1. Introduction
In the industrial production of concentrators, due to the long-term use of dynamic water rich in calcium and magnesium ions such as lime, scaling is a common occurrence in heat exchange equipment, cooling water systems and several types of transmission pipelines [
1]. This type of scaling phenomenon can easily cause pipe wall corrosion, pipe blockage and slurry transportation problems, thus affecting the mineral separation process, safe operation of the concentrator and economic benefits. To ensure the efficient production of concentrators, the dynamic water system often requires descaling [
2]. Therefore, for energy conservation and environmental protection purposes, it has become increasingly important to seek economic and efficient methods of scale prevention and descaling to provide technical support for normal mineral separation and index stability operations [
3,
4,
5].
The water quality used in the concentrator contains Ca
2+ and Mg
2+, which react with CO
2 and CO
32− in water and form inorganic salts such as CaCO
3; these salts are difficult to dissolve in water and gradually become sediment [
6,
7]. Considering the causes of scale formation, the primary methods of scale removal are mechanical, chemical reagents and high-pressure water gun sprays. These methods have certain effects on scale prevention and removal; however, there are a few problems such as a high investment cost, a high treatment cost and secondary pollutants [
6,
8,
9].
Several researchers have performed more detailed studies on magnetization scale prevention [
10,
11,
12]. For example, Liu et al. [
13] researched the synergistic scale inhibition effects of rare-earth permanent magnetic materials and polyaspartic acid and optimized this effect based on the magnetic field strength, temperature, water hardness and reagent dosage. The results indicated that magnetization treatment can enhance the scale inhibition effects of polyaspartic acid. Seung Koo et al. discussed the effects of ion and particle mechanisms of a magnetic field on the precipitation of calcium carbonate and found that the particle mechanism can reduce the concentration of calcium ions in the solution during the magnetization process whereas the ionic mechanism can reduce the formation of CaCO
3 crystals during the magnetization separation process [
14]. Chang et al. conducted a series of growth experiments on calcium carbonate polycrystalline vaterite in a fluidized bed crystallizer subjected to a magnetic field [
7]. The results indicated that under room temperature conditions, the seeds grew when they were not magnetized but they did not grow when subjected to a magnetic field. Furthermore, it was found that as the intensity of the magnetic field intensity increased, the action time decreased. Vaterite crystals grew faster in a lower pH environment whereas calcite hardly grew. Additionally, it was found that the high supersaturation and nonuniform activity ratio was beneficial to vaterite growth.
Consequently, a few scholars have also explored the practical application of magnetization scale prevention. Wang et al. designed a dynamic electromagnetic test device [
15]. By analyzing and comparing the characteristics of conductivity over time, the metastable curves of calcium carbonate crystals under different influencing factors (such as solution concentration and electromagnetic field strength) were obtained. Deng et al. researched the effects of an adjustable constant magnetic field with a maximum flux density of 4100 MT on the precipitation process of calcium carbonate in hard water without impurity ions [
16]. The results indicated that the magnetic field changed the dehydration process of CaCO
3 as the precursor of a crystal nucleus by affecting the hydration process of ions in the solution, which then changed the structure of the water mass and affected the precipitation process of CaCO
3.
To solve the above-mentioned problems, new and efficient scale prevention and removal technologies such as magnetization scale prevention and scale removal technology have gradually attracted the attention of researchers [
10,
17,
18]. The primary advantage of this technology is that there is no secondary pollution of chemical agents and the application range is wide [
19]. In addition, the magnesium ions and calcium ions in the water are elements of the same main group, which will also have a certain impact on the crystallization process of calcium carbonate in the pulp. However, there are certain problems in this process such as ideal laboratory indexes and unstable industrial application effects. Additionally, the adaptability of the process to different industries varies. Thus, using anti-scaling and descaling of the pipeline in the concentrator as the breakthrough point, the variation law of the solubility of magnetized calcium carbonate is characterized by the detection methods of conductivity, pH value and calcium ion concentration. This study can not only provide a theoretical basis for scale prevention and descaling of slurry pipelines in concentrators but also offer a reference for the application of magnetization technology in different fields.
3. Results and Discussion
3.1. Effects of Mg2+ Ions on the pH of the Solution
The effects of different magnesium salt hydrolyses on the solution’s pH value were studied in both static and flowing water and the results are shown in
Figure 2.
