Eudialyte Group Minerals from the Lovozero Alkaline Massif, Russia: Occurrence, Chemical Composition, and Petrogenetic Significance
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
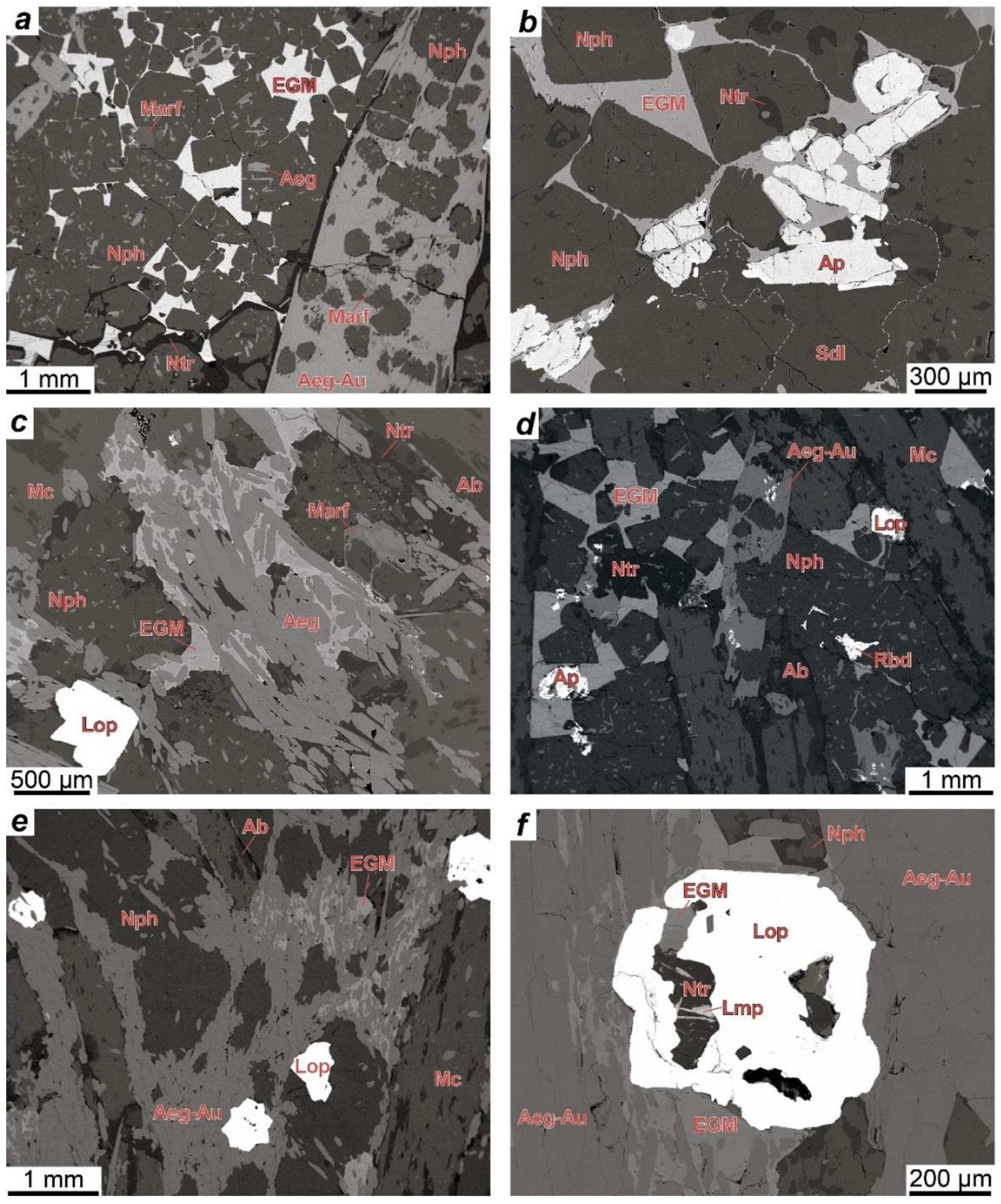
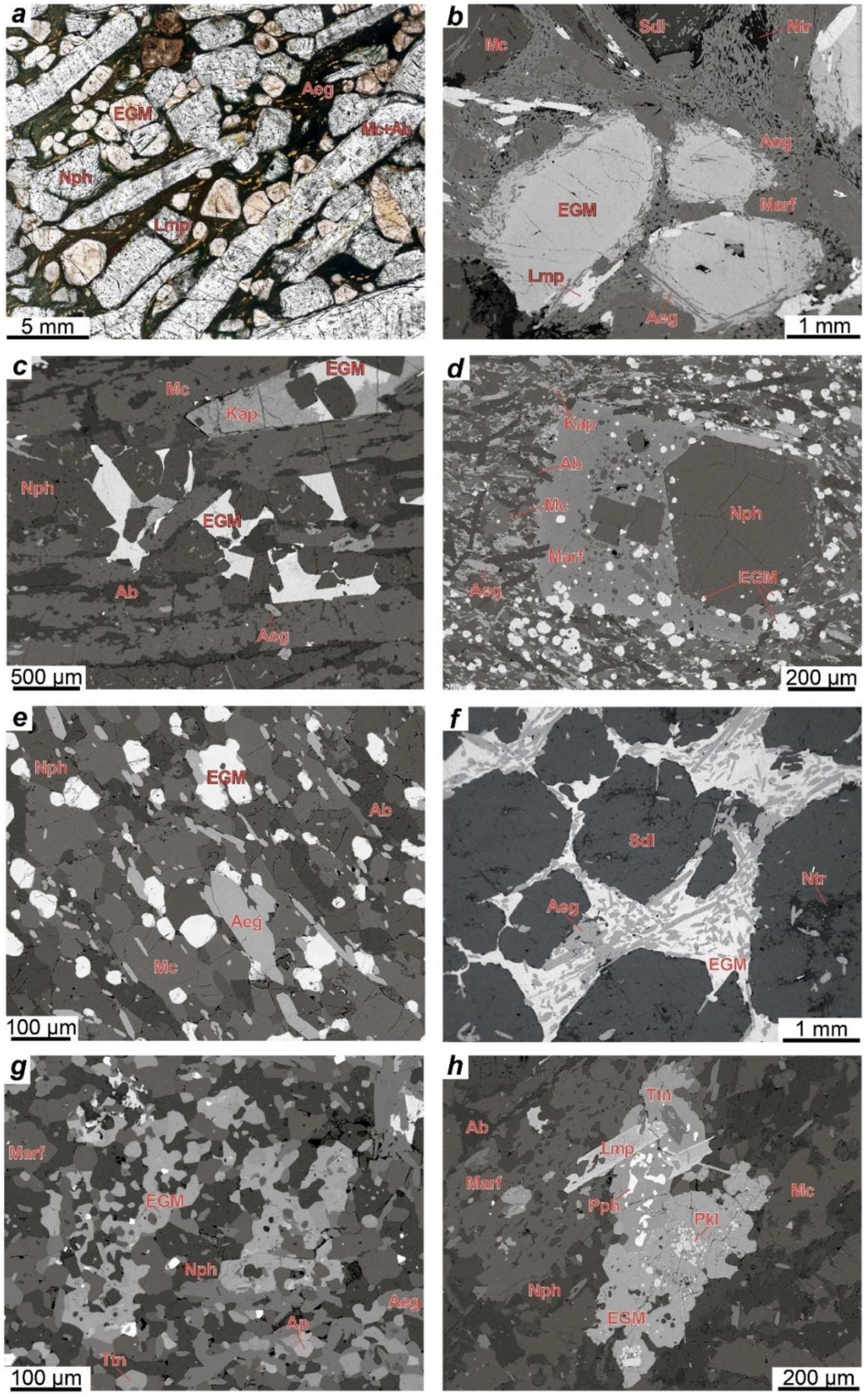
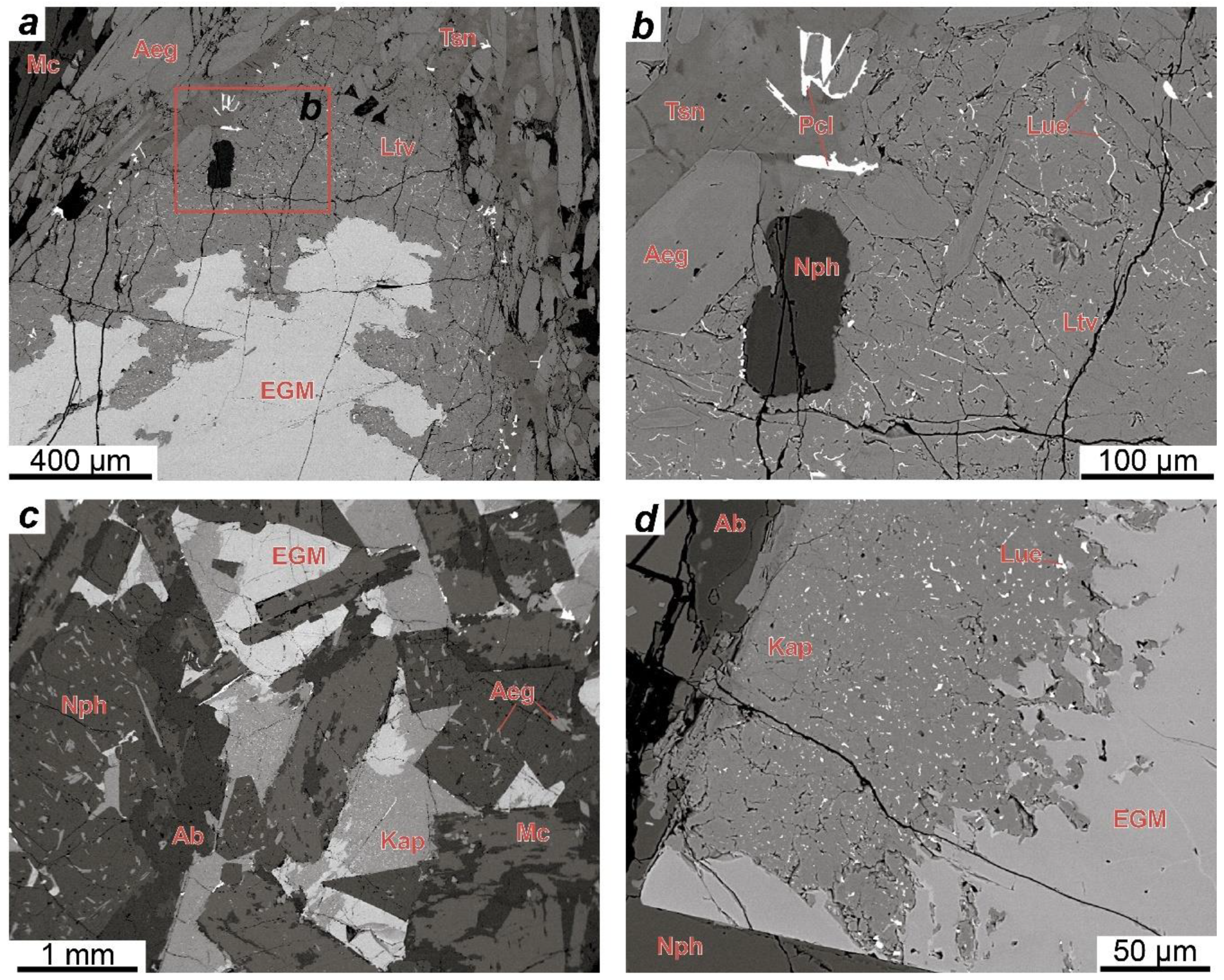
4.1. Petrography
4.1.1. Rocks of the Layered Complex
4.1.2. Rocks of the Eudialyte Complex
4.1.3. Poikilitic Foid Syenites
4.1.4. Metasomatized Volcaniclastic Rock
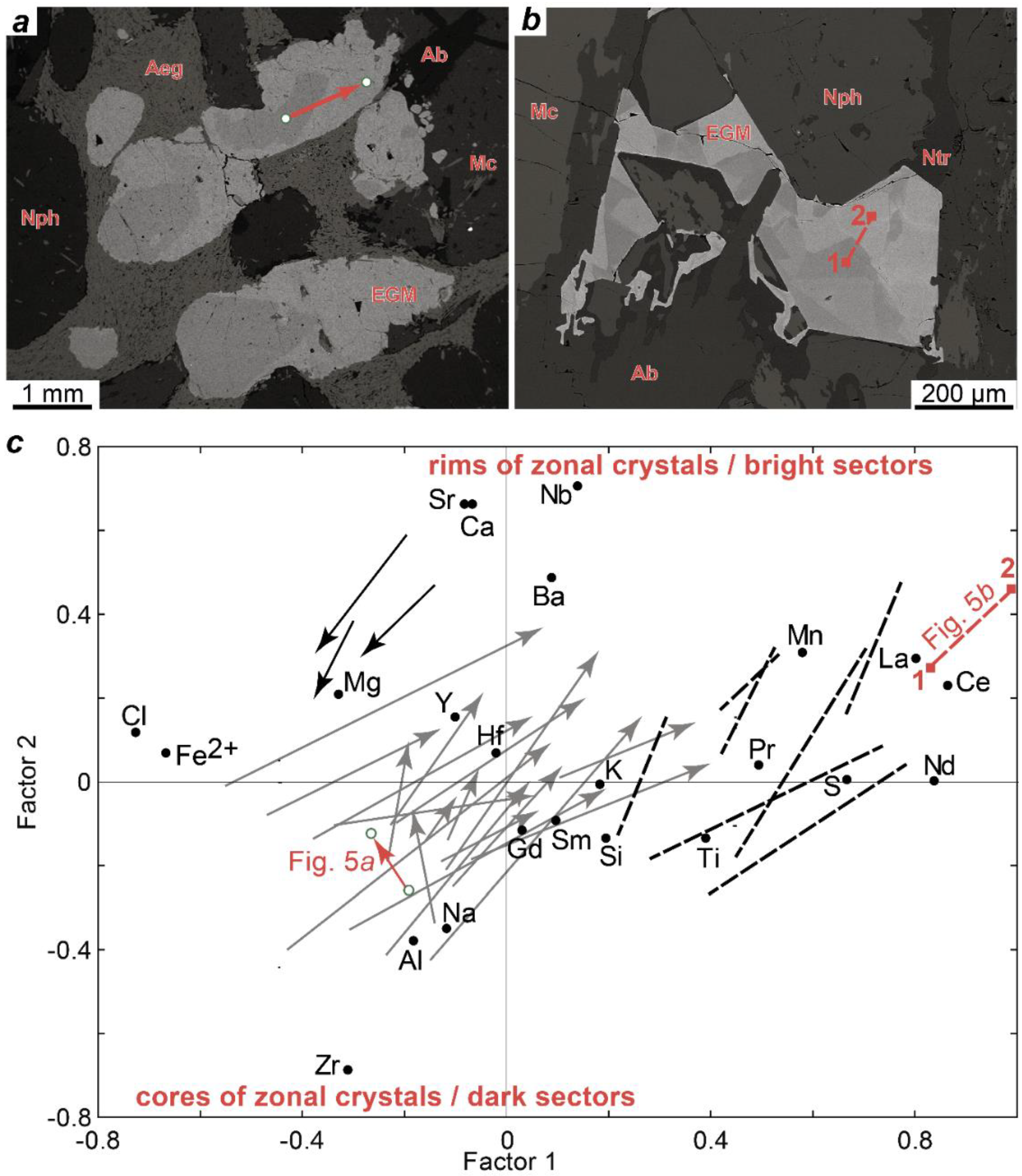
4.2. Crystal Structure and Chemical Composition of the EGM
