Bioleaching for the Removal of Arsenic from Mine Tailings by Psychrotolerant and Mesophilic Microbes at Markedly Continental Climate Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Samples
2.2. Analytical Analysis
2.3. Bioleaching Experiments
3. Results and Discussion
3.1. Ore Sample Characterization
3.2. Bioleaching Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leist, M.; Casey, R.J.; Caridi, D. The management of arsenic wastes: Problems and prospects. J. Hazard. Mater. 2000, 76, 125–138. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Belluck, D.A.; Benjamin, S.L.; Baveye, P.; Sampson, J.; Johnson, B. Re: “Widespread arsenic contamination of soils in residential areas and public spaces: An emerging regulatory or medical crisis”. Int. J. Toxicol. 2003, 22, 473. [Google Scholar]
- Lim, K.T.; Shukor, M.Y.; Wasoh, H. Physical, chemical, and biological methods for the removal of arsenic compounds. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Chakraborti, D.; Rahman, M.M.; Paul, K.; Chowdhury, U.K.; Sengupta, M.K.; Lodh, D.; Chanda, C.R.; Saha, K.C.; Mukherjee, S.C. Arsenic calamity in the Indian subcontinent: What lessons have been learned? Talanta 2002, 58, 3–22. [Google Scholar] [CrossRef]
- Smith, E.; Smith, J.; Smith, L.; Biswas, T.; Correll, R.; Naidu, R. Arsenic in Australian environment: An overview. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2003, 38, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Choong, T.S.Y.; Chuah, T.G.; Robiah, Y.; Gregory Koay, F.L.; Azni, I. Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination 2007, 217, 139–166. [Google Scholar] [CrossRef]