When magnetized ultra-pure water was used as a solvent, the magnesium carbonate was weakly alkaline and the magnesium sulfate was weakly acidic so the pH value of the MgCO
3 solution increased with an increase in the MgCO
3 concentration whereas the MgSO
4 solution decreased with an increase in the MgSO
4 concentration (
Figure 2a). In static water, the pH value of the MgCO
3 solution first increased rapidly at a low concentration then increased slowly and eventually stabilized at approximately 10.1. In flowing water, the increasing trend of the pH value of the MgCO
3 solution was faster than in static water. When the concentration of MgCO
3 was high (4% and 5%), the pH value of the MgCO
3 solution was stable at approximately 10.0 in both static and flowing water. In the MgSO
4 solution, the pH value of the MgSO
4 solution decreased slowly with an increase in concentration regardless of whether it was static or flowing water. It should be noted that the decrease between the pH values was consistent; i.e., the pH value in static water was always approximately 0.6 higher than that in flowing water.
However, in the magnetized CaCl
2-NaHCO
3 mixed solution, the hydrolysis of magnesium salts had minimal effects on the pH value of the solution (
Figure 2b). With an increase in the magnesium salt concentration, the pH value of the solution in static water was stable between 7.0–7.1. The pH value of flowing water was slightly higher than that of static water, which was stable at approximately 7.4. This phenomenon indicated that CaCl
2-NaHCO
3 acts as an extremely suitable buffer for the pH of the solution. One likely reason was that the anions in the added magnesium salts reacted with the calcium ions in the solution to form the insoluble precipitate.
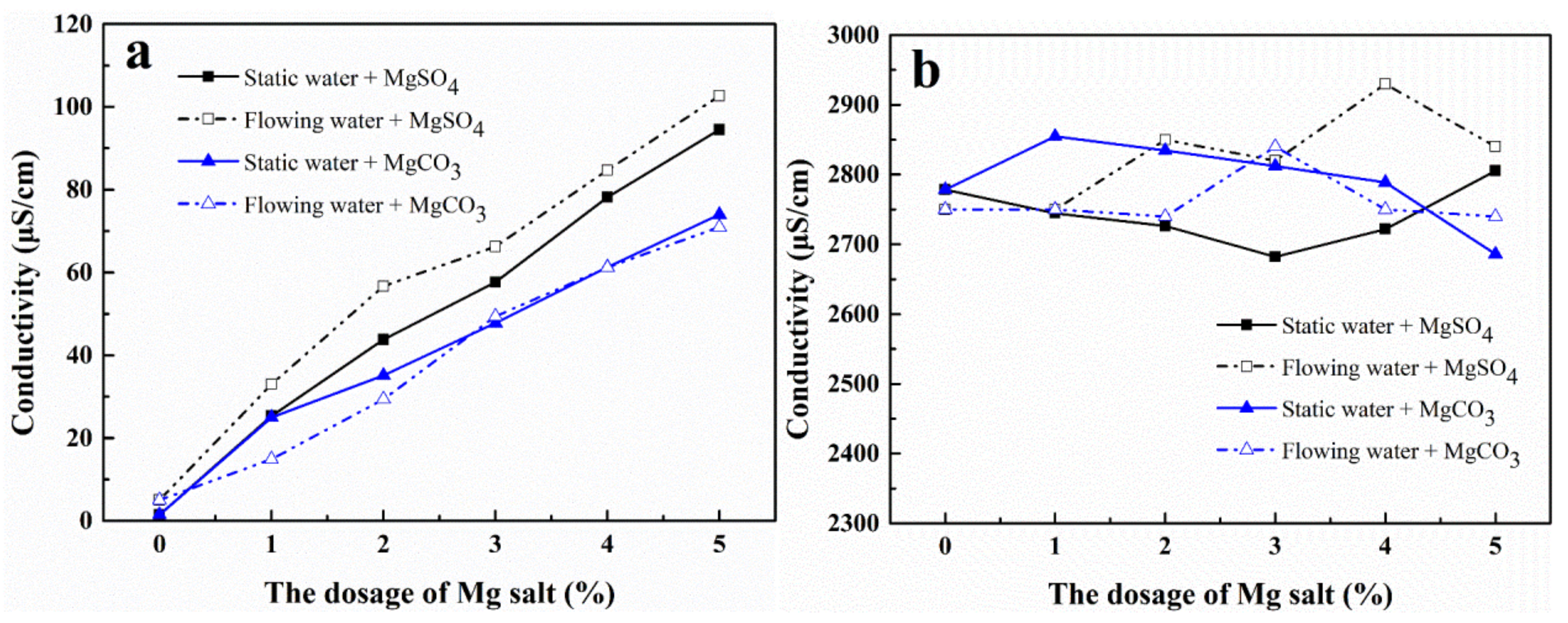
3.2. Effects of Mg2+ Ions on the Conductivity of the Solution
The relationship between the conductivity of the solution and the concentration of magnesium salts was investigated for both static and flowing water and the results are shown in
Figure 3.