4.3. Secondary Substitutions of the EGM
4.4. Associated Minerals: Clinopyroxenes
5. Discussion
6. Conclusions
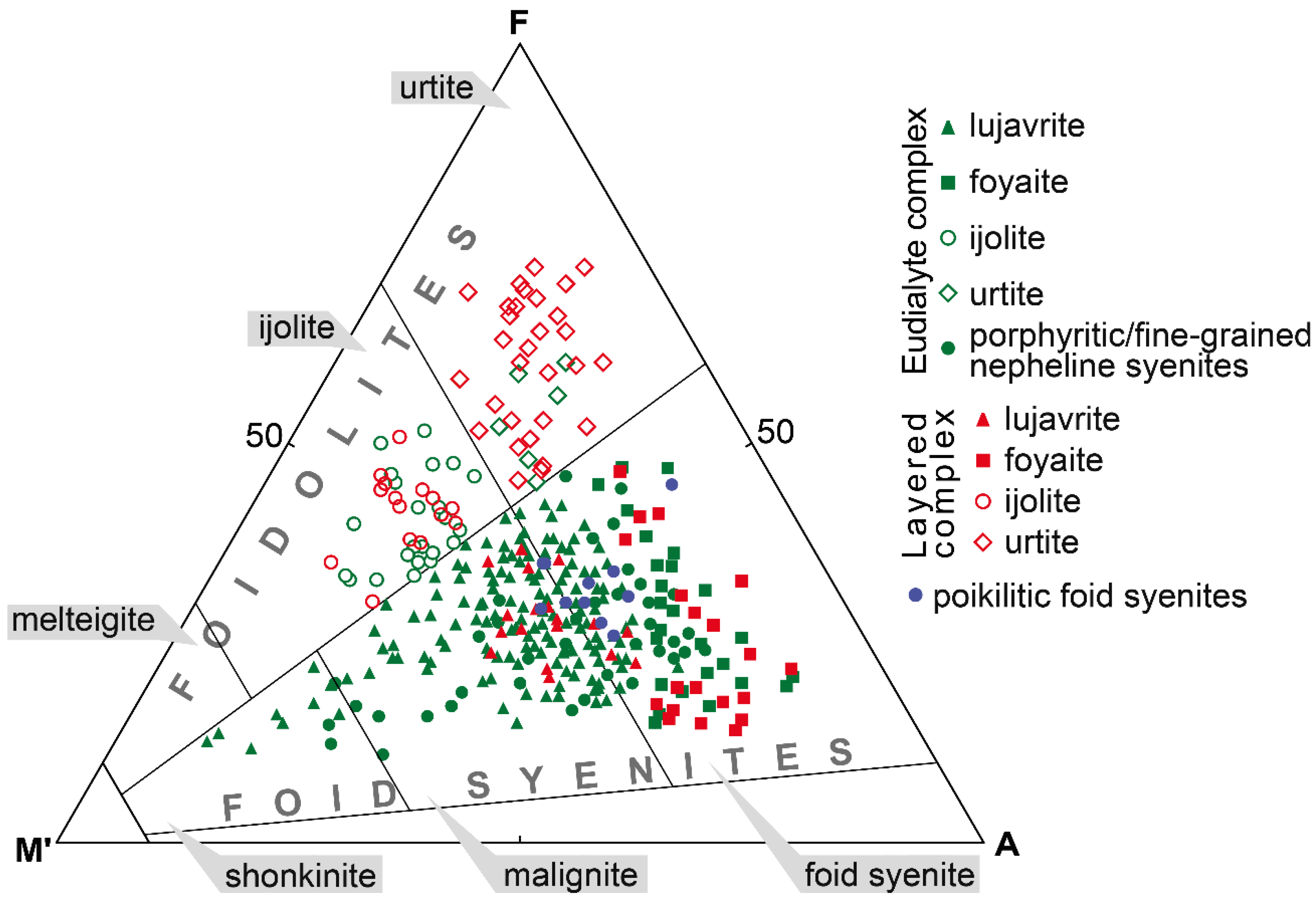
- The minerals of eudialyte group (EGM) are a characteristic accessory and sometimes rock-forming minerals of the Lovozero alkaline massif rocks. In all types of the rocks, they form at the late magmatic stage. In the rocks of the Layered complex, the EGM crystallize later than alkaline clinopyroxenes and amphiboles, but in the Eudialyte complex, crystallization of the EGM begins simultaneously with clinopyroxenes and ends after their formation.