- Chowdhury, T.R.; Mandal, B.K.; Samanta, G.; Basu, G.K.; Chowdhury, P.P.; Chanda, C.R.; Karan, N.K.; Lodh, D.; Dhar, R.K.; Das, D.; et al. Arsenic in groundwater in six districts of West Bengal, India: The biggest arsenic calamity in the world: The status report up to August, 1995. In Arsenic; Springer: Dordrecht, The Netherlands, 1997; pp. 93–111. [Google Scholar]
- Wegelin, M.; Gechter, D.; Hug, S.; Mahmud, A. SORAS—A simple arsenic removal process. In Water, Sanitation and Hygiene—Challenges of the Millennium, Proceedings of the 26th WEDC Conference, Dhaka, Bangladesh, 2000; WEDC: Loughborough, UK, 2001; Volume 2, pp. 255–258. [Google Scholar]
- Novikov, V.; Simonett, O.; Kirby, A.; Hughes, G. Waste and Chemicals in Central Asia: A Visual Synthesis; Eigenmann, G., Ed.; Zoï Environment Network (Zoi): Bern, Switzerland; Geneva, Switzerland, 2013. [Google Scholar]
- Ko, M.S.; Park, H.S.; Kim, K.W.; Lee, J.U. The role of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in arsenic bioleaching from soil. Environ. Geochem. Health 2013, 35, 727–733. [Google Scholar] [CrossRef]
- Hinwood, A.; Bannister, R.; Shugg, A.; Sim, M. Environmental arsenic in rural Victoria: An update. Water 1998, 25, 34–36. [Google Scholar]
- Tsai, S.L.; Singh, S.; Chen, W. Arsenic metabolism by microbes in nature and the impact on arsenic remediation. Curr. Opin. Biotechnol. 2009, 20, 659–667. [Google Scholar] [CrossRef]
- Simeonova, D.D.; Micheva, K.; Muller, D.A.E.; Lagarde, F.; Lett, M.C.; Groudeva, V.I.; Lièvremont, D. Arsenite oxidation in batch reactors with alginate-immobilized ULPAs1 strain. Biotechnol. Bioeng. 2005, 91, 441–446. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, M.H.; Park, H.J.; Lee, J.U. Bioleaching of arsenic and heavy metals from mine tailings by pure and mixed cultures of Acidithiobacillus spp. J. Ind. Eng. Chem. 2015, 21, 451–458. [Google Scholar] [CrossRef]
