), plagioclase (75%), quartz (10%) and K-feldspar (5%). |
| FS12-02 | Altered quartz diorite consists of medium-grained, green amphibole (5%), plagioclase (75%), K-feldspar (10%) and quartz (10%). | |
| FS12-03 | Less altered diorite consists of medium-grained, euhedral black-green amphibole (20%), biotite (5%) and plagioclase (70%) with K-feldspar and quartz less than 5%. | |
| FS12-04 | Altered diorite consists of medium-grained, green amphibole (15%), biotite (3%) and plagioclase (75%) with K-feldspar and quartz less than 7%. | |
| FS12-05 | Fresh hornblende diorite consists of coarse-grained, euhedral black-green amphibole (40%) and plagioclase (55%) with pyroxene and quartz less than 5%. | |
| BJ0818-21 | Intensively altered monzodiorite with porphyritic-like texture, consists of plagioclase (80%) and K-feldspar (10%) with quartz and pyroxene less 10%. | |
| BJ0818-23 | Less altered monzodiorite with equigranular texture, consists of medium-grained, euhedral black-green amphibole (20%) and plagioclase (75%) with K-feldspar and quartz less than 5%. | |
| BJ0818-27 | Most intensively altered monzodiorite with porphyritic-like texture, close to the skarn; consists of coarse-grained yellow-green epidote (10%) and plagioclase (80%) with quartz and pyroxene less than 10%. | |
| Skarn | FS12-08 | Skarn with taxitic structure mainly consists of coarse-grained garnet (80%) and fined-grained diopside (15%) with quartz and calcite less than 5%. |
| FS12-09 | Skarn with taxitic to massive structure mainly consists of medium-grained diopside (85%) and coarse-grained garnet (10%) with quartz and calcite less than 5%. | |
| BJ0818-28 | Skarn with taxitic to massive structure mainly consists of medium-grained epidote (80%) and diopside (10%) with quartz and calcite less than 10%. | |
| BJ0818-30 | Skarn with taxitic structure mainly consists of medium-grained epidote (70%) and garnet (20%) with biotite, quartz and calcite less than 10%. | |
| Ores | FS12-13 | Medium-fine-grained ore with dense disseminated structure, contains 45% magnetite; gangue minerals consist of diopside, garnet, epidote, calcite and minor sulfides. |
| FS12-17 | Medium-fine-grained ore with massive structure, contains 80% magnetite; gangue minerals consist of diopside, garnet, epidote, calcite and minor sulfides. | |
| BJ0818-36 | Fine-grained ore with taxitic-massive structure, contains 60% magnetite; gangue minerals consist of epidote, diopside, calcite and minor sulfides. |
| Sample | Description | SiO2 | TiO2 | Al2O3 | ∑Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | LOI | TOTAL | FeO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fushan district | % | % | % | % | % | % | % | % | % | % | % | % | % | |
| FS12-01 | Quartz Diorite | 65.4 | 0.38 | 16.7 | 3.29 | 0.05 | 1.04 | 4.04 | 4.66 | 2.68 | 0.16 | 0.78 | 99.1 | 1.08 |
| FS12-02 | Altered Quartz Diorite | 61.4 | 0.39 | 18.4 | 2.02 | 0.06 | 1.96 | 2.53 | 8.39 | 1.50 | 0.25 | 2.32 | 99.2 | 1.23 |