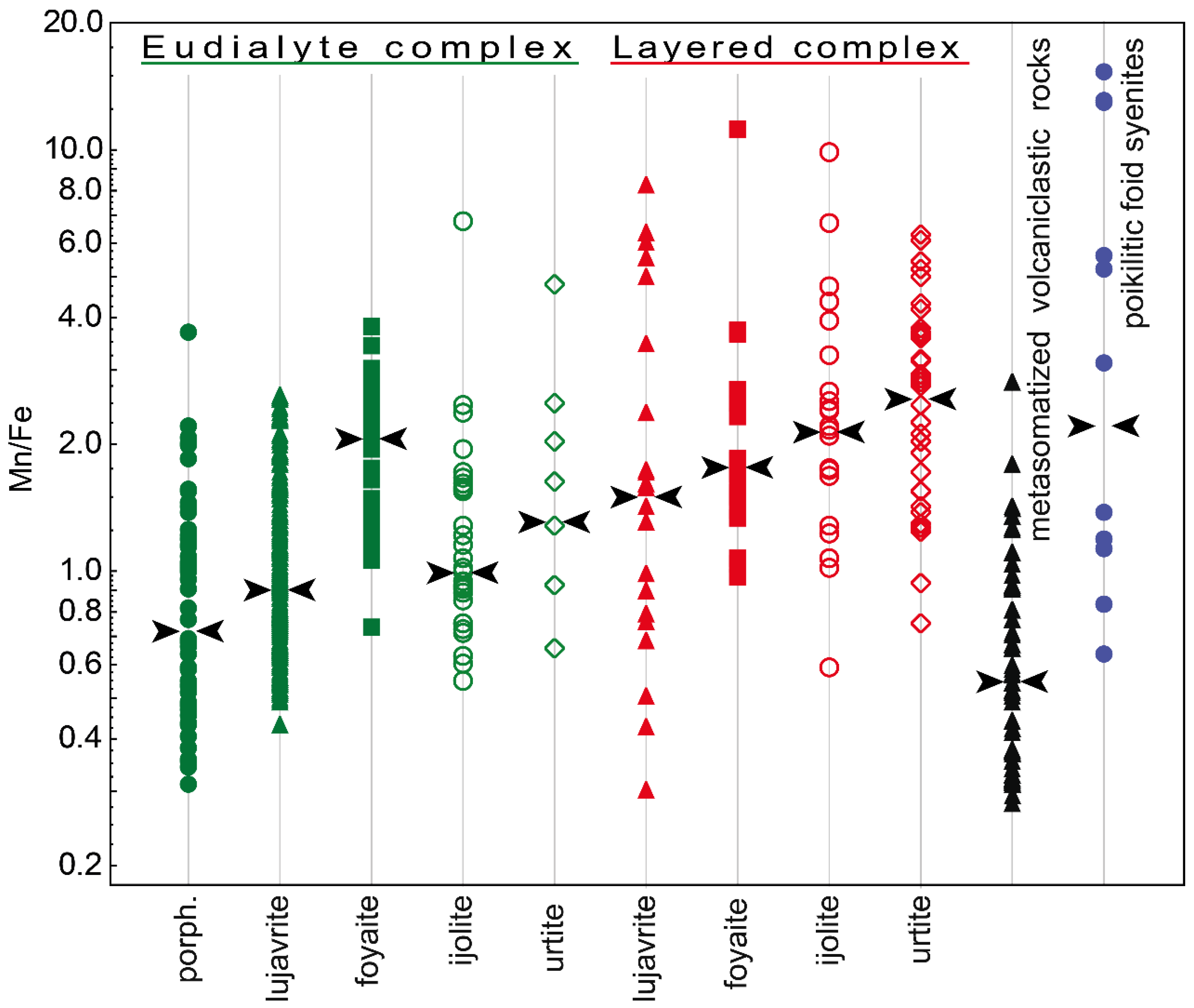
- In the Layered complex, the maximum modal content of EGM is found in leucocratic rocks (foyaite and urtite). In the Eudialyte complex, the largest amount of EGM is concentrated in melanocratic rocks (lujavrite), while leucocratic rocks contain primary minerals of the lovozerite group. This distribution occurs due to fractional crystallization by the lujavrite → foyaite → urtite path, in which the sodium (and HFSE) concentration in the residual liquid progressively increases.
