Phase Relations in the FeO-Fe3C-Fe3N System at 7.8 GPa and 1350 °C: Implications for Oxidation of Native Iron at 250 km
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Composition
2.2. High-Pressure Apparatus
2.3. Analytical Methods
3. Results
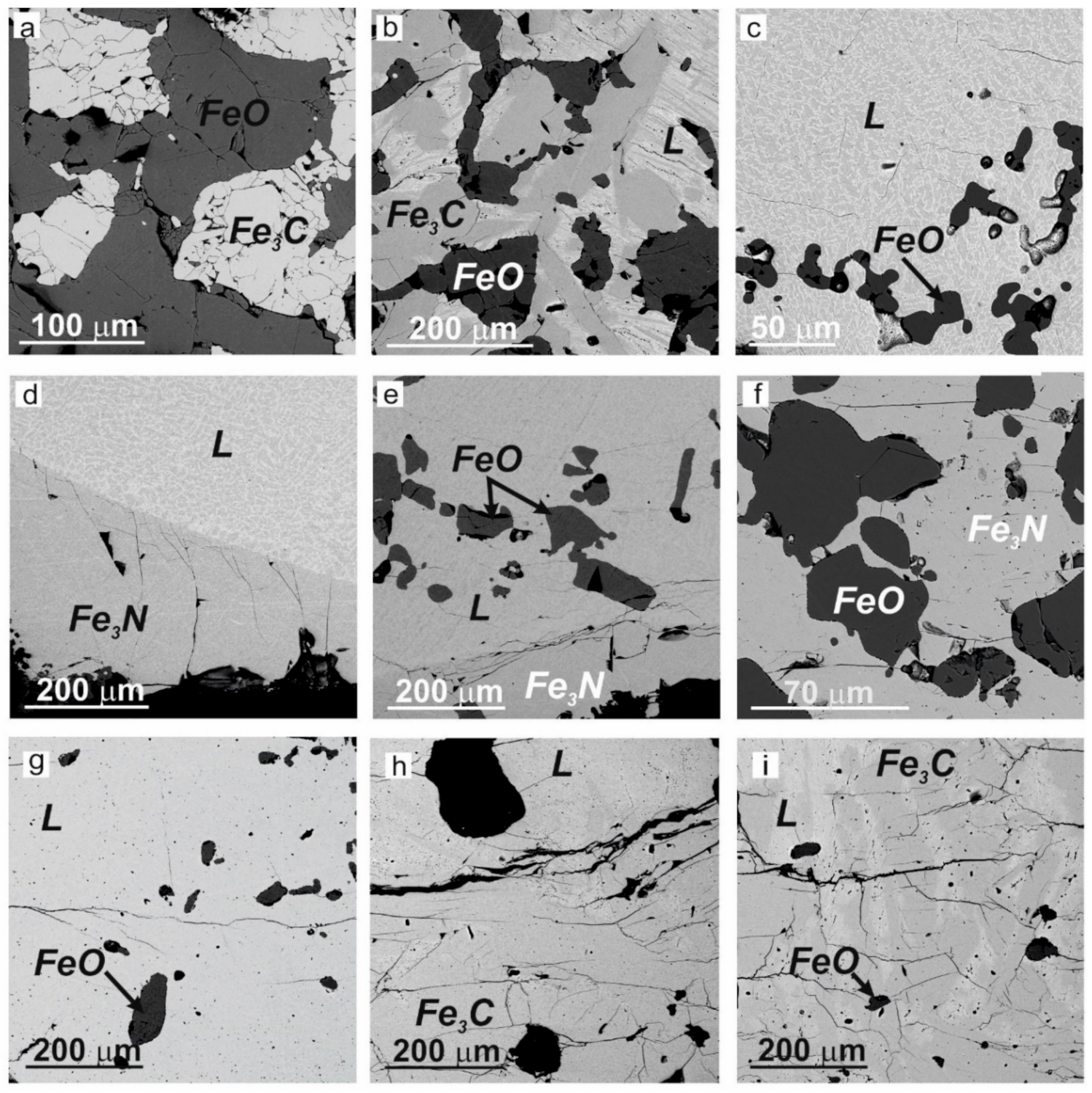
3.1. Textures of Experimental Samples
- one phase: granular solid FeO, with straight grain boundaries and equilibrium 120° dihedral angles;
- two phases: granular solid FeO + Fe3C or FeO + Fe3N (Figure 2a,f);
- two phases: anhedral FeO + quenched melt (Figure 2c);
- three phases: anhedral or subhedral Fe3C + anhedral FeO + quenched melt (Figure 2b);
- three phases: anhedral FeO + anhedral Fe3N + quenched melt (Figure 2d,e);
- four phases: non-equilibrium association of anhedral FeO + Fe3C + few Fe7C3 grains (X-ray powder diffraction data) + quenched melt, obtained in a series of experiments with excess oxygen (Figure 2h,i).