| FS12-03 | Less Altered Diorite | 58.4 | 0.69 | 16.3 | 7.56 | 0.14 | 2.82 | 5.91 | 3.72 | 2.78 | 0.30 | 0.84 | 99.5 | 3.53 |
| FS12-04 | Altered Diorite | 55.0 | 0.73 | 13.6 | 4.95 | 0.06 | 7.83 | 8.96 | 4.22 | 0.98 | 0.25 | 2.30 | 98.8 | 3.03 |
| FS12-05 | Hornblende Diorite | 52.4 | 1.21 | 14.6 | 10.3 | 0.12 | 6.54 | 7.20 | 4.96 | 0.84 | 0.27 | 1.08 | 99.5 | 4.65 |
| FS12-09 | Gt-Di Skarn | 40.2 | 0.92 | 12.3 | 8.99 | 0.23 | 16.8 | 13.3 | 1.73 | 0.94 | 0.41 | 3.14 | 98.9 | 3.99 |
| Wu’an district | ||||||||||||||
| BJ0818-21 | Altered Monzodiorite | 60.3 | 0.55 | 16.9 | 2.54 | 0.07 | 3.03 | 6.05 | 8.28 | 0.22 | 0.22 | 2.16 | 100.3 | 1.67 |
| BJ0818-22 | Altered Monzodiorite | 61.0 | 0.50 | 16.5 | 2.75 | 0.08 | 3.25 | 5.81 | 8.34 | 0.10 | 0.21 | 1.56 | 100.1 | 1.71 |
| BJ0818-23 | Less altered Monzodiorite | 58.5 | 0.55 | 16.5 | 5.87 | 0.08 | 3.37 | 4.24 | 5.66 | 3.68 | 0.23 | 0.96 | 99.5 | 2.85 |
| BJ0818-24 | Altered Monzodiorite | 60.1 | 0.56 | 16.4 | 2.59 | 0.07 | 2.86 | 5.95 | 5.86 | 3.64 | 0.25 | 1.08 | 99.3 | 1.45 |
| BJ0818-25 | Altered Monzodiorite | 59.5 | 0.51 | 16.7 | 2.18 | 0.06 | 2.93 | 6.38 | 8.33 | 0.24 | 0.24 | 2.72 | 99.8 | 1.44 |
| BJ0818-25P | Altered Monzodiorite | 59.6 | 0.51 | 16.7 | 2.21 | 0.06 | 2.93 | 6.38 | 8.29 | 0.22 | 0.24 | 2.66 | 99.8 | 1.44 |
| BJ0818-26 | Altered Monzodiorite | 61.3 | 0.53 | 16.4 | 2.28 | 0.06 | 2.95 | 6.20 | 8.34 | 0.34 | 0.25 | 1.84 | 100.5 | 1.50 |
| BJ0818-27 | Altered Monzodiorite | 60.6 | 0.52 | 16.7 | 2.34 | 0.07 | 3.05 | 7.16 | 7.48 | 0.62 | 0.25 | 1.04 | 99.8 | 1.38 |
| BJ0818-28 | Epidote Skarn | 51.8 | 0.42 | 4.56 | 5.97 | 0.13 | 11.9 | 18.6 | 0.67 | 2.76 | 0.43 | 2.04 | 99.3 | 3.90 |
| BJ0818-30 | Gt-Ep Skarn | 46.3 | 0.07 | 2.46 | 4.34 | 0.07 | 16.0 | 19.1 | 0.18 | 1.37 | 0.48 | 9.43 | 99.7 | 3.11 |
| BJ0818-36 | Iron Ore | 21.6 | 0.04 | 0.52 | 62.0 | 0.06 | 8.29 | 6.48 | 0.08 | 0.06 | 0.06 | −1.02 | 98.1 | na |
| BJ0818-50 | Limestone | 2.20 | 0.02 | 0.41 | 0.22 | 0.01 | 0.46 | 55.5 | 0.06 | 0.03 | 0.01 | 42.1 | 101.0 | na |
| BJ0818-51 | Limestone | 2.50 | 0.04 | 0.80 | 0.42 | 0.02 | 2.27 | 52.2 | 0.20 | 0.09 | 0.02 | 42.1 | 100.7 | na |
| Sample | Description | δ56Fe | 2SE | δ57Fe | 2SE | n |
|---|---|---|---|---|---|---|
| Bulk Samples | ||||||
| BCR-2 | Basalt Standard | 0.110 | 0.028 | 0.144 | 0.062 | 4 |
| GSP-2 | Granodiorite Standard | 0.151 | 0.034 | 0.223 | 0.040 | 4 |
| FS12-01 | Quartz Diorite | 0.167 | 0.028 | 0.233 | 0.062 | 4 |
| FS12-02 | Altered Quartz Diorite | −0.026 | 0.028 | 0.007 | 0.065 | 4 |
| FS12-03 | Less Altered Diorite | 0.082 | 0.028 | 0.161 | 0.065 | 4 |
| FS12-04 | Altered Diorite | 0.051 | 0.028 | 0.097 | 0.062 | 4 |
| FS12-05 | Hornblende Diorite | 0.172 | 0.028 | 0.246 | 0.062 | 4 |
| FS12-06 | Garnet Skarn | 0.008 | 0.028 | 0.037 | 0.062 | 4 |
| FS12-08 | Di-Gt Skarn | −0.006 | 0.028 | −0.018 | 0.062 | 4 |