- Jafari, M.; Abdollahi, H.; Shafaei, S.Z.; Gharabaghi, M.; Jafari, H.; Akcil, A.; Panda, S. Acidophilic bioleaching: A Review on the Process and Effect of Organic–inorganic Reagents and Materials on its Efficiency. Miner. Process. Extr. Metall. Rev. 2019, 40, 87–107. [Google Scholar] [CrossRef]
- Zhappar, N.K.; Shaikhutdinov, V.M.; Kanafin, Y.N.; Ten, O.A.; Balpanov, D.S.; Korolkov, I.V.; Collinson, S.R.; Erkasov, R.S.; Bakibaev, A.A. Bacterial and chemical leaching of copper-containing ores with the possibility of subsequent recovery of trace silver. Chem. Pap. 2019, 73, 1357–1367. [Google Scholar] [CrossRef]
- Brierley, C.L. Bacterial succession in bioheap leaching. Hydrometallurgy 2001, 59, 249–255. [Google Scholar] [CrossRef]
- Coram, N.; Rawlings, D.E. Molecular Relationship between Two Groups of the Genus Leptospirillum and the Finding that Leptospirillum ferriphilum sp. nov. Dominates South African Commercial Biooxidation Tanks That Operate at 40 °C. Appl. Environ. Microbiol. 2002, 68, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, P.D.; Haddad, C.M.; Hawkes, R.B.; Robertson, W.J.; Plumb, J.J. Effects of temperature on the rates of iron and sulfur oxidation by selected bioleaching Bacteria and Archaea: Application of the Ratkowsky equation. Miner. Eng. 2005, 18, 1304–1314. [Google Scholar] [CrossRef]
- Park, J.; Han, Y.; Lee, E.; Choi, U.; Yoo, K.; Song, Y.; Kim, H. Bioleaching of highly concentrated arsenic mine tailings by Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2014, 133, 291–296. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- D’Hugues, P.; Cezac, P.; Cabral, T.; Battaglia, F.; Truong-Meyer, X.M.; Morin, D. Bioleaching of a cobaltiferous pyrite: A continuous laboratory-scale study at high solids concentration. Miner. Eng. 1997, 10, 507–527. [Google Scholar] [CrossRef]