In magnetized ultra-pure water, the conductivity of the solution had a good linear correlation with the concentration of the magnesium salts (
Figure 3a). The conductivity of the MgSO
4 solution in the dynamic water flow was higher than that in the static water flow whereas the conductivity of the MgCO
3 solution had a minor relationship with the flow state. At the same concentration, the conductivity of the MgSO
4 solution was higher than that of the MgCO
3 solution. It is possible that the solubility of MgSO
4 was higher than that of MgCO
3, which led to the high concentration of magnesium ions in the solution and good conductivity.
In the magnetized CaCl
2-NaHCO
3 mixed solution, the effects of MgCO
3 and MgSO
4 on the conductivity of the solution were different (
Figure 3b). In static water, the conductivity of the MgCO
3 solution first increased and then decreased slowly with an increase in the MgCO
3 concentration whereas the conductivity of the MgSO
4 solution had opposing results. When the concentration of MgCO
3 was 1%, the static water solution reached its maximum conductivity (2853 μS·cm
−1). When the concentration of MgSO
4 was 3%, the minimum conductivity (2681 μS·cm
−1) of the static aqueous solution was obtained.
In the dynamic flow, when the concentration of MgCO3 was between 2–4%, the conductivity of the solution first increased and then decreased. When the concentration of MgCO3 was less than 2 or greater than 4, the conductivity of the solution remained unchanged. However, the conductivity of the MgSO4 solution increased with an increase in the concentration in flowing water. When the concentration of MgSO4 was 4%, the conductivity of the solution reached its maximum value (2937 μS·cm−1). As the conductivity of the inorganic salt solution was positively correlated with the concentration of charged ions in the solution, as the conductivity increased, the concentration of Ca2+ ions in the solution increased. Therefore, MgSO4 could promote the dissolution of magnetized CaCO3 more effectively than MgCO3.
3.3. Effects of Mg2+ Ions on the Ca2+ Concentration of the Solution
The effects of the magnesium salts on the concentration of Ca
2+ ions in the magnetized CaCl
2-NaHCO
3 mixed solution is shown in
Figure 4 for both static and flowing water.
In static water, the concentration of Ca
2+ ions decreased with an increase in the MgCO
3 concentration whereas it first decreased and then increased with an increase in the MgSO
4 concentration (
Figure 3a). With an increase in the MgCO
3 concentration, the Ca
2+ ion concentration of the solution decreased from 239.74 mg·L
−1 to 220.19 mg·L
−1. With an increase in the MgSO
4 concentration, the concentration of the Ca
2+ ions in the solution first decreased and then increased. When the concentration of MgSO
4 was 3.0%, the minimum concentration of the Ca
2+ ions in the solution was 219.62 mg·L
−1.
In flowing water, the concentration of the Ca2+ ions in the solution increased with an increase in the MgSO4 concentration. When the concentration of MgSO4 was 4.0%, the maximum concentration of the Ca2+ ions in the solution was 240.38 mg·L−1, which indicated an increase of 5.93%. However, with an increase in the MgCO3 concentration, the concentration of the Ca2+ ions in the solution first increased and then decreased. When the concentration of MgCO3 was more than 3.0%, the concentration of the Ca2+ ions in the solution decreased significantly. When the concentration of MgCO3 was 5.0%, the minimum concentration of the Ca2+ ions in the solution was 207.69 mg·L−1, which indicated a decrease of 22.19%. As the solubility of magnesium carbonate in water is greater than that of calcium carbonate, magnesium carbonate will hinder the dissolution of calcium carbonate and reduce the calcium ion concentration.