| FS12-09 | Gt-Di Skarn | −0.203 | 0.028 | −0.282 | 0.062 | 4 |
| FS12-10 | Ep-Gt Skarn | 0.160 | 0.028 | 0.223 | 0.062 | 4 |
| FS12-13 | Iron Ore | −0.037 | 0.028 | −0.007 | 0.062 | 4 |
| FS12-15 | Garnet Skarn | 0.124 | 0.028 | 0.223 | 0.062 | 4 |
| FS12-17 | Iron Ore | 0.167 | 0.028 | 0.223 | 0.062 | 4 |
| BJ0818-21 | Altered Monzodiorite | 0.043 | 0.019 | 0.047 | 0.037 | 4 |
| BJ0818-22 | Altered Monzodiorite | 0.048 | 0.019 | 0.055 | 0.037 | 4 |
| BJ0818-23 | Less altered Monzodiorite | 0.166 | 0.019 | 0.261 | 0.037 | 4 |
| BJ0818-24 | Altered Monzodiorite | 0.108 | 0.019 | 0.167 | 0.037 | 4 |
| BJ0818-25 | Altered Monzodiorite | 0.054 | 0.019 | 0.103 | 0.037 | 4 |
| BJ0818-26 | Altered Monzodiorite | 0.073 | 0.019 | 0.066 | 0.037 | 4 |
| BJ0818-27 | Altered Monzodiorite | 0.086 | 0.019 | 0.126 | 0.037 | 4 |
| BJ0818-28 | Epidote Skarn | 0.083 | 0.019 | 0.088 | 0.037 | 4 |
| BJ0818-28P | Epidote Skarn | 0.071 | 0.019 | 0.105 | 0.037 | 4 |
| BJ0818-30 | Gt-Ep Skarn | 0.058 | 0.019 | 0.087 | 0.037 | 4 |
| BJ0818-36 | Iron Ore | 0.145 | 0.019 | 0.223 | 0.037 | 4 |
| BJ0818-50 | Limestone | −0.157 | 0.019 | −0.238 | 0.037 | 4 |
| BJ0818-51 | Limestone | 0.042 | 0.019 | 0.063 | 0.037 | 4 |
| Mineral separates | ||||||
| FS12-12 | Magnetite | 0.088 | 0.023 | 0.131 | 0.053 | 6 |
| FS12-13 | Magnetite | 0.052 | 0.023 | 0.097 | 0.053 | 6 |
| FS12-14 | Magnetite | 0.091 | 0.023 | 0.145 | 0.053 | 6 |
| FS12-14 | Magnetite | 0.071 | 0.028 | 0.055 | 0.065 | 4 |
| FS12-16 | Magnetite | 0.105 | 0.028 | 0.149 | 0.065 | 4 |
| FS12-17 | Magnetite | 0.147 | 0.028 | 0.209 | 0.065 | 4 |
| FS12-06 | Pyrite | 0.359 | 0.023 | 0.542 | 0.053 | 6 |
| FS12-13 | Pyrite | 0.293 | 0.023 | 0.441 | 0.053 | 6 |
| FS12-14 | Pyrite | 0.293 | 0.028 | 0.423 | 0.065 | 4 |
| FS12-16 | Pyrite | 0.222 | 0.028 | 0.326 | 0.065 | 4 |
| FS12-05 | Amphibole | 0.094 | 0.023 | 0.140 | 0.053 | 6 |
| FS12-06 | Garnet | 0.105 | 0.025 | 0.130 | 0.058 | 5 |
| FS12-08 | Garnet | 0.152 | 0.023 | 0.222 | 0.053 | 6 |
| FS12-10 | Garnet | 0.158 | 0.023 | 0.230 | 0.053 | 6 |
| FS12-08 | Diopside | −0.028 | 0.023 | −0.032 | 0.053 | 6 |
| FS12-09 | Diopside | −0.210 | 0.023 | −0.317 | 0.053 | 6 |
| FS12-12 | Diopside | −0.177 | 0.023 | −0.262 | 0.053 | 6 |
| FS12-08 | Epidote | 0.218 | 0.023 | 0.343 | 0.053 | 6 |
| BJ0818-36 | Magnetite | 0.127 | 0.019 | 0.205 | 0.037 | 4 |
| BJ0818-38 | Magnetite | 0.146 | 0.019 | 0.245 | 0.037 | 4 |
| BJ0818-43 | Magnetite | 0.137 | 0.019 | 0.207 | 0.037 | 4 |
| BJ0818-46 | Magnetite | 0.143 | 0.019 | 0.190 | 0.037 | 4 |
| BJ0818-59 | Magnetite | 0.114 | 0.019 | 0.162 | 0.037 | 4 |
| BJ0818-59 | Pyrite | 0.270 | 0.019 | 0.365 | 0.037 | 4 |
| BJ0818-59P | Pyrite | 0.273 | 0.025 | 0.408 | 0.065 | 4 |
| BJ0818-59 | Pyrrhotite | 0.242 | 0.025 | 0.363 | 0.065 | 4 |
| Sample | Description | ∑Fe2O3 | FeO | TiO2 | Fe2+/Fe3+ | ∑Fe2O3/TiO2 | δ56Fe | 2SE | LOI | F | αB-A | δ56FeLeached |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FS12-01 | Quartz Diorite | 3.29 | 1.08 | 0.38 | 0.57 | 8.66 | 0.167 | 0.028 | 0.78 | 1.000 | ||