- Quatrini, R.; Johnson, D.B. Acidithiobacillus ferrooxidans. Trends Microbiol. 2019, 27, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, K.B.; González-Toril, E.; Johnson, D.B. Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 2010, 14, 9–19. [Google Scholar] [CrossRef]
- Dopson, M.; Halinen, A.-K.; Rahunen, N.; Özkaya, B.; Sahinkaya, E.; Kaksonen, A.H.; Lindström, E.B.; Puhakka, J.A. Mineral and iron oxidation at low temperatures by pure and mixed cultures of acidophilic microorganisms. Biotechnol. Bioeng. 2007, 97, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Kamde, K.; Pandey, R.A.; Thul, S.T.; Dahake, R.; Shinde, V.M.; Bansiwal, A. Microbially assisted arsenic removal using Acidothiobacillus ferrooxidans mediated by iron oxidation. Environ. Technol. Innov. 2018, 10, 78–90. [Google Scholar] [CrossRef]
- Talla, E.; Hedrich, S.; Mangenot, S.; Ji, B.; Johnson, D.B.; Barbe, V.; Bonnefoy, V. Insights into the pathways of iron- and sulfur-oxidation, and biofilm formation from the chemolithotrophic acidophile Acidithiobacillus ferrivorans CF27. Res. Microbiol. 2014, 165, 753–760. [Google Scholar] [CrossRef]
- Liljeqvist, M.; Rzhepishevska, O.I.; Dopson, M. Gene identification and substrate regulation provide insights into sulfur accumulation during bioleaching with the psychrotolerant acidophile Acidithiobacillus ferrivorans. Appl. Environ. Microbiol. 2013, 79, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Halinen, A.K.; Rahunen, N.; Kaksonen, A.H.; Puhakka, J.A. Heap bioleaching of a complex sulfide ore: Part II. Effect of temperature on base metal extraction and bacterial compositions. Hydrometallurgy 2009, 98, 101–107. [Google Scholar] [CrossRef]