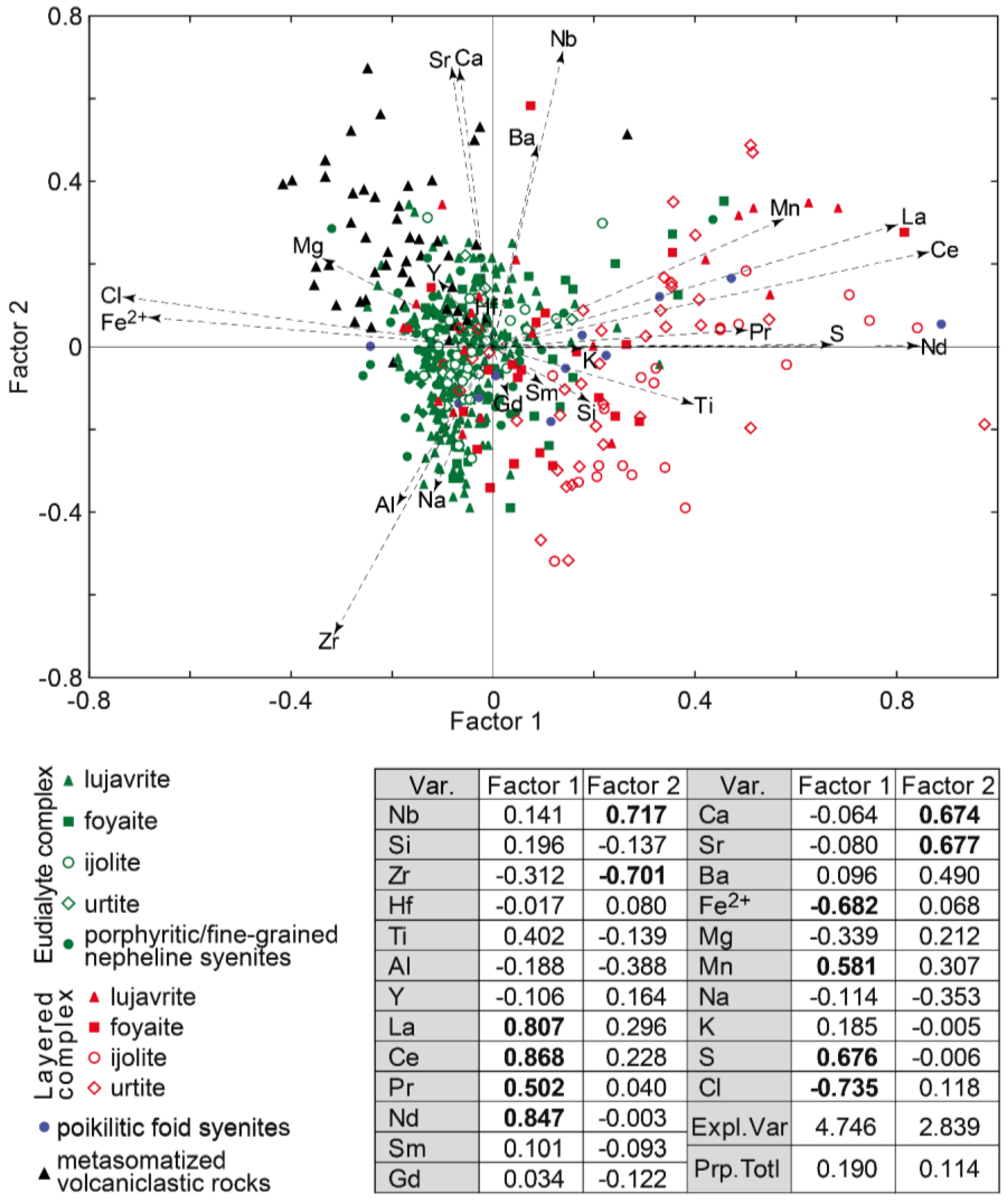
- Most of the EGM samples (70%) from the rocks of the Lovozero massif are hyperzirconium, i.e., their Zr content exceeds 3 apfu. Since the excess zirconium is included in the M(2B) position, the generally accepted formula calculation based on (Si + Zr + Ti + Nb + Al + Hf + W + Ta) = 29 cations cannot be applied. To trace changes in the composition of EGM during magmatic evolution, the ratio of the atomic amounts of cations can be used.
- In the lujavrite-foyaite-urtite series in both Layered and Eudialyte complexes, the Mn/Fe ratio in the EGM increases. However, the Mn/Fe ratio in the EGM from rocks of the Eudialyte complex is significantly lower due to the high iron content. The maximum iron contents and, accordingly, the minimum Mn/Fe values are typical of the EGM, which crystallized simultaneously with clinopyroxenes (aegirine and aegirine-augite). The reason for this is that, during the crystallization of alkali clinopyroxenes in the melt/solution, the concentration of ferrous iron sharply increases.
- At the post-magmatic stage, the EGM are replaced by minerals of the lovozerite group. Which mineral from this group will replace the EGM depends on the alkalinity of the residual solution. The EGM from melanocratic rocks (lujavrite and ijolite) are replaced by litvinskite, and the EGM from leucocratic rocks (urtite and foyaite) are usually replaced by kapustinite.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stromeyer, F. Summary of meeting 16 December 1819. Göttingische Gelehrte Anz. 1819, 3, 1993–2000. [Google Scholar]
- Rastsvetaeva, R.K. Structural mineralogy of the eudialyte group: A review. Crystallogr. Rep. 2007, 52, 47–64. [Google Scholar] [CrossRef]
- Davris, P.; Stopic, S.; Balomenos, E.; Panias, D.; Paspaliaris, I.; Friedrich, B. Leaching of rare earth elements from eudialyte concentrate by suppressing silica gel formation. Miner. Eng. 2017, 108, 115–122. [Google Scholar] [CrossRef]
- Lebedev, V.N. Sulfuric Acid Technology for Processing of Eudialyte Concentrate. Russ. J. Appl. Chem. 2003, 76, 1559–1563. [Google Scholar] [CrossRef]
- Schønwandt, H.K.; Barnes, G.B.; Ulrich, T. A Description of the World-Class Rare Earth Element Deposit, Tanbreez, South Greenland. Rare Earths Ind. 2016, 73–85. [Google Scholar] [CrossRef]
- McLemore, V.; Reservation, M.I. Background and perspectives on the Pajarito Mountain yttrium-zirconium deposit, Mescalero Apache Indian Reseruation, Otero Gounty, New Mexico. Am. Mineral. 1990, 72, 801–811. [Google Scholar]
- Kogarko, L.N. Ore-forming potential of alkaline magmas. Lithos 1990, 26, 167–175. [Google Scholar] [CrossRef]
- Sørrensen, H. Agpaitic nepheline syenites: A potential source of rare elements. Appl. Geochem. 1992, 7, 417–427. [Google Scholar] [CrossRef]
- Schilling, J.; Marks, M.A.W.; Wenzel, T.; Vennemann, T.; Horváth, L.; Tarassoff, P.; Jacob, D.E.; Markl, G. The Magmatic to Hydrothermal Evolution of the Intrusive Mont Saint-Hilaire Complex: Insights into the Late-stage Evolution of Peralkaline Rocks. J. Pet. 2011, 52, 2147–2185. [Google Scholar] [CrossRef]
- Sjöqvist, A.S.; Cornell, D.; Andersen, T.B.; Erambert, M.; Ek, M.; Leijd, M. Three Compositional Varieties of Rare-Earth Element Ore: Eudialyte-Group Minerals from the Norra Kärr Alkaline Complex, Southern Sweden. Minerals 2013, 3, 94–120. [Google Scholar] [CrossRef]
- Olivo, G.R.; Williams-Jones, A.E. Hydrothermal REE-rich eudialyte from the Pilanesberg complex, South Africa. Can. Mineral. 1999, 37, 653–663. [Google Scholar]
- Mitchell, R.H.; Liferovich, R.P. Subsolidus deuteric/hydrothermal alteration of eudialyte in lujavrite from the Pilansberg alkaline complex, South Africa. Lithos 2006, 91, 352–372. [Google Scholar] [CrossRef]
- Schilling, J.; Marks, M.A.; Wenzel, T.; Märkl, G. Reconstruction of magmatic to subsolidus processes in an agpaitic system using eudialyte textures and composition: A case study from Tamazeght, Morocco. Can. Mineral. 2009, 47, 351–365. [Google Scholar] [CrossRef]
- Ratschbacher, B.C.; Marks, M.A.; Bons, P.D.; Wenzel, T.; Markl, G. Emplacement and geochemical evolution of highly evolved syenites investigated by a combined structural and geochemical field study: The lujavrites of the Ilímaussaq complex, SW Greenland. Lithos 2015, 231, 62–76. [Google Scholar] [CrossRef]
- Atanasova, P.; Marks, M.A.W.; Heinig, T.; Krause, J.; Gutzmer, J.; Markl, G. Distinguishing Magmatic and Metamorphic Processes in Peralkaline Rocks of the Norra Kärr Complex (Southern Sweden) Using Textural and Compositional Variations of Clinopyroxene and Eudialyte-group Minerals. J. Pet. 2017, 58, 361–384. [Google Scholar] [CrossRef]
- Borst, A.M.; Friis, H.; Andersen, T.; Nielsen, T.F.D.; Waight, T.E.; Smit, M.A. Zirconosilicates in the kakortokites of the Ilímaussaq complex, South Greenland: Implications for fluid evolution and high-field-strength and rare-earth element mineralization in agpaitic systems. Mineral. Mag. 2016, 80, 5–30. [Google Scholar] [CrossRef]
- Borst, A.M.; Friis, H.; Nielsen, T.F.D.; Waight, T.E. Bulk and Mush Melt Evolution in Agpaitic Intrusions: Insights from Compositional Zoning in Eudialyte, Ilímaussaq Complex, South Greenland. J. Pet. 2018, 59, 589–612. [Google Scholar] [CrossRef]
- Sørensen, H. The agpaitic rocks—An overview. Mineral. Mag. 1997, 61, 485–498. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Lazutkina, L.N.; Romanchev, B.P. The origin of eudialyte mineralization. Geochem. Int. 1982, 19, 128–145. [Google Scholar]