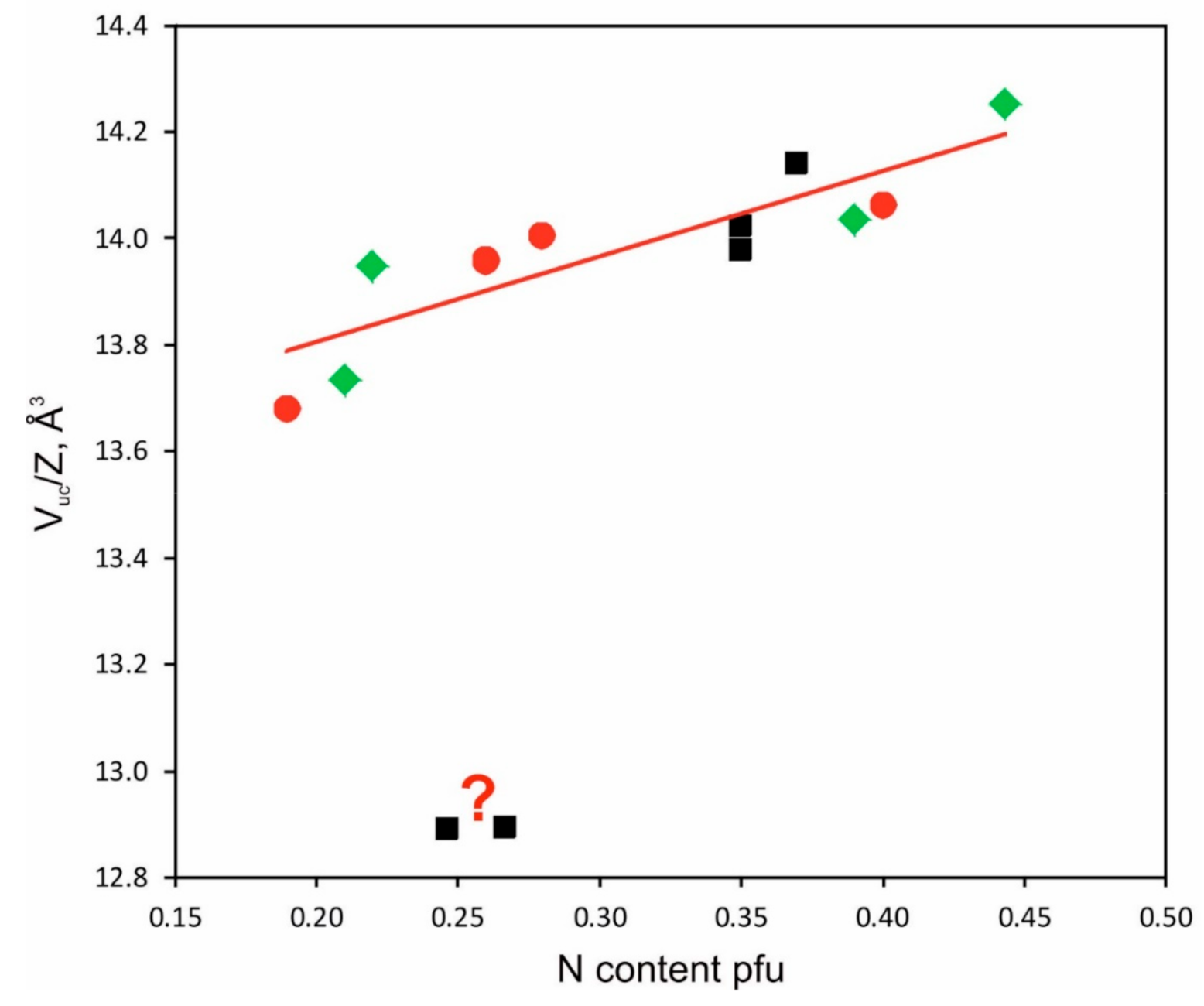
3.2. Crystal Structure and Compositions of Phases
4. Discussion
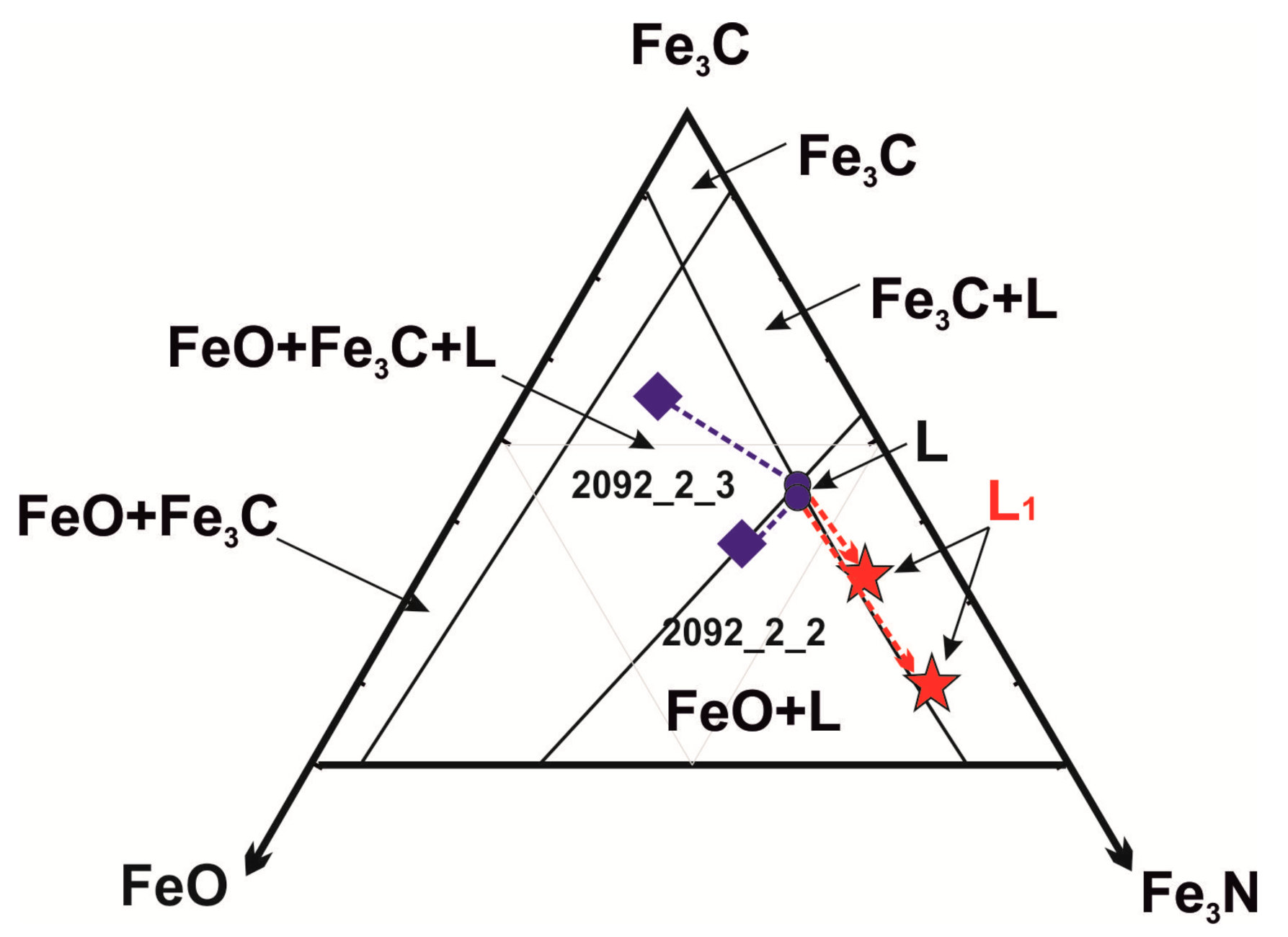
4.1. Phase Relations in System FeO-Fe3C-Fe3N
4.2. Formation Conditions of Iron Oxide, Carbide, and Nitride Inclusions in Natural Diamonds
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frost, D.J.; Liebske, C.; Langenhorst, F.; McCammon, C.A.; Trønnes, R.G.; Rubie, D.C. Experimental evidence for the existence of iron-rich metal in the Earth’s lower mantle. Nature 2004, 428, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A.; Ballhaus, C.; Golla-Schindler, U.; Ulmer, P.; Kamenetsky, V.S.; Kuzmin, D.V. Metal saturation in the upper mantle. Nature 2007, 449, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A.; Schmidt, M.W. Redox freezing and melting in the Earth’s deep mantle resulting from carbon-iron redox coupling. Nature 2011, 472, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Stagno, V.; Ojwang, D.O.; McCammon, C.A.; Frost, D.J. The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature 2013, 493, 84. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, N.V.; Efimova, E.S.; Pospelova, L.N. Native iron in Yakutian diamonds and its mineral assemblage. Sov. Geol. Geophys. 1981, 22, 25–28. [Google Scholar]
- Bulanova, G.P. The formation of diamond. J. Geochem. Explor. 1995, 53, 1–23. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib. Mineral. Petrol. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Jacob, D.E.; Kronz, A.; Viljoen, K.S. Cohenite, native iron and troilite inclusions in garnets from polycrystalline diamond aggregates. Contrib. Mineral. Petrol. 2004, 146, 566–576. [Google Scholar] [CrossRef]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.; Richardson, S.H.; Wang, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 354, 1403–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shatsky, V.S.; Ragozin, A.L.; Logvinova, A.M.; Wirth, R.; Kalinina, V.V.; Sobolev, N.V. Diamond-rich placer deposits from iron-saturated mantle beneath the northeastern margin of the Siberian Craton. Lithos 2020, 364–365, 1–12. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Wirth, R. Iron carbide inclusions in lower-mantle diamond from Juina, Brazil. Can. Mineral. 2011, 49, 555–572. [Google Scholar] [CrossRef] [Green Version]