- Peng, T.; Chen, L.; Wang, J.; Miao, J.; Shen, L.; Yu, R.; Gu, G.; Qiu, G.; Zeng, W. Dissolution and passivation of chalcopyrite during bioleaching by acidithiobacillus ferrivorans at low temperature. Minerals 2019, 9, 332. [Google Scholar] [CrossRef]
- Escobar, B.; Buccicardi, S.; Morales, G.; Wiertz, J. Biooxidation of ferrous iron and sulphide at low temperatures: Implications on acid mine drainage and bioleaching of sulphide minerals. Hydrometallurgy 2010, 104, 454–458. [Google Scholar] [CrossRef]
- Elberling, B.; Schippers, A.; Sand, W. Bacterial and chemical oxidation of pyritic mine tailings at low temperatures. J. Contam. Hydrol. 2000, 41, 225–238. [Google Scholar] [CrossRef]
- Zeng, W.M.; Peng, Y.P.; Peng, T.J.; Nan, M.H.; Chen, M.; Qiu, G.Z.; Shen, L. Electrochemical studies on dissolution and passivation behavior of low temperature bioleaching of chalcopyrite by Acidithiobacillus ferrivorans YL15. Miner. Eng. 2020, 155, 106416. [Google Scholar] [CrossRef]
- Silverman, M.P.; Lungren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Filippova, N.A. Phazovy Analiz Rud i Produktov ikh Pererabotk, 2nd ed.; Khimiya: Moscow, Russia, 1975. (In Russian) [Google Scholar]
- Vakhromeyev, S.A. Manual on Mineragraphy, 3rd ed.; Irkutsk Mining and Metallurgical Institute: Irkutsk, Russia, 1956. (In Russian) [Google Scholar]
- Zhao, K.; Gu, G.; Qiu, G.; Wang, X. Study on the jarosite mediated by bioleaching of pyrrhotite using Acidthiobacillus ferrooxidans. Biosci. J. 2017, 33, 721–729. [Google Scholar] [CrossRef]
- Ngoma, E.; Borja, D.; Smart, M.; Shaik, K.; Kim, H.; Petersen, J.; Harrison, S.T.L. Bioleaching of arsenopyrite from Janggun mine tailings (South Korea) using an adapted mixed mesophilic culture. Hydrometallurgy 2018, 181, 21–28. [Google Scholar] [CrossRef]