| FS12-02 | Altered Quartz Diorite | 2.02 | 1.23 | 0.39 | 2.02 | 5.18 | −0.026 | 0.028 | 2.32 | 0.598 | 1.00038 | 0.455 |
| FS12-03 | Less Altered Diorite | 7.56 | 3.53 | 0.69 | 1.05 | 11.0 | 0.082 | 0.028 | 0.84 | 1.000 | ||
| FS12-04 | Altered Diorite | 4.95 | 3.03 | 0.73 | 2.07 | 6.78 | 0.051 | 0.028 | 2.30 | 0.619 | 1.00006 | 0.131 |
| FS12-05 | Hornblende Diorite | 10.3 | 4.65 | 1.21 | 0.99 | 8.50 | 0.172 | 0.028 | 1.08 | 1.000 | ||
| BJ0818-21 | Altered Monzodiorite | 2.54 | 1.67 | 0.55 | 2.63 | 4.64 | 0.043 | 0.019 | 2.16 | 0.438 | 1.00015 | 0.262 |
| BJ0818-22 | Altered Monzodiorite | 2.75 | 1.71 | 0.50 | 2.15 | 5.49 | 0.048 | 0.019 | 1.56 | 0.518 | 1.00018 | 0.293 |
| BJ0818-23 | Less altered Monzodiorite | 5.87 | 2.85 | 0.55 | 1.15 | 10.6 | 0.166 | 0.019 | 0.96 | 1.000 | ||
| BJ0818-24 | Altered Monzodiorite | 2.59 | 1.45 | 0.56 | 1.60 | 4.61 | 0.108 | 0.019 | 1.08 | 0.435 | 1.00007 | 0.210 |
| BJ0818-25 | Altered Monzodiorite | 2.20 | 1.44 | 0.51 | 2.57 | 4.29 | 0.054 | 0.019 | 2.72 | 0.404 | 1.00012 | 0.242 |
| BJ0818-26 | Altered Monzodiorite | 2.28 | 1.50 | 0.53 | 2.62 | 4.26 | 0.073 | 0.019 | 1.84 | 0.402 | 1.00010 | 0.228 |
| BJ0818-27 | Altered Monzodiorite | 2.34 | 1.38 | 0.52 | 1.84 | 4.54 | 0.086 | 0.019 | 1.04 | 0.428 | 1.00009 | 0.226 |
| WA12-07 | Altered Monzodiorite | 6.72 | 2.10 | 0.65 | 0.52 | 10.3 | 0.130 | 0.030 | 0.68 | 0.935 | 1.00112 | 1.285 |
| WA12-08 | Altered Monzodiorite | 4.94 | 1.54 | 0.61 | 0.52 | 8.10 | 0.101 | 0.030 | 1.90 | 0.732 | 1.00033 | 0.489 |
| WA12-13 | Altered Monzodiorite | 3.29 | 1.58 | 0.63 | 1.12 | 5.22 | 0.035 | 0.030 | 3.89 | 0.472 | 1.00023 | 0.357 |
| WA12-14 | Less altered Monzodiorite | 5.86 | 1.77 | 0.53 | 0.50 | 11.1 | 0.205 | 0.030 | 1.08 | 1.000 | ||
| WA12-23 | Altered Monzodiorite | 2.48 | 1.26 | 0.57 | 1.28 | 4.35 | −0.071 | 0.030 | 2.98 | 0.394 | 1.00030 | 0.384 |
| WA12-31 | Altered Monzodiorite | 4.72 | 1.12 | 0.60 | 0.35 | 7.80 | 0.056 | 0.030 | 1.92 | 0.706 | 1.00043 | 0.562 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Zhang, H.; Santosh, M.; Su, B.; Zhang, P.; Han, C.; He, Y. Iron Isotopes Constrain the Metal Sources of Skarn Deposits: A Case Study from the Han-Xing Fe Deposit, China. Minerals 2020, 10, 951. https://doi.org/10.3390/min10110951
Zhu B, Zhang H, Santosh M, Su B, Zhang P, Han C, He Y. Iron Isotopes Constrain the Metal Sources of Skarn Deposits: A Case Study from the Han-Xing Fe Deposit, China. Minerals. 2020; 10(11):951. https://doi.org/10.3390/min10110951
Chicago/Turabian StyleZhu, Bin, Hongfu Zhang, M. Santosh, Benxun Su, Pengfei Zhang, Chunming Han, and Yongsheng He. 2020. "Iron Isotopes Constrain the Metal Sources of Skarn Deposits: A Case Study from the Han-Xing Fe Deposit, China" Minerals 10, no. 11: 951. https://doi.org/10.3390/min10110951