- Kaminsky, F.; Wirth, R. Nitrides and carbonitrides from the lowermost mantle and their importance in the search for Earth’s “lost” nitrogen. Am. Min. 2017, 102, 1667–1676. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Bayukov, O.A. The role of rocks saturated with metallic iron in the formation of ferric carbonate-silicate melts: Experimental modeling under PT-conditions of lithospheric mantle. Russ. Geol. Geophys. 2015, 56, 143–154. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle–slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef] [Green Version]
- Rohrbach, A.; Ghosh, S.; Schmidt, M.W.; Wijbrans, C.H.; Klemme, S. The stability of Fe-Ni carbides in the Earth’s mantle: Evidence for a low Fe-Ni-C melt fraction in the deep mantle. Earth Planet. Sci. Lett. 2014, 388, 211–221. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Poli, S. Devolatilization during Subduction. Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 669–697. [Google Scholar]
- Lord, O.T.; Walter, M.J.; Dasgupta, R.; Walker, D.; Clark, S.M. Melting in the Fe-C system to 70 GPa. Earth Planet. Sci. Lett. 2009, 284, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Roskosz, M.; Bouhifd, M.A.; Jephcoat, A.P.; Marty, B.; Mysen, B.O. Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochim. Cosmochim. Acta 2013, 121, 15–28. [Google Scholar] [CrossRef]
- Dalou, C.; Hirschmann, M.M.; von der Handt, A.; Mosenfelder, J.; Armstrong, L.S. Nitrogen and carbon fractionation during core–mantle differentiation at shallow depth. Earth Planet. Sci. Lett. 2017, 458, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Sokol, A.G.; Palyanov, Y.u.N.; Tomilenko, A.A.; Bul’bak, T.A.; Palyanova, G.A. Carbon and nitrogen speciation in nitrogen-rich C-O-H-N fluids at 5.5–7.8 GPa. Earth Planet. Sci. Lett. 2017, 460, 234–243. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Kruk, A.N.; Sokol, I.A.; Palyanov, Y.N. Fate of fluids at the base of subcratonic lithosphere: Experimental constraints at 5.5–7.8 GPa and 1150–1350 deg C. Lithos. 2018, 318, 419–433. [Google Scholar] [CrossRef]
- Grewal, D.S.; Dasgupta, R.; Sun, C.; Tsuno, K.; Costin, G. Delivery of carbon, nitrogen, and sulfur to the silicate Earth by a giant impact. Sci. Adv. 2019, 5, eaau3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasterok, D.; Chapman, D.S. Heat production and geotherms for the continental lithosphere. Earth Planet. Sci. Lett. 2011, 307, 59–70. [Google Scholar] [CrossRef]