- Estrade, G.; Salvi, S.; Béziat, D.; Estrade, G. Crystallization and destabilization of eudialyte-group minerals in peralkaline granite and pegmatite: A case study from the Ambohimirahavavy complex, Madagascar. Mineral. Mag. 2018, 82, 375–399. [Google Scholar] [CrossRef]
- Harris, C.; Rickard, R.S. Rare-earth-rich eudialyte and dalyite from a peralkaline granite dyke at Straumsvola, Dronning Maud Land, Antarctica. Can. Mineral. 1987, 25, 755–762. [Google Scholar]
- Marks, M.A.; Hettmann, K.; Schilling, J.; Frost, B.R.; Markl, G. The Mineralogical Diversity of Alkaline Igneous Rocks: Critical Factors for the Transition from Miaskitic to Agpaitic Phase Assemblages. J. Pet. 2011, 52, 439–455. [Google Scholar] [CrossRef]
- Marks, M.A.; Markl, G. A global review on agpaitic rocks. Earth Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Zadov, A.E.; Korovushkin, V.V.; Ekimenkova, I.A.; Rastsvetaeva, R.K. Ikranite (Na, H3O)15 (Ca, Mn, REE)6 Fe23+ Zr3 (□, Zr)(□, Si) Si24 O66 (O, OH)6 Cl· nH2O and raslakite Na15 Ca3 Fe3 (Na, Zr)3 Zr3 (Si, Nb)(Si25 O73)(OH, H2O)3 (Cl, OH)—New eudialyte-group minerals from the Lovozero Massif. ZRMO 2003, 5, 22–33. [Google Scholar]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastsvetaeva, R.K. Dualite, Na30 (Ca,Na,Ce,Sr)12 (Na,Mn,Fe,Ti)6 Zr3 Ti3 Mn Si51 O144 (OH, H2O, Cl)9, a new zircono-titanosilicate with a modular eudialyte-like structure from the Lovozero alkaline Pluton, Kola Peninsula, Russia. Geol. Ore Depos. 2008, 50, 574–582. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastsvetaeva, R.K. Voronkovite, Na15 (Na,Ca,Ce)3 (Mn,Ca)3 Fe3 Zr3 Si26 O72 (OH,O)4 Cl · H2O, a new mineral species of the eudialyte group from the Lovozero alkaline pluton, Kola Peninsula, Russia. Geol. Ore Depos. 2009, 51, 750–756. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Pekov, I.V.; Belakovskiy, D.I.; Vozchikova, S.A.; Britvin, S.N. Sergevanite, Na15 (Ca3Mn3) (Na2Fe) Zr3Si26O72 (OH)3 · H2O, a new eudialyte-group mineral from the Lovozero alkaline massif, Kola Peninsula. Can. Mineral. 2020, 58, 421–436. [Google Scholar] [CrossRef]
- Kramm, U.; Kogarko, L. Nd and Sr isotope signatures of the Khibina and Lovozero agpaitic centres, Kola Alkaline province, Russia. Lithos 1994, 32, 225–242. [Google Scholar] [CrossRef]
- Gerasimovsky, V.I.; Volkov, V.P.; Kogarko, L.N.; Polyakov, A.I.; Saprykina, T.V.; Balashov, Y.A. Geochemistry of the Lovozero Alkaline Massif; Nauka: Moscow, Russia, 1966. (In Russian) [Google Scholar]
- Bussen, I.V.; Sakharov, A.S. Petrology of the Lovozero Alkaline Massif; Nauka: Leningrad, Russia, 1972. (In Russian) [Google Scholar]
- StatSoft Inc. Statistica 13. Available online: www.statsoft.ru (accessed on 19 June 2018).
- Reyment, R.A.; Jvreskog, K.G. Applied Factor Analysis in the Natural Sciences; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Le Maitre, R.W. (Ed.) Igneous Rocks: A Classification and Glossary of Terms: Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks; Cambridge University Press: Cambridge, UK, 2005; ISBN 9780521662154. [Google Scholar]
- Johnsen, O.; Grice, J.D. The crystal chemistry of the eudialyte group. Can. Mineral. 1999, 37, 865–891. [Google Scholar]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Aksenov, S.M. Minerals of the Eudialyte Group: Crystal Chemistry, Properties, Genesis; NSU: Nizhny Novgorod, Russia, 2012. (In Russian) [Google Scholar]
- Golyshev, V.M.; Simonov, V.I.; Belov, N.V. About the crystal structure of eudialyte. Crystallography 1971, 16, 93–98. [Google Scholar]
- Giuseppetti, G.; Mazzi, F.; Tadini, C. The crystal structure of eudialyte. Mineral. Pet. 1971, 16, 105–127. [Google Scholar] [CrossRef]
- Ferraris, G.; Makovicky, E.; Merlino, S. Crystallography of Modular Materials; Oxford University Press: Oxford, UK, 2004. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastsvetaeva, R.K. Alluaivite, Na19 (Ca,Mn)6 (Ti,Nb)3 Si26 O74 Cl · H2O, a new titanosilicate with a eudialyte-like structure. Zap. VMO 1990, 119, 117–120. (In Russian) [Google Scholar]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastvetaeva, R.K. Labyrinthite (Na,K,Sr)35 Ca12 Fe3 Zr6 Ti Si51 O144 (O,OH,H2O)9 Cl3, a new mineral with the modular eudialyte-like structure from Khibiny alkaline massif, Kola Peninsula. Zap. RMO 2006, 135, 38–48. (In Russian) [Google Scholar]
- Khomyakov, A.P.; Nechelyustov, G.N.; Arakcheeva, A.V. Rastsvetaevite, Na27 K8 Ca12 Fe3 Zr6 Si4 [Si3O9]4 [Si9O27]4 (O,OH,H2O)6 Cl2, a new mineral with a modular eudialyte-like structure and crystal-chemical systematics of the eudialyte group. Zap. RMO 2006, 135, 49–65. (In Russian) [Google Scholar]
- Osipov, A.S.; Antonov, A.A.; Panikorovskii, T.L.; Zolotarev, A.A. Hydrated CO3-Bearing Analog of Manganoeudialyte from Alkali Pegmatites of the Konder Pluton, Khabarovsk Krai. Geol. Ore Depos. 2018, 60, 726–735. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V. Classification of eudialyte-group minerals. Geol. Ore Depos. 2012, 54, 487–497. [Google Scholar] [CrossRef]