- Zhang, D.R.; Xia, J.L.; Nie, Z.Y.; Chen, H.R.; Liu, H.C.; Deng, Y.; Zhao, Y.D.; Zhang, L.L.; Wen, W.; Yang, H.Y. Mechanism by which ferric iron promotes the bioleaching of arsenopyrite by the moderate thermophile Sulfobacillus thermosulfidooxidans. Process Biochem. 2019, 81, 11–21. [Google Scholar] [CrossRef]
- Astudillo, C.; Acevedo, F. Adaptation of Sulfolobus metallicus to high pulp densities in the biooxidation of a flotation gold concentrate. Hydrometallurgy 2008, 92, 11–15. [Google Scholar] [CrossRef]
- Daoud, J.; Karamanev, D. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans. Miner. Eng. 2006, 19, 960–967. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.; Xu, B.; Li, L.; Wu, B. Surface characterization of arsenopyrite during chemical and biological oxidation. Sci. Total Environ. 2018, 626, 349–356. [Google Scholar] [CrossRef]
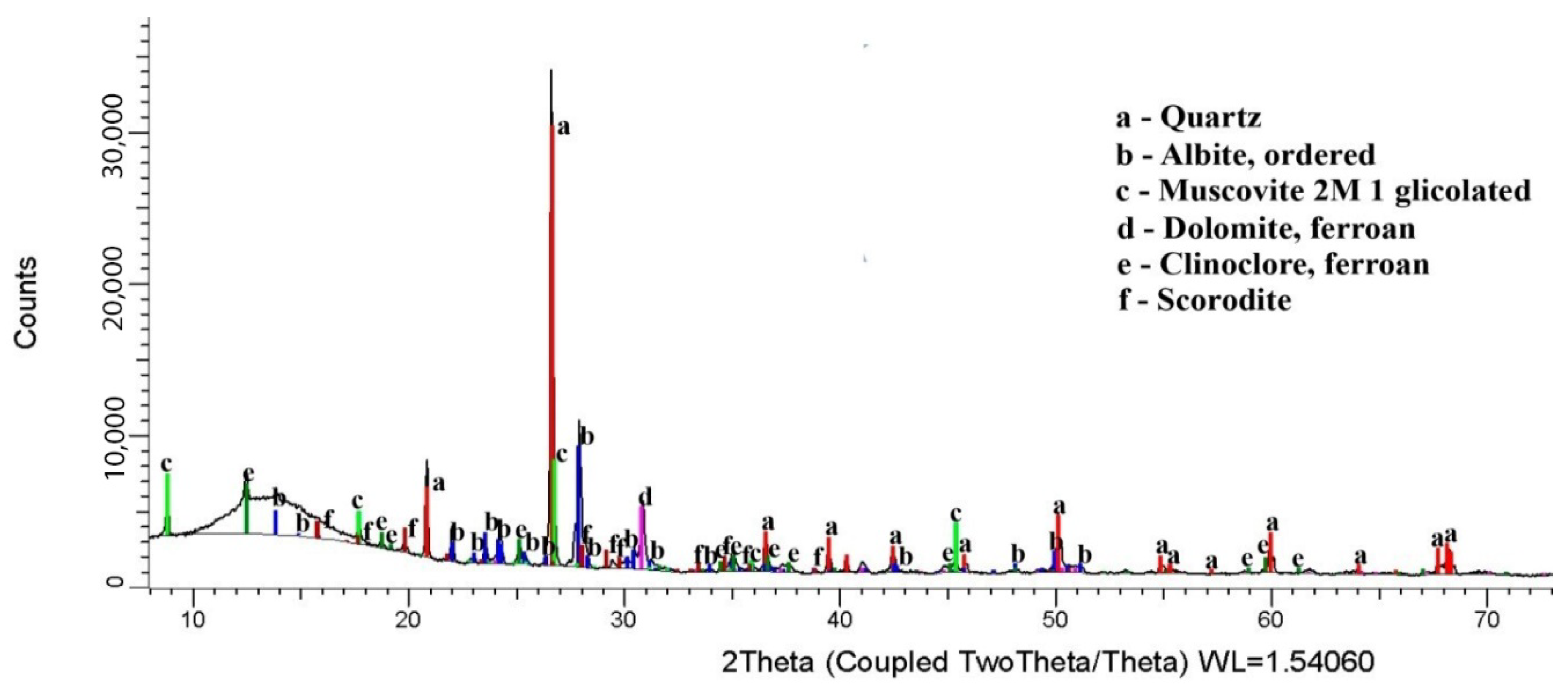
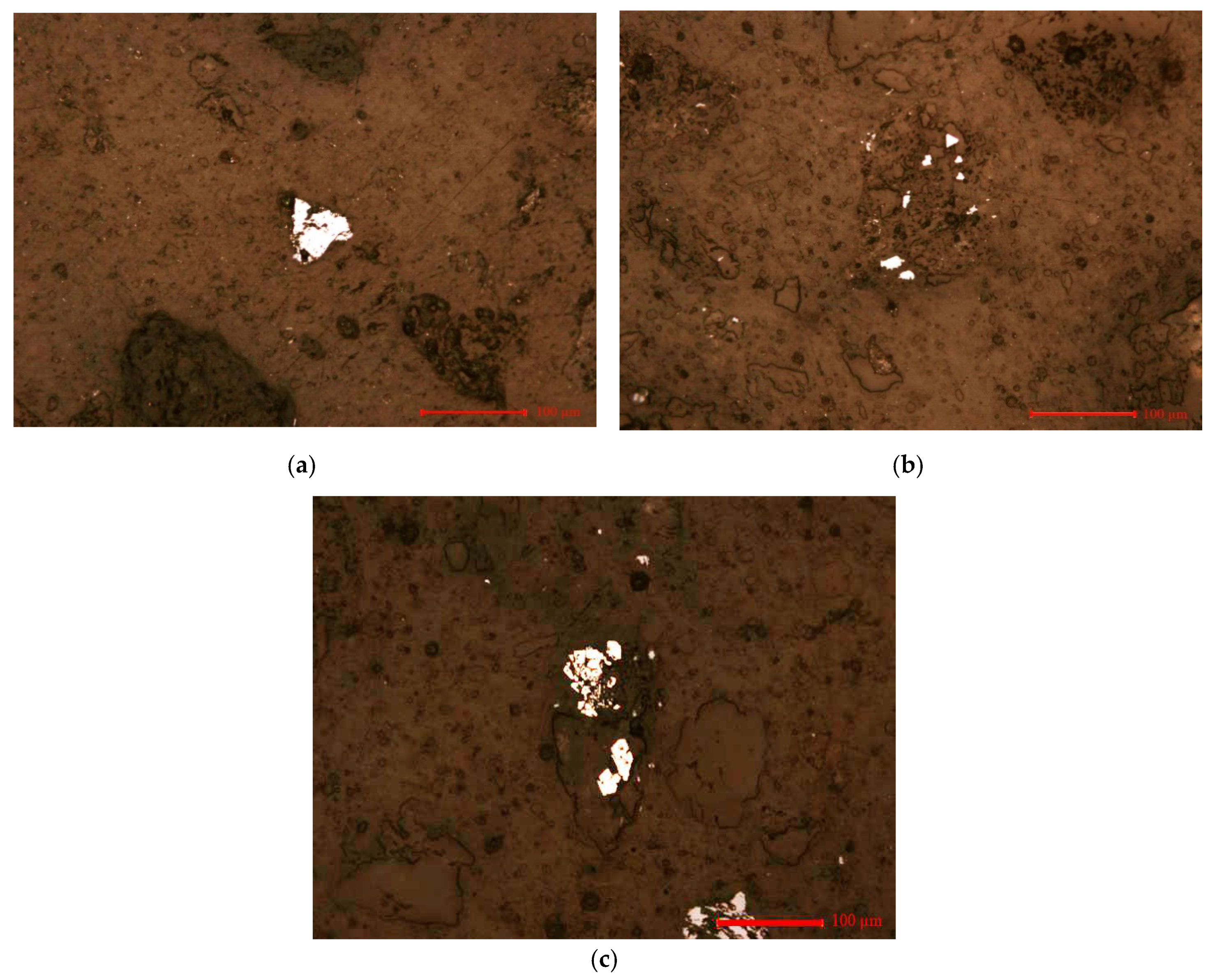


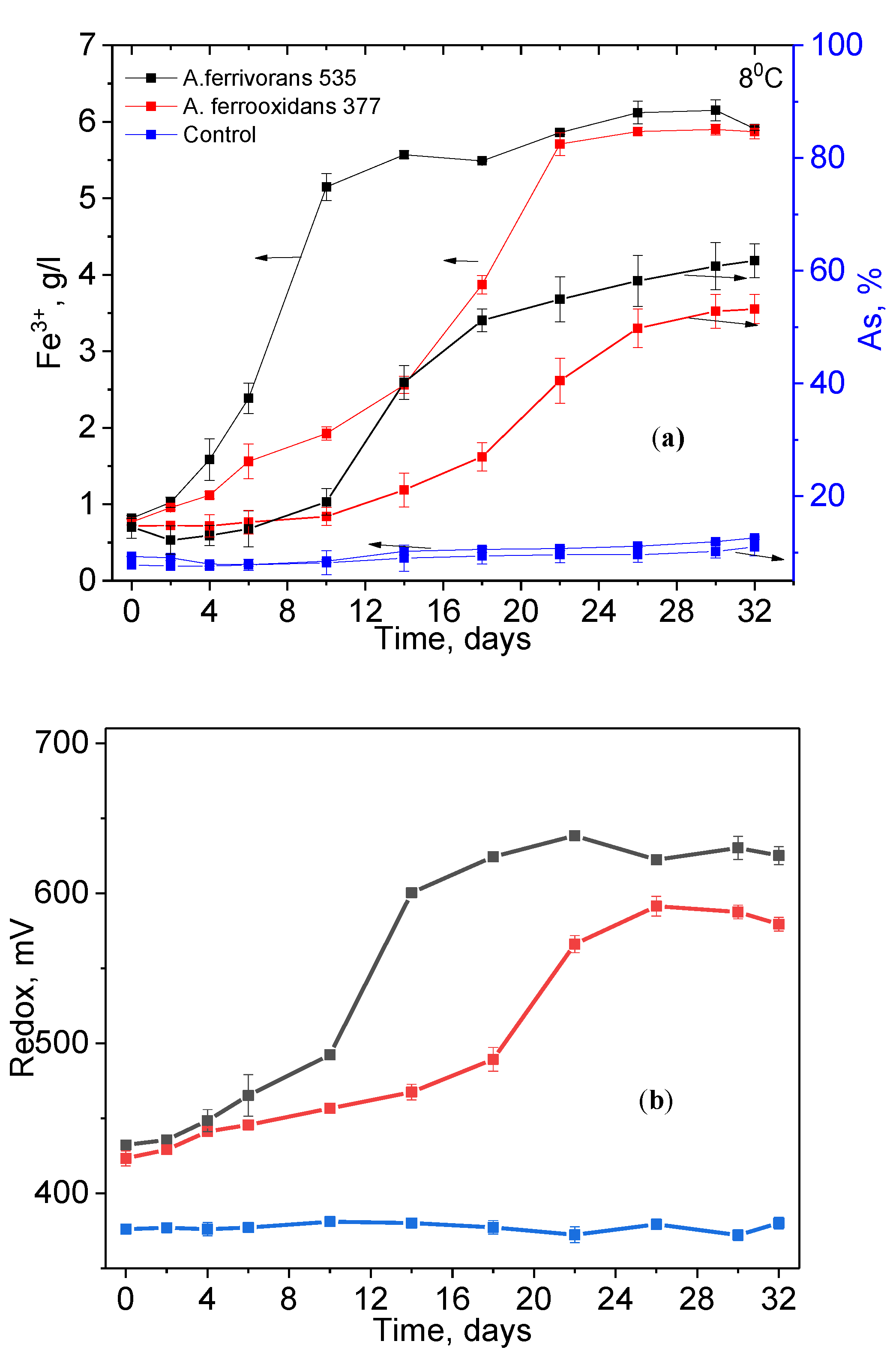

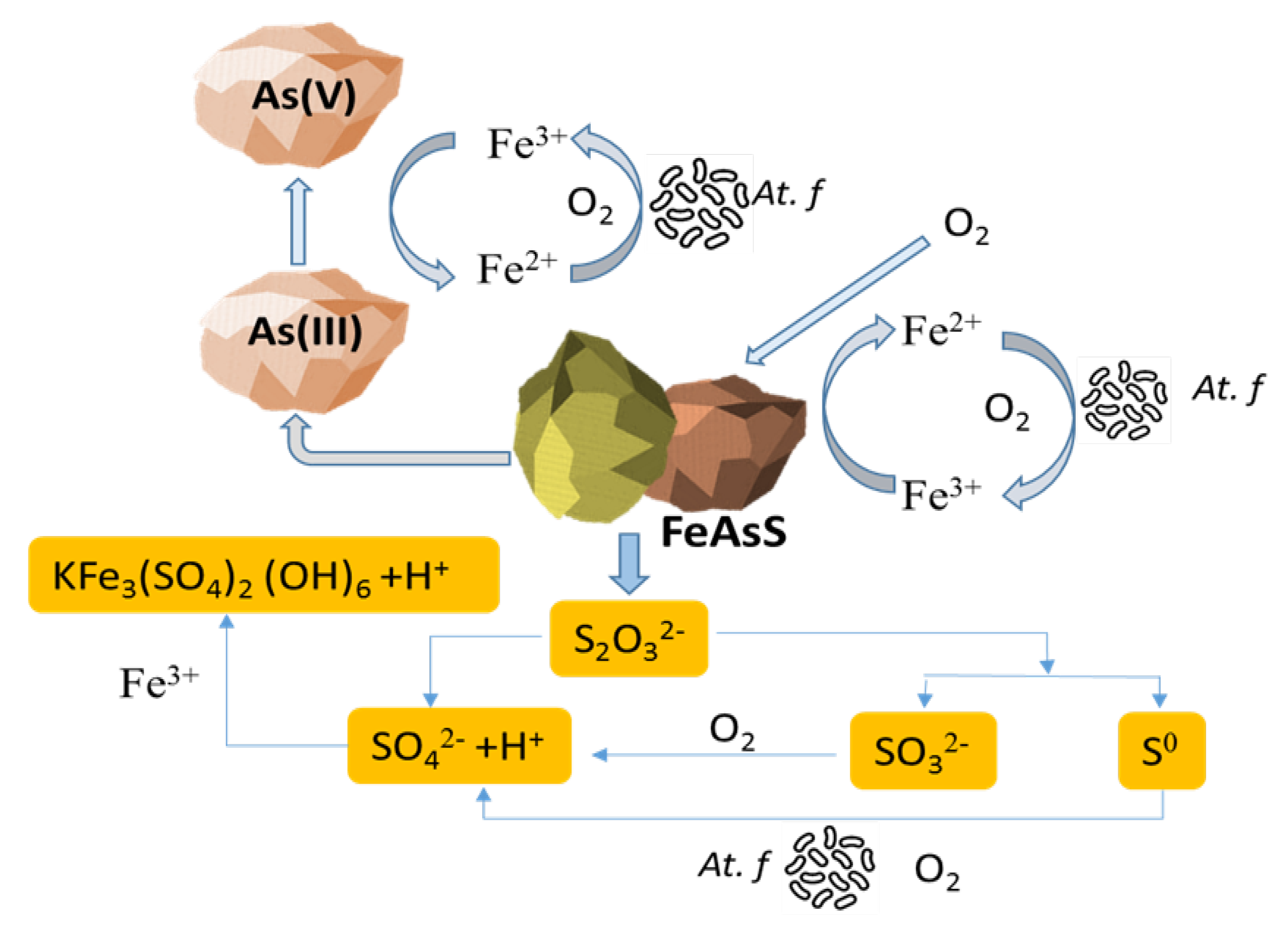
| Elements | As | Fe | S | Zn |
|---|---|---|---|---|
| Composition (%) | 0.045 | 3.10 | 0.15 | <0.05 |
| As Phases | Mass Fraction (%) | ||||||
|---|---|---|---|---|---|---|---|
| Before Leaching | 8 °C | 28 °C | |||||
| 377 | 535 | Control | 377 | 535 | Control | ||
| as oxide | <0.005 | ND ** | ND ** | ND ** | ND ** | ND ** | ND ** |
| as elemental | <0.005 | ND ** | ND ** | ND ** | ND ** | ND ** | ND ** |
| associated with Zn | <0.005 | ND ** | ND ** | ND ** | ND ** | ND ** | ND ** |
| as sulfide | <0.005 | ND ** | ND ** | 0.006 | ND ** | ND ** | 0.007 |
| as difficult to decompose * | 0.022 | 0.022 | 0.019 | 0.023 | 0.015 | 0.013 | 0.022 |