- Pearson, D.G.; Canil, D.; Shirey, S.B. Mantle samples included in volcanic rocks: Xenoliths and diamonds. Treatise Geochem. (Second Ed.) 2014, 3, 169–253. [Google Scholar]
- Ohtani, E.; Ringwood, A.E.; Hibberson, W. Composition of the core, II. Effect of high pressure on solubility of FeO in molten iron. Earth Planet. Sci. Lett. 1984, 71, 94–103. [Google Scholar] [CrossRef]
- Wriedt, H.A. The Fe-O (Iron-Oxygen) System. J. Phase Equilibria 1991, 12, 170–200. [Google Scholar] [CrossRef]
- Komabayashi, T. Thermodynamics of melting relations in the system Fe-FeO at high pressure: Implications for oxygen in the Earth’s core. J. Geophys. Res. Solid Earth 2014, 119, 4164–4177. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Sokol, A.G. Effect of nitrogen impurity on diamond crystal growth processes. Cryst. Growth Des. 2010, 10, 3169–3175. [Google Scholar] [CrossRef]
- Turkin, A.I. Lead selenide as a continuous internal indicator of pressure in solid-media cells of high-pressure apparatus in the range of 4–6.8 GPa. High Temp.-High Press. 2004, 36, 371–376. [Google Scholar] [CrossRef]
- Day, H.W. A revised diamond-graphite transition curve. Am. Min. 2012, 97, 52–62. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.u.M.; Palyanov, Y.u.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high-pressure apparatus. High. Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Sokol, A.G.; Kruk, A.N.; Seryotkin, Y.V.; Korablin, A.A.; Palyanov, Y.N. Phase relations in the Fe-Fe3C-Fe3N system at 7.8 GPa and 1350 °C: Implications for carbon and nitrogen hosts in Fe0-saturated upper mantle. Phys. Earth Plan. Int. 2017, 265, 43–53. [Google Scholar] [CrossRef]
- Wells, A. Metallographic analysis of compound layers on ferritic nitrocarburized plain low carbon steel. J. Mater. Sci. 1985, 20, 2439–2445. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, v. 1.1; Rigaku Corporation: Oxford, UK, 2016. [Google Scholar]
- Sheldrick, G. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Jack, K.H. Binary and Ternary Interstitial Alloys. II. The Iron-Carbon-Nitrogen System. Proc. R. Soc. Lond. A. 1948, 195, 41. [Google Scholar]
- Strong, H.M.; Chrenko, R.M. Diamond growth rates and physical properties of laboratory-made diamond. J. Phys. Chem. 1971, 75, 1838–1843. [Google Scholar] [CrossRef]