- Pfaff, K.; Wenzel, T.; Schilling, J.; Marks, M.A.; Markl, G. A fast and easy-to-use approach to cation site assignment for eudialyte-group minerals. Neues Jahrb. Mineral. Abh. J. Mineral. Geochem. 2010, 187, 69–81. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Khomyakov, A.P. Crystal structure of a hyperzirconium analogue of eudialyte. Crystallogr. Rep. 2000, 45, 219–221. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Rastsvetaeva, R.K. Crystal-structure refinement of zirconium-rich eudialyte and its place among calcium-poor eudialyte-group minerals. Crystallogr. Rep. 2013, 58, 671–677. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Verin, I.A. Crystal structure of hyperzirconium sulfate analogue of eudialyte. Dokl. Earth Sci. 2006, 409, 985–989. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Mikhailova, J.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal Chemistry of the Eudialyte Group Minerals from the Lovozero Eudialyte Complex, Kola Peninsula, Russia. Magmat. Earth Relat. Strateg. Met. Depos. 2019, 36, 221–223. [Google Scholar]
- Nivin, V. Free hydrogen-hydrocarbon gases from the Lovozero loparite deposit (Kola Peninsula, NW Russia). Appl. Geochem. 2016, 74, 44–55. [Google Scholar] [CrossRef]
- Nivin, V. Variations in the composition and origin of hydrocarbon gases from inclusions in minerals of the Khibiny and Lovozero plutons, Kola Peninsula, Russia. Geol. Ore Depos. 2011, 53, 699–707. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Williams, C.T.; Woolley, A.R. Compositional evolution and cryptic variation in pyroxenes of the peralkaline Lovozero intrusion, Kola Peninsula, Russia. Mineral. Mag. 2006, 70, 347–359. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Korovushkin, V.V.; Perfiliev, Y.D.; Cherepanov, V.M. Location, valence states, and oxidation mechanisms of iron in eudialyte-group minerals from Mössbauer spectroscopy. Phys. Chem. Mineral. 2010, 37, 543–554. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Ivanyuk, G.Y.; Kalashnikov, A.O.; Pakhomovsky, Y.A.; Bazai, A.; Yakovenchuk, V.N. Petrogenesis of the Eudialyte Complex of the Lovozero Alkaline Massif (Kola Peninsula, Russia). Minerals 2019, 9, 581. [Google Scholar] [CrossRef]
- Williams, C.T.; Kogarko, L.N.; Woolley, A.R. Chemical evolution and petrogenetic implications of loparite in the layered, agpaitic Lovozero complex, Kola Peninsula, Russia. Mineral. Pet. 2002, 74, 1–24. [Google Scholar] [CrossRef]
- Kogarko, L.N. Problems of the Genesis of the Agpaitic Magmas; Nauka: Moscow, Russia, 1977. (In Russian) [Google Scholar]
- Jones, A.P.; Peckett, A. Zirconium-bearing aegirines from Motzfeldt, South Greenland. Contrib. Mineral. Pet. 1981, 75, 251–255. [Google Scholar] [CrossRef]
- Larsen, L.M. Clinopyroxenes and Coexisting Mafic Minerals from the Alkaline Ilimaussaq Intrusion, South Greenland. J. Pet. 1976, 17, 258–290. [Google Scholar] [CrossRef]
- Marks, M.; Halama, R.; Wenzel, T.; Markl, G. Trace element variations in clinopyroxene and amphibole from alkaline to peralkaline syenites and granites: Implications for mineral–melt trace-element partitioning. Chem. Geol. 2004, 211, 185–215. [Google Scholar] [CrossRef]
- Johnsen, O.; Gault, R.A. Chemical variation in eudialyte. Neues Jahrb. Mineral. 1997, 171, 215–237. [Google Scholar]
- Schilling, J.; Wu, F.-Y.; McCammon, C.; Wenzel, T.; Marks, M.A.W.; Pfaff, K.; Jacob, D.E.; Markl, G. The compositional variability of eudialyte-group minerals. Mineral. Mag. 2011, 75, 87–115. [Google Scholar] [CrossRef]
- Pfaff, K.; Krumrei, T.; Marks, M.; Wenzel, T.; Rudolf, T.; Markl, G. Chemical and physical evolution of the ‘lower layered sequence’ from the nepheline syenitic Ilímaussaq intrusion, South Greenland: Implications for the origin of magmatic layering in peralkaline felsic liquids. Lithos 2008, 106, 280–296. [Google Scholar] [CrossRef]
- Mann, U.; Marks, M.; Markl, G. Influence of oxygen fugacity on mineral compositions in peralkaline melts: The Katzenbuckel volcano, Southwest Germany. Lithos 2006, 91, 262–285. [Google Scholar] [CrossRef]
- Marks, M.; Markl, G. Fractionation and Assimilation Processes in the Alkaline Augite Syenite Unit of the Ilímaussaq Intrusion, South Greenland, as Deduced from Phase Equilibria. J. Pet. 2001, 42, 1947–1969. [Google Scholar] [CrossRef]
- Korobeinikov, A.N.; Laajoki, K. Petrological aspects of the evolution of clinopyroxene composition in the intrusive rocks of the Lovozero alkaline massif. Geochem. Int. 1994, 31, 69–76. [Google Scholar]
- Markl, G.; Marks, M.A.; Frost, B.R. On the Controls of Oxygen Fugacity in the Generation and Crystallization of Peralkaline Melts. J. Pet. 2010, 51, 1831–1847. [Google Scholar] [CrossRef]
- Bailey, D.K.; Schairer, J.F. The System Na2O-Al2O3-Fe2O3-SiO2 at 1 atmosphere, and the petrogenesis of alkaline rocks. J. Petrol. 1966, 7, 114–170. [Google Scholar] [CrossRef]
- Khomyakov, A.P. Mineralogy of Hyperagpaitic Alkaline Rocks; Oxford University Press: Oxford, MS, USA, 1995. [Google Scholar]
- Mikhailova, J.A.; Men’Shikov, Y.P.; Pakhomovskii, Y.A.; Yakovenchuk, V.N.; Ivanyuk, G.Y. Trap formation of the Kola peninsula. Petrology 2011, 19, 87–101. [Google Scholar] [CrossRef]




| Symbol | Mineral Name | Symbol | Mineral Name |
|---|---|---|---|
| Aeg | aegirine | Mc | microcline(-perthite) |
| Aeg-Au | aegirine-augite | Nph | nepheline |
| Ab | albite | Ntr | natrolite |
| Ap | fluorapatite | Pcl | pyrochlore |
| Kap | kapustinite | Pkl | parakeldyshite |
| Lmp | lamprophillite | Pph | pyrophanite |
| Lop | loparite-(Ce) | Sdl | sodalite |
| Ltv | litvinskite | Rbd | rhabdophan-(Ce) |
| Lue | lueshite | Tsn | tisinalite |
| Marf | magnesioarfvedsonite | Ttn | titanite |
| Rock | Lujavrite | Foyaite | Ijolite | Urtite | ||||
|---|---|---|---|---|---|---|---|---|
| Sample | LV-XI-5 | LV-I-7-5 | LV-IV-3-5 1 | LV-IV-3-5 1 | LV-X-3 | LV-X-3 | LV-I-7-1 | LV-X-2 |
| l.s. | d.s. | l.s. | d.s. | |||||
| Nb2O5 | 1.95 | 0.28 | 1.43 | 0.57 | 1.28 | 0.92 | 0.48 | 0.72 |