| Total | 0.045 | 0.022 | 0.019 | 0.039 | 0.015 | 0.013 | 0.034 |
| Fe Phase | |||||||
| as oxide | 3.1 | 2.11 | 2.03 | 1.59 | 1.71 | 1.86 | 1.4 |
| as sulfide | <0.005 | 0.48 | 0.48 | 0.73 | 2.41 | 2.55 | 0.09 |
| as difficult to decompose * | 3.1 | 0.012 | 0.007 | 0.014 | 0.026 | 0.008 | 0.02 |
| Total | 0.045 | 2.59 | 2.51 | 2.32 | 4.12 | 4.41 | 1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitkamal, K.N.; Zhappar, N.K.; Shaikhutdinov, V.M.; Shibayeva, A.K.; Ilyas, S.; Korolkov, I.V.; Kim, H. Bioleaching for the Removal of Arsenic from Mine Tailings by Psychrotolerant and Mesophilic Microbes at Markedly Continental Climate Temperatures. Minerals 2020, 10, 972. https://doi.org/10.3390/min10110972
Seitkamal KN, Zhappar NK, Shaikhutdinov VM, Shibayeva AK, Ilyas S, Korolkov IV, Kim H. Bioleaching for the Removal of Arsenic from Mine Tailings by Psychrotolerant and Mesophilic Microbes at Markedly Continental Climate Temperatures. Minerals. 2020; 10(11):972. https://doi.org/10.3390/min10110972
Chicago/Turabian StyleSeitkamal, Kuanysh N., Nariman K. Zhappar, Valentin M. Shaikhutdinov, Aigerim K. Shibayeva, Sadia Ilyas, Ilya V. Korolkov, and Hyunjung Kim. 2020. "Bioleaching for the Removal of Arsenic from Mine Tailings by Psychrotolerant and Mesophilic Microbes at Markedly Continental Climate Temperatures" Minerals 10, no. 11: 972. https://doi.org/10.3390/min10110972
APA StyleSeitkamal, K. N., Zhappar, N. K., Shaikhutdinov, V. M., Shibayeva, A. K., Ilyas, S., Korolkov, I. V., & Kim, H. (2020). Bioleaching for the Removal of Arsenic from Mine Tailings by Psychrotolerant and Mesophilic Microbes at Markedly Continental Climate Temperatures. Minerals, 10(11), 972. https://doi.org/10.3390/min10110972