- Sokol, A.G.; Khokhryakov, A.F.; Borzdov, Y.M.; Kupriyanov, I.N.; Palyanov, Y.N. Solubility of carbon and nitrogen in a sulfur-bearing iron melt: Constraints for siderophile behavior at upper mantle conditions. Am. Min. 2019, 104, 1857–1865. [Google Scholar] [CrossRef]
- Li, Y.; Wiedenbeck, M.; Shcheka, S.; Keppler, S. Nitrogen solubility in upper mantle minerals. Earth Planet. Sci. Lett. 2013, 377–378, 311–323. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Khokhryakov, A.F.; Borzdov, Y.M.; Doroshev, A.M.; Tomilenko, A.A.; Sobolev, N.V. Inclusions in synthetic diamond. Dokl. Akad. Nauk. 1996, 338, 78–80. [Google Scholar]
- Frost, D.J.; Wood, B.J. Experimental measurements of the properties of H2O-CO2 mixtures at high pressures and temperatures. Geochim. Cosmochim. Acta 1997, 61, 3301–3309. [Google Scholar] [CrossRef]
- Dasgupta, R.; Buono, A.; Whelan, G.; Walker, D. High-pressure melting relations in Fe-C-S systems: Implications for formation, evolution, and structure of metallic cores in planetary bodies. Geochim. Cosmochim. Acta 2009, 73, 6678–6691. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Bataleva, Y.V.; Kupriyanov, I.N. Effect of sulfur on diamond growth and morphology in metal-carbon systems. CrystEngComm 2020, 22, 5497–5508. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Kupriyanov, I.N.; Bataleva, Y.V.; Nechaev, D.V. Effect of Oxygen of Diamond Crystallization in Metal-Carbon Systems. ACS Omega 2020, 5, 18376–18383. [Google Scholar] [CrossRef]
- Pal’Yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F.; Shatsky, A.F.; Sobolev, N.V. The diamond growth from Li2CO3, Na2CO3, K2CO3 and Cs2CO3 solvent-catalysts at P = 7 GPa and T = 1700–1750 °C. Diam. Relat. Mater. 1999, 8, 1118–1124. [Google Scholar] [CrossRef]
- Kumar, M.S.; Akaishi, M.; Yamaoka, S. Formation of diamond from supercritical H2O-CO2 fuid at high pressure and high temperature. J. Cryst. Growth. 2000, 213, 203–206. [Google Scholar] [CrossRef]
- Yamaoka, S.; Kumar, M.D.S.; Akaishi, M.; Kanda, H. Reactions between carbon and water under diamond-stable high pressure and high tempreture conditions. Diam. Relat. Mater. 2000, 9, 1480–1486. [Google Scholar] [CrossRef]
- Sokol, A.G.; Pal’Yanov, Y.N.; Pal’Yanova, G.A.; Khokhryakov, A.F.; Borzdov, Y.M. Diamond and graphite crystallization from C-O-H fluids under high pressure and high temperature conditions. Diam. Relat. Mater. 2001, 10, 2131–2136. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G. The effect of composition of mantle fluids/melts on diamond formation processes. Lithos 2009, 112, 690–700. [Google Scholar] [CrossRef]