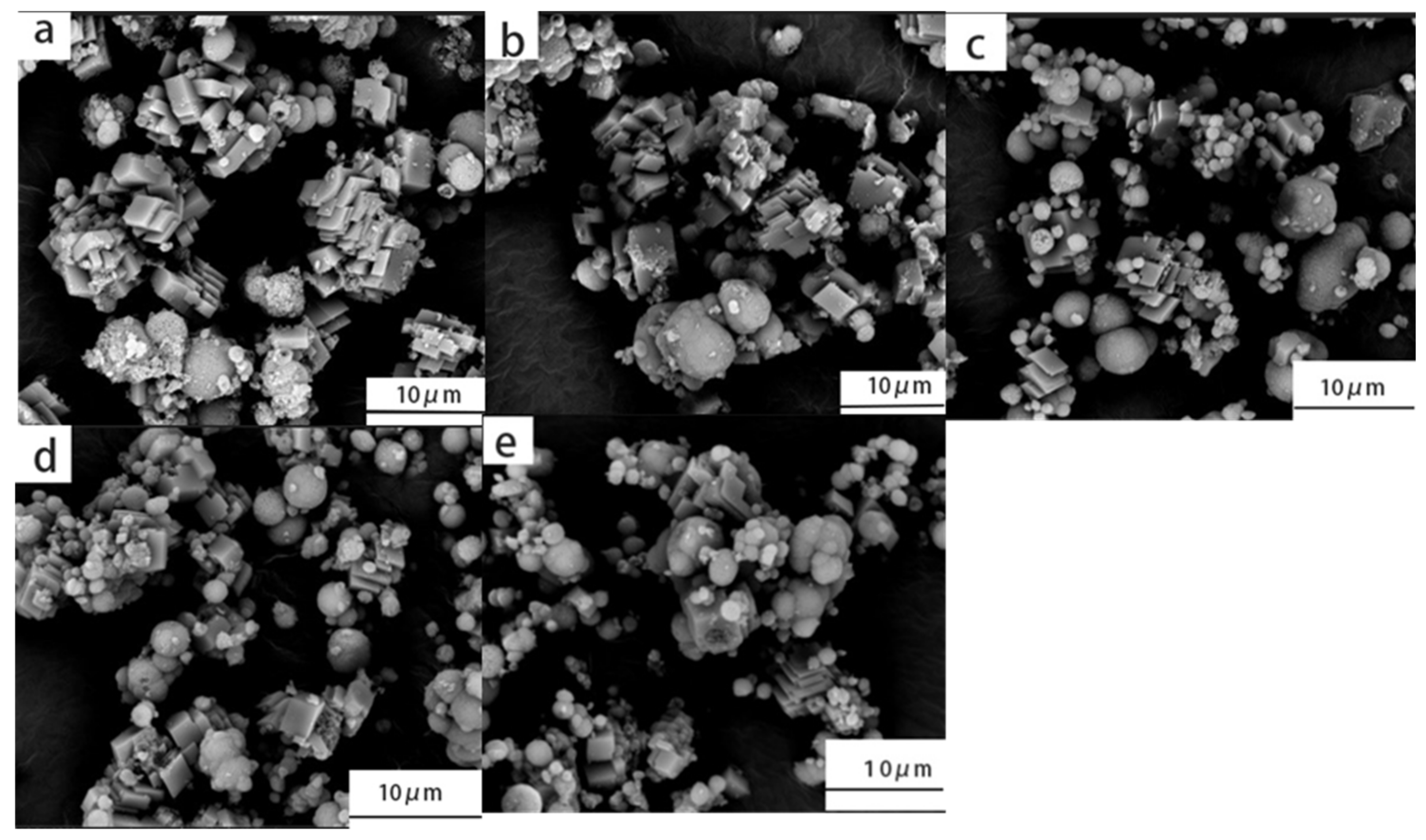
3.4. Morphology Analysis of Magnesium Sulfate for Magnetization Anti-Scaling Products
According to the above experimental results, magnesium sulfate can promote the dissolution of calcium carbonate.
Figure 5 shows the morphology changes of different concentrations of the magnesium sulfate solution on the dissolution behavior of calcium carbonate after magnetization.