| SiO2 | 49.18 | 50.78 | 49.47 | 49.97 | 49.68 | 52.26 | 51.45 | 51.09 |
| ZrO2 | 10.78 | 13.37 | 9.83 | 10.42 | 10.93 | 16.13 | 14.62 | 10.32 |
| TiO2 | 0.64 | 0.44 | 0.72 | 0.50 | 0.44 | 0.57 | 0.74 | 0.66 |
| Al2O3 | 0.08 | 0.16 | 0.11 | 0.08 | 0.10 | 0.35 | 0.29 | 0.11 |
| Y2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.27 |
| La2O3 | 1.09 | 0.39 | 0.88 | 0.77 | 0.99 | 0.47 | 0.38 | 0.46 |
| Ce2O3 | 2.35 | 1.19 | 1.92 | 1.54 | 3.01 | 1.42 | 1.11 | 1.67 |
| Pr2O3 | 0.20 | b.d. | 0.07 | 0.18 | 0.52 | 0.25 | b.d. | b.d. |
| Nd2O3 | 0.91 | 0.63 | 0.68 | 0.56 | 0.53 | 0.45 | 0.42 | 0.74 |
| Sm2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| FeO | 0.76 | 2.35 | 0.70 | 0.71 | 0.54 | 1.05 | 0.66 | 0.71 |
| MnO | 4.50 | 3.30 | 3.84 | 3.46 | 3.55 | 2.62 | 2.08 | 2.07 |
| MgO | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| CaO | 7.90 | 5.94 | 7.97 | 8.43 | 8.41 | 6.71 | 7.64 | 9.70 |
| SrO | 2.48 | 1.41 | 3.03 | 2.51 | 1.31 | 0.66 | 1.28 | 2.31 |
| BaO | 0.68 | b.d. | 0.74 | 0.83 | b.d. | b.d. | b.d. | b.d. |
| Na2O | 14.03 | 15.23 | 13.31 | 15.05 | 15.33 | 14.69 | 17.42 | 15.89 |
| K2O | 0.25 | 0.14 | 0.21 | 0.15 | 0.19 | 0.19 | 0.10 | 0.14 |
| Cl | 0.43 | 0.36 | 0.24 | 0.24 | 0.37 | 0.34 | 0.56 | 0.11 |
| SO3 | 0.71 | 0.30 | 0.69 | 0.51 | 1.02 | 0.76 | b.d. | 0.98 |
| O=Cl | 0.10 | 0.08 | 0.05 | 0.05 | 0.08 | 0.08 | 0.13 | 0.03 |
| sum | 98.82 | 96.15 | 95.80 | 96.40 | 98.08 | 99.79 | 99.10 | 97.93 |
| Formula based on Σ(Si + Al + Zr + Ti + Hf + Nb + Ta + W) normalized to 29 apfu | ||||||||
| Nb | 0.46 | 0.06 | 0.34 | 0.13 | 0.30 | 0.20 | 0.11 | 0.17 |
| Si | 25.51 | 25.42 | 25.81 | 25.98 | 25.71 | 24.69 | 24.99 | 25.96 |
| Zr | 2.73 | 3.26 | 2.50 | 2.64 | 2.76 | 3.72 | 3.46 | 2.56 |
| Ti | 0.25 | 0.16 | 0.28 | 0.20 | 0.17 | 0.20 | 0.27 | 0.25 |
| Al | 0.05 | 0.09 | 0.07 | 0.05 | 0.06 | 0.20 | 0.17 | 0.07 |
| Y | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 0.07 |
| La | 0.21 | 0.07 | 0.17 | 0.15 | 0.19 | 0.08 | 0.07 | 0.09 |
| Ce | 0.45 | 0.22 | 0.37 | 0.29 | 0.57 | 0.25 | 0.20 | 0.31 |
| Pr | 0.04 | ‒ | 0.01 | 0.03 | 0.10 | 0.04 | ‒ | ‒ |
| Nd | 0.17 | 0.11 | 0.13 | 0.10 | 0.10 | 0.08 | 0.07 | 0.13 |
| Fe2+ | 0.33 | 0.98 | 0.31 | 0.31 | 0.23 | 0.42 | 0.27 | 0.30 |
| Mn | 1.98 | 1.40 | 1.70 | 1.52 | 1.55 | 1.05 | 0.86 | 0.89 |
| Ca | 4.39 | 3.18 | 4.46 | 4.69 | 4.66 | 3.40 | 3.98 | 5.28 |
| Sr | 0.75 | 0.41 | 0.92 | 0.76 | 0.39 | 0.18 | 0.36 | 0.68 |
| Ba | 0.14 | ‒ | 0.15 | 0.17 | ‒ | ‒ | ‒ | ‒ |
| Na | 14.11 | 14.78 | 13.46 | 15.18 | 15.38 | 13.46 | 16.41 | 15.65 |
| K | 0.16 | 0.09 | 0.14 | 0.10 | 0.12 | 0.11 | 0.06 | 0.09 |
| Cl | 0.38 | 0.30 | 0.21 | 0.21 | 0.32 | 0.27 | 0.46 | 0.10 |
| S | 0.28 | 0.11 | 0.27 | 0.20 | 0.39 | 0.27 | ‒ | 0.37 |
| Eudialyte Complex | Met. vol.-sed. Rocks | Poik. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rock | Lujavrite | Foyaite | Ijolite | Porph. | |||||||
| Sample | LV-117-121 | LV-160-42 | LV-222-437 | LV-153-186 | LV-222-151 1 | LV-157-137 | LV-44-168 | LV-222-448 | |||
| core | rim | core | rim | core | rim | ||||||
| Nb2O5 | 0.60 | 0.81 | 0.91 | 0.59 | 0.69 | 0.38 | 0.40 | 0.67 | 1.79 | 0.77 | 0.92 |
| SiO2 | 49.66 | 49.83 | 52.16 | 50.62 | 49.09 | 51.31 | 50.15 | 51.43 | 49.35 | 53.50 | 48.73 |
| ZrO2 | 13.53 | 12.00 | 12.33 | 15.74 | 14.73 | 12.66 | 13.16 | 12.55 | 10.40 | 11.46 | 12.47 |
| TiO2 | 0.62 | 0.53 | 0.41 | 0.66 | 0.69 | 0.46 | 0.47 | 0.54 | 0.40 | 0.42 | 0.66 |
| Al2O3 | 0.22 | 0.10 | 0.12 | 0.24 | 0.33 | 0.17 | 0.15 | 0.22 | 0.21 | 0.24 | 0.21 |
| Y2O3 | b.d. | b.d. | 0.62 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| La2O3 | 0.32 | 0.57 | 0.43 | 0.27 | 0.19 | 0.20 | 0.27 | 0.23 | 0.13 | 0.16 | 0.30 |
| Ce2O3 | 0.62 | 1.06 | 0.88 | 0.67 | 0.53 | 0.69 | 0.81 | 0.75 | 0.67 | 0.40 | 0.94 |
| Pr2O3 | 0.25 | 0.28 | 0.15 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| Nd2O3 | 0.29 | 0.39 | 0.43 | 0.27 | 0.26 | 0.33 | 0.27 | 0.32 | 0.15 | 0.06 | 0.34 |
| Sm2O3 | b.d. | b.d. | 0.15 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| FeO | 3.33 | 3.33 | 1.94 | 3.54 | 1.86 | 3.10 | 2.40 | 2.89 | 2.26 | 2.95 | 2.38 |
| MnO | 2.23 | 2.58 | 3.07 | 1.94 | 2.33 | 2.30 | 2.39 | 1.68 | 2.04 | 1.49 | 2.79 |
| MgO | 0.08 | 0.07 | 0.08 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.07 | 0.04 | b.d. |
| CaO | 7.00 | 7.60 | 7.87 | 5.62 | 5.73 | 8.58 | 8.40 | 8.13 | 9.86 | 10.03 | 7.34 |
| SrO | 1.69 | 1.98 | 2.51 | 0.89 | 1.22 | 0.98 | 1.17 | 2.25 | 2.34 | 1.71 | 1.36 |
| BaO | b.d. | b.d. | 0.47 | 0.11 | 0.16 | 0.10 | 0.24 | 0.69 | 0.38 | 0.77 | b.d. |
| Na2O | 16.15 | 15.78 | 8.29 | 14.90 | 15.67 | 14.89 | 15.47 | 13.52 | 10.87 | 10.26 | 14.90 |
| K2O | 0.31 | 0.29 | 0.21 | 0.24 | 0.31 | 0.30 | 0.23 | 0.25 | 0.18 | 0.24 | 0.29 |
| Cl | 1.34 | 1.48 | 1.43 | 0.82 | 0.97 | 0.85 | 1.23 | 1.54 | 1.32 | 1.52 | 1.10 |
| SO3 | b.d. | b.d. | 0.12 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| O=Cl | 0.30 | 0.33 | 0.32 | 0.18 | 0.22 | 0.19 | 0.28 | 0.35 | 0.30 | 0.34 | 0.25 |
| sum | 97.96 | 98.33 | 94.24 | 96.95 | 94.53 | 97.08 | 96.93 | 97.30 | 92.13 | 95.65 | 94.44 |
| Formula based on Σ(Si + Al + Zr + Ti + Hf + Nb + Ta + W) normalized to 29 apfu | |||||||||||
| Nb | 0.14 | 0.19 | 0.20 | 0.13 | 0.16 | 0.09 | 0.09 | 0.15 | 0.42 | 0.17 | 0.22 |
| Si | 25.15 | 25.55 | 25.63 | 24.74 | 24.77 | 25.57 | 25.39 | 25.49 | 25.66 | 25.85 | 25.25 |
| Zr | 3.34 | 3.00 | 2.95 | 3.75 | 3.62 | 3.08 | 3.25 | 3.03 | 2.64 | 2.70 | 3.15 |
| Ti | 0.24 | 0.20 | 0.15 | 0.24 | 0.26 | 0.17 | 0.18 | 0.20 | 0.16 | 0.15 | 0.26 |