- Bureau, H.; Langenhorst, F.; Auzende, A.L.; Frost, D.J.; Estève, I.; Siebert, J. The growth of fibrous, cloudy and polycrystalline diamonds. Geochim. Cosmochim. Acta 2012, 7, 202–214. [Google Scholar] [CrossRef]
- Bureau, H.; Frost, D.J.; Bolfan-Casanova, N.; Leroy, C.; Esteve, I.; Cordier, P. Diamond growth in mantle fluids. Lithos 2016, 265, 4–15. [Google Scholar] [CrossRef] [Green Version]


| Fe2O3 | ||||
|---|---|---|---|---|
| Run ID | Fe3C | Fe3N | FeO | O |
| 1940_2_1 | 11.5 | 80.2 | 8.3 | - |
| 1940_2_4 | 41 | 38.8 | 20.3 | - |
| 1930_2_1 | 40.7 | 47.5 | 11.8 | - |
| 1982_2_6 | 0 | 85 | 15 | - |
| 1930_2_4 | 79.2 | 11 | 9.8 | - |
| 1930_2_5 | 92.4 | 4 | 3.7 | - |
| 1963_2_3 | 12.8 | 40 | 47.2 | - |
| 1982_2_3 | 15 | 29 | 56 | - |
| 1982_2_4 | 55 | 14 | 31 | - |
| 1982_2_2 | 0 | 31.25 | 68.75 | - |
| 1963_2_1 | 44.1 | 52.9 | 2.9 | - |
| 1982_2_5 | 5 | 95 | 0 | - |
| 1930_2_2 | 86.4 | 13.6 | 0 | - |
| 1940_2_2 | 11.4 | 8.1 | 80.5 | - |
| 1963_2_4 | 12.8 | 17 | 70.3 | - |
| 1963_2_5 | 30.1 | 2.9 | 67 | - |
| 1963_2_6 | 30.1 | 17 | 52.9 | - |
| 1930_2_8 | 35.1 | 6.1 | 58.7 | - |
| 2092_2_1 | 36 | 43 | 14 | 7 |
| 2092_2_2 | 63 | 18 | 12 | 6 |
| 2092_2_3 | 74 | 8 | 12 | 6 |
| 1606_3_5 | 20 | 3 | 51 | 25 |
| 1606_3_6 | 2 | 23 | 52 | 26 |
| Run ID | Phase | Number of Analyses | Fe | C | N | O | Total |
|---|---|---|---|---|---|---|---|
| 1963_2_1 | Cohenite | 7 | 92.6(3) | 6.5(5) | - | 0.6(3) | 99.7 |
| Wüstite | 9 | 75.0(3) | - | - | 24.3(1) | 99.3 | |
| 1982_2_5 | Cohenite | 8 | 93.7(4) | 6.3(5) | - | 0.5(3) | 100.5 |
| Wüstite | 8 | 76.2(1) | - | - | 24.2(1) | 100.4 | |
| 1930_2_2 | Cohenite | 8 | 93.4(3) | 6.6(4) | - | 0.3(5) | 100.3 |
| Wüstite | 9 | 75.7(3) | - | - | 25.0(1) | 100.7 | |
| 1940_2_2 | Iron nitride | 8 | 91.8(1) | 2.7(1) | 5.3(4) | 0.4(1) | 100.2 |
| Wüstite | 7 | 75.5(3) | - | - | 25.3(4) | 100.8 | |
| 1963_4_2 | Iron nitride | 7 | 91.4(5) | 2.7(1) | 4.9(3) | 0.5(3) | 99.6 |
| Wüstite | 7 | 76.9(5) | - | - | 22(3) | 98.9 | |
| 1963_2_5 | Iron nitride | 8 | 92.2(3) | 2.1(5) | 5.4(2) | 0.4(1) | 100.1 |
| Wüstite | 7 | 76.4(2) | - | - | 22.6(1) | 99 | |
| 1963_2_6 | Iron nitride | 9 | 91.7(3) | 2.3(5) | 5.3(5) | 0.4(5) | 99.7 |