With an increase in the magnesium sulfate concentration, the precipitate of calcium carbonate in the solution gradually transformed from cubic to spherical. The content of calcium carbonate precipitation in the solution decreased gradually. This phenomenon was consistent with that reported in the literature [
22]; i.e., magnesium sulfate can promote the dissolution of cubic calcium carbonate under magnetization conditions and form a vaterite such as calcium carbonate. With an increase in the magnesium sulfate concentration, the vaterite-type calcium carbonate content increased gradually. When the concentration of magnesium sulfate was 5.0%, the size of the cubic calcite calcium carbonate increased and a few crystal defects appeared. Simultaneously, the agglomeration of vaterite-type calcium carbonate was more significant. Therefore, the appropriate amount of magnesium sulfate can promote the formation of vaterite-type calcium carbonate with high solubility, prevent its agglomeration and improve the magnetization anti-scaling effects under flowing water conditions.
3.5. Material Composition Analysis of Magnesium Sulfate for Magnetization Anti-Scaling Products
Based on the analysis of the morphology of magnetized anti-scaling products when subjected to magnesium sulfate, the effects of magnesium sulfate on the chemical structure of calcium carbonate precipitation were studied using Raman spectroscopy. The results are shown in
Figure 6.
Before and after adding magnesium sulfate, the Raman shift peak appeared at 1080–1090 cm
−1 (
Figure 6). Based on the standard spectrum, the standard Raman shift peak corresponding to the calcite-type calcium carbonate was 1086 cm
−1 whereas that of vaterite was 1089 cm
−1. To further analyze the crystal structure and the content of calcium carbonate in the precipitation particles, we conducted a thorough analysis of the Raman spectrum and the results are shown in
Figure 6b–f and
Table 1.
In the fitting peaks in
Figure 6b–f, only two Raman characteristic shift peaks of calcite and vaterite were found and the integral area of these two peaks changed with an increase in the magnesium sulfate concentration.
With an increase in the magnesium sulfate content, the total peak area of calcium carbonate increased gradually (
Table 1). The calcite-type calcium carbonate content, which hindered the anti-scaling effect, gradually decreased whereas the vaterite-type calcium carbonate content, which promoted the anti-scaling effect, gradually increased. When the concentration of magnesium sulfate was 5.0%, the vaterite-type calcium carbonate content was higher than the calcite-type calcium carbonate content; however, the agglomeration phenomenon was more significant and the size of the calcite-type calcium carbonate product was higher. These phenomena not only affected its solubility in water but also made it easier to deposit scales. The magnetization anti-scaling effect was optimal when the concentration of magnesium sulfate was 4.0%.
3.7. Material Composition Analysis of Magnesium Carbonate for Magnetization Anti-Scaling Products
To further study the effect of magnesium carbonate on the structure of magnetized anti-scaling products, Raman spectroscopy was used to detect and analyze the magnetized anti-scaling products. The results are shown in
Figure 8 and
Table 2.
It can be seen from
Figure 8 that the magnetized anti-scaling products after the addition of magnesium carbonate were a mixture of calcite and vaterite. With an increase in the magnesium carbonate concentration, the proportion of the calcite-type calcium carbonate increased gradually. When the mass fraction of magnesium carbonate was 5.0%, the calcite-type calcium carbonate content reached 79.27%, which was considerably higher than the vaterite-type calcium carbonate content. As the vaterite-type calcium carbonate can promote dissolution of the scale, it was necessary to create conditions in the solution to achieve the highest possible vaterite-type calcium carbonate content. Therefore, as the concentration of magnesium carbonate in the solution increased, the magnetization and scale prevention became less favorable.
4. Conclusions
(1) Under dynamic water conditions, 4.0% MgSO4 had the greatest anti-scaling effect, which could increase the calcium ion concentration of the calcium chloride sodium bicarbonate solution by 5.93%. However, the synergistic effects of 5.0% magnesium carbonate on calcium carbonate scaling were the strongest, which could reduce the calcium ion concentration of the calcium chloride sodium bicarbonate solution by 22.19%.
(2) Magnesium carbonate reduced the effects of magnetization because it inhibited the formation of vaterite-type calcium carbonate and promoted the formation of calcite-type calcium carbonate crystals. Magnesium sulfate can improve the effects of magnetization because magnesium sulfate promoted the formation of vaterite calcium carbonate with a high solubility.