| Wüstite | 9 | 75.4(2) | - | - | 23.5(5) | 98.9 | |
| 1930_2_8 | Iron nitride | 9 | 92.0(3) | 2.3(3) | 5.0(1) | 0.4(4) | 99.7 |
| Wüstite | 10 | 74.9(1) | - | - | 24.8(5) | 99.7 | |
| 1940_2_1 | Cohenite | 9 | 92.0(1) | 6.8(2) | - | 0.5(1) | 99.3 |
| Wüstite | 7 | 74.5(2) | - | - | 25.6(3) | 100.1 | |
| Quenched melt | 9 | 93.6(3) | 3.8(3) | 1.2(5) | 0.7(3) | 99.3 | |
| 1940_2_4 | Cohenite | 9 | 91.9(4) | 6.6(4) | - | 0.3(3) | 98.8 |
| Wüstite | 8 | 75.3(2) | - | - | 24.2(4) | 99.5 | |
| Quenched melt | 9 | 94.3(1) | 3.7(5) | 1.3(2) | 0.6(2) | 99.9 | |
| 1930_2_1 | Cohenite | 7 | 93.9(3) | 6.4(3) | - | 0.4(2) | 100.7 |
| Wüstite | 10 | 74.9(2) | - | - | 25.1(4) | 100 | |
| Quenched melt | 9 | 95.4(3) | 3.7(3) | 1.3(5) | 0.5(5) | 100.9 | |
| 1982_2_6 | Cohenite | 7 | 93.6(2) | 6.7(1) | 0.1(2) | 0.5(4) | 100.9 |
| Wüstite | 9 | 75.0(1) | - | - | 25.8(4) | 100.8 | |
| Quenched melt | 7 | 94.2(3) | 3.8(4) | 1.4(2) | 1.0(4) | 100.4 | |
| 1930_2_4 | Wüstite | 7 | 75.1(2) | - | - | 25.6(1) | 100.7 |
| Quenched melt | 8 | 94.2(1) | 3.8(2) | 2.0(4) | 0.6(2) | 100.6 | |
| 1930_2_5 | Wüstite | 10 | 74.8(2) | - | - | 25.5(4) | 100.3 |
| Quenched melt | 10 | 94(3) | 3.6(4) | 1.9(5) | 0.5(2) | 100 | |
| 1963_2_3 | Wüstite | 7 | 75.2(1) | - | - | 24.4(3) | 99.6 |
| Quenched melt | 7 | 91.3(3) | 3.7(3) | 3(3) | 0.6(3) | 98.6 | |
| 1982_2_3 | Iron nitride | 7 | 92.2(2) | 2.4(3) | 4.6(3) | 0.5(5) | 99.7 |
| Wüstite | 10 | 75.3(3) | - | - | 23.5(1) | 98.8 | |
| Quenched melt | 7 | 92.0(4) | 3.0(2) | 3.5(1) | 0.6(2) | 99.1 | |
| 1982_2_4 | Wüstite | 10 | 75.3(2) | - | - | 23.7(3) | 99 |
| Iron nitride | 8 | 92.1(1) | 2.4(2) | 4.6(3) | 0.5(2) | 99.6 | |
| Quenched melt | 8 | 92.1(3) | 2.8(2) | 3.5(1) | 0.6(3) | 99 | |
| 1982_2_2 | Iron nitride | 7 | 91.9(2) | 2.1(2) | 4.5(2) | 0.5(3) | 99 |
| Wüstite | 9 | 75.2(4) | - | - | 24.1(3) | 99.3 | |
| Quenched melt | 10 | 92.5(5) | 2.9(2) | 3.6(1) | 0.6(3) | 99.6 | |
| 2092_2_1 | Wüstite | 7 | 74.9 (6) | - | - | 24.0 (2) | 99.1 |
| Quenched melt | 16 | 92.7 (2) | 3.1 (3) | 3.5 (1) | 0.40 (4) | 99.7 | |
| 2092_2_2 | Cohenite | 8 | 91.8 (1) | 6.5 (1) | 0.3 (1) | 0.4 (1) | 99.6 |
| Wüstite | 5 | 74.3 (2) | - | - | 24.6 (2) | 99.1 | |
| Quenched melt | 19 | 93.3 (3) | 3.9 (3) | 2.0 (1) | 0.4 (2) | 100.4 | |
| 2092_2_3 | Cohenite | 8 | 91.9 (5) | 6.7 (2) | 0.21 (3) | 0.31 (2) | 99.1 |
| Wüstite | 5 | 75.0 (5) | - | - | 24.1 (5) | 99.1 | |
| Quenched melt | 12 | 93.9 (3) | 4.0 (4) | 1.5 (2) | 0.31 (6) | 99.4 | |
| 1606_3_5 | Cohenite | 9 | 91.9 (9) | 7.0 (5) | 0.3 (1) | 0.3 (1) | 99.5 |
| Wüstite | 5 | 74.5 (7) | - | - | 24.6 (6) | 99.2 | |
| 1606_3_6 | Iron nitride | 8 | 89.8 (9) | 2.4 (3) | 6.5 (4) | 0.5 (1) | 99.2 |
| Wüstite | 5 | 73.5 (4) | - | - | 23.7 (7) | 97.2 | |
| Standard | Fe3C | 10 | 93.6(2) | 6.6(2) | - | 0.1(1) | 100.2 |
| Standard | Fe3N | 10 | 92.1(2) | - | 7.6(1) | 0.1(1) | 99.8 |
| Sample | Unit-Cell Parameters, Space Group, Formula Unit Number | Formula Unit * | Vuc/Z (Å3) |
|---|---|---|---|
| 1963_2_1 | a = 5.0814(6), b = 6.7465(8), c = 4.5173(6) Å, V = 154.86(3) Å3, Pnma, Z = 12 | FeC0.293(4) | 12.905 |
| 1982_2_3 | a = 2.6901(7), c = 4.3658(13) Å, V = 27.361(18) Å3, P63/mmc, Z = 2 | FeN0.19(4) | 13.681 |
| 1963_2_4 | a = 2.7199(3), c = 4.3717(6) Å, V = 28.008(6) Å3, P63/mmc, Z = 2 | FeN0.28(2) | 14.004 |
| 1963_2_5 | a = 2.7241(2), c = 4.3760(5) Å, V = 28.123(5) Å3, P63/mmc, Z = 2 | FeN0.40(2) | 14.062 |
| 1963_2_6 | a = 2.7152(2), c = 4.3730(5) Å, V = 27.921(4) Å3, P63/mmc, Z = 2 | FeN0.26(6) | 13.961 |
| 1940_2_2 | a = 2.7158(3), c = 4.3697(5) Å, V = 27.911(5) Å3, P63/mmc, Z = 2 | FeN0.26(2) | 13.956 |
| Sample | Unit-Cell Parameters, Space Group, Formula Unit Number | Formula Unit * |
|---|---|---|
| 1963_2_1 | a = 4.3135(2) Å, V = 80.259(12) Å3, , Z = 4 | Fe0.946(7)O |
| 1940_2_1 | a = 4.31845(11) Å, V = 80.535(6) Å3, , Z = 4 | Fe1.000(5)O |
| 1940_2_4 | a = 4.3204(2) Å, V = 80.642(12) Å3, , Z = 4 | Fe0.863(8)O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, A.N.; Sokol, A.G.; Seryotkin, Y.V.; Palyanov, Y.N. Phase Relations in the FeO-Fe3C-Fe3N System at 7.8 GPa and 1350 °C: Implications for Oxidation of Native Iron at 250 km. Minerals 2020, 10, 984. https://doi.org/10.3390/min10110984
Kruk AN, Sokol AG, Seryotkin YV, Palyanov YN. Phase Relations in the FeO-Fe3C-Fe3N System at 7.8 GPa and 1350 °C: Implications for Oxidation of Native Iron at 250 km. Minerals. 2020; 10(11):984. https://doi.org/10.3390/min10110984
Chicago/Turabian StyleKruk, Aleksei N., Alexander G. Sokol, Yurii V. Seryotkin, and Yuri N. Palyanov. 2020. "Phase Relations in the FeO-Fe3C-Fe3N System at 7.8 GPa and 1350 °C: Implications for Oxidation of Native Iron at 250 km" Minerals 10, no. 11: 984. https://doi.org/10.3390/min10110984