| Al | 0.13 | 0.06 | 0.07 | 0.14 | 0.19 | 0.10 | 0.09 | 0.13 | 0.13 | 0.13 | 0.13 |
| Y | ‒ | ‒ | 0.16 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| La | 0.06 | 0.11 | 0.08 | 0.05 | 0.03 | 0.04 | 0.05 | 0.04 | 0.03 | 0.03 | 0.06 |
| Ce | 0.11 | 0.20 | 0.16 | 0.12 | 0.10 | 0.13 | 0.15 | 0.14 | 0.13 | 0.07 | 0.18 |
| Pr | 0.05 | 0.05 | 0.03 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Nd | 0.05 | 0.07 | 0.07 | 0.05 | 0.05 | 0.06 | 0.05 | 0.06 | 0.03 | 0.01 | 0.06 |
| Sm | ‒ | ‒ | 0.03 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Fe2+ | 1.41 | 1.43 | 0.80 | 1.45 | 0.78 | 1.29 | 1.02 | 1.20 | 0.98 | 1.19 | 1.03 |
| Mn | 0.96 | 1.12 | 1.28 | 0.80 | 1.00 | 0.97 | 1.03 | 0.70 | 0.90 | 0.61 | 1.22 |
| Mg | 0.06 | 0.05 | 0.05 | ‒ | ‒ | ‒ | ‒ | ‒ | 0.06 | 0.03 | ‒ |
| Ca | 3.80 | 4.17 | 4.14 | 2.94 | 3.10 | 4.58 | 4.56 | 4.32 | 5.49 | 5.19 | 4.07 |
| Sr | 0.50 | 0.59 | 0.71 | 0.25 | 0.36 | 0.28 | 0.34 | 0.65 | 0.71 | 0.48 | 0.41 |
| Ba | ‒ | ‒ | 0.09 | 0.02 | 0.03 | 0.02 | 0.05 | 0.13 | 0.08 | 0.15 | ‒ |
| Na | 15.86 | 15.69 | 7.90 | 14.11 | 15.33 | 14.38 | 15.18 | 13.00 | 10.96 | 9.61 | 14.97 |
| K | 0.20 | 0.19 | 0.13 | 0.15 | 0.20 | 0.19 | 0.15 | 0.16 | 0.12 | 0.14 | 0.19 |
| Cl | 1.15 | 1.29 | 1.19 | 0.68 | 0.83 | 0.71 | 1.05 | 1.29 | 1.16 | 1.24 | 0.96 |
| S | ‒ | ‒ | 0.04 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Comp. | wt.% | Mol. Weight | Mol. Amount | Atomic Amount | Formula Based on | ||
|---|---|---|---|---|---|---|---|
| Cation | Anion | Si = 25.40 | (Si + Zr + Ti + Nb + Al + Hf + W + Ta) = 29 | ||||
| Na2O | 15.97 | 61.98 | 0.2577 | 0.5153 | 0.2577 | Na16.02 | Na15.62 |
| K2O | 0.48 | 94.20 | 0.0051 | 0.0102 | 0.0051 | K0.32 | K0.31 |
| CaO | 5.64 | 56.08 | 0.1006 | 0.1006 | 0.1006 | Ca3.13 | Ca3.05 |
| SrO | 0.69 | 103.62 | 0.0067 | 0.0067 | 0.0067 | Sr0.21 | Sr0.20 |
| MgO | 0.28 | 40.30 | 0.0069 | 0.0069 | 0.0069 | Mg0.22 | Mg0.21 |
| MnO | 2.01 | 70.94 | 0.0283 | 0.0283 | 0.0283 | Mn0.88 | Mn0.85 |
| FeO | 5.02 | 71.85 | 0.0699 | 0.0699 | 0.0699 | Fe2.17 | Fe2.10 |
| Al2O3 | 0.26 | 101.96 | 0.0026 | 0.0051 | 0.0078 | Al0.16 | Al0.15 |
| La2O3 | 0.44 | 325.81 | 0.0014 | 0.0027 | 0.0042 | La0.08 | La0.08 |
| Ce2O3 | 0.87 | 328.23 | 0.0027 | 0.0053 | 0.0081 | Ce0.16 | Ce0.16 |
| Nd2O3 | 0.42 | 336.48 | 0.0012 | 0.0025 | 0.0036 | Nd0.08 | Nd0.08 |
| SiO2 | 49.10 | 60.09 | 0.8171 | 0.8171 | 1.6342 | Si25.40 | Si24.77 |
| TiO2 | 0.37 | 79.86 | 0.0046 | 0.0046 | 0.0092 | Ti0.14 | Ti0.14 |
| ZrO2 | 15.07 | 123.22 | 0.1223 | 0.1223 | 0.2446 | Zr3.80 | Zr3.71 |
| HfO2 | 0.43 | 210.49 | 0.0020 | 0.0020 | 0.0040 | Hf0.06 | Hf0.06 |
| Nb2O5 | 0.71 | 265.81 | 0.0027 | 0.0053 | 0.0135 | Nb0.17 | Nb0.16 |
| Cl | 1.34 | 35.45 | 0.0378 | 0.0378 | Cl1.18 | Cl1.14 | |
| H2O | 1.35 | 0.0749 | 0.1499 | 0.0749 | H4.66 | H4.54 | |
| –O = (F,Cl)2 | −0.30 | ||||||
| sum | 100.15 | ||||||
| Sample | LV-28-261 | LV-31-181 | LV-228-176 | LV-228-176 | LV-33-32 | LV-33-32 | LV-VI-3 |
|---|---|---|---|---|---|---|---|
| Mineral | Ltv | Ltv | Ltv | Tsn | Kap | Kap | Kap |
| Nb2O5 | 0.19 | 0.88 | b.d. | 0.37 | 0.51 | 0.23 | 0.26 |
| SiO2 | 58.44 | 58.61 | 58.56 | 57.88 | 50.34 | 51.00 | 49.58 |
| TiO2 | 0.83 | 0.63 | 0.72 | 3.95 | 0.49 | 0.37 | 0.45 |
| ZrO2 | 12.99 | 13.36 | 12.75 | 5.83 | 14.35 | 13.51 | 13.52 |
| Fe2O3 | 0.68 | 0.72 | 1.03 | 2.57 | 0.05 | 0.21 | 0.17 |
| Al2O3 | b.d. | b.d. | b.d. | b.d. | 0.55 | 0.08 | b.d. |
| La2O3 | b.d. | 0.12 | b.d. | b.d. | 0.15 | b.d. | 0.34 |
| Ce2O3 | 0.49 | 0.58 | 0.31 | b.d. | 0.60 | 0.32 | 0.88 |
| Nd2O3 | 0.35 | 0.36 | 0.23 | b.d. | 0.33 | 0.24 | 0.28 |
| MnO | 3.73 | 2.29 | 3.39 | 4.75 | 0.70 | 1.50 | 1.10 |
| CaO | 1.10 | 1.15 | 1.46 | 1.27 | 0.26 | 0.47 | 0.37 |
| SrO | b.d. | 1.15 | 0.23 | b.d. | b.d. | b.d. | b.d. |
| Na2O | 10.50 | 8.09 | 12.17 | 15.70 | 26.65 | 27.99 | 28.80 |
| K2O | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.06 |
| Sum | 89.29 | 87.94 | 90.85 | 92.32 | 95.00 | 95.91 | 95.80 |
| Formula based on Si6(O,OH)18, apfu | |||||||
| Si | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Nb | 0.01 | 0.04 | ‒ | 0.02 | 0.03 | 0.01 | 0.01 |
| Ti | 0.06 | 0.05 | 0.06 | 0.31 | 0.04 | 0.03 | 0.04 |
| Zr | 0.65 | 0.67 | 0.64 | 0.3 | 0.83 | 0.78 | 0.8 |
| Fe3+ | 0.05 | 0.05 | 0.08 | 0.2 | 0.01 | 0.02 | 0.01 |
| Al | ‒ | ‒ | ‒ | ‒ | 0.08 | 0.01 | ‒ |
| La | ‒ | ‒ | ‒ | ‒ | 0.01 | ‒ | 0.02 |
| Ce | 0.02 | 0.02 | 0.01 | ‒ | 0.03 | 0.01 | 0.04 |
| Nd | 0.01 | 0.01 | 0.01 | ‒ | 0.01 | 0.01 | 0.01 |
| Mn | 0.32 | 0.2 | 0.29 | 0.42 | 0.07 | 0.15 | 0.11 |
| Ca | 0.12 | 0.13 | 0.16 | 0.14 | 0.03 | 0.06 | 0.05 |
| Sr | ‒ | 0.07 | 0.01 | ‒ | ‒ | ‒ | ‒ |
| Na | 2.09 | 1.61 | 2.42 | 3.16 | 6.16 | 6.38 | 6.76 |
| K | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 0.01 |
| OH | 5.87 | 6.26 | 5.58 | 4.63 | 1.6 | 1.75 | 1.24 |
| O | 12.13 | 11.74 | 12.42 | 13.37 | 16.4 | 16.25 | 16.76 |
| H2O | 0.73 | 0.99 | 0.34 | 0.34 | 1.19 | 0.73 | 1.08 |
| Rock | Lujavrite | Foyaite | Ijolite | Urtite | ||||
|---|---|---|---|---|---|---|---|---|
| Sample | LV-III-5-2 | LV-XI-1 | LV-309 | LV-IV-1-5 | LV-IV-3-2 | LV-IV-1-2 | LV-327-6 | LV-IV-2-3 |
| SiO2 | 52.17 | 52.62 | 52.09 | 50.78 | 52.84 | 51.52 | 52.48 | 52.14 |
| TiO2 | 2.31 | 1.93 | 2.45 | 1.94 | 1.84 | 1.85 | 4.47 | 2.02 |
| ZrO2 | 0.57 | 0.65 | 0.74 | 0.52 | 0.75 | 0.79 | 0.58 | 0.57 |
| Al2O3 | 0.99 | 0.93 | 0.87 | 0.80 | 0.92 | 0.82 | 0.75 | 1.09 |
| CaO | 5.15 | 6.62 | 3.04 | 2.24 | 4.46 | 5.55 | 2.05 | 3.57 |
| MgO | 2.88 | 3.06 | 1.87 | 1.56 | 2.48 | 2.29 | 1.28 | 2.23 |
| FeO | 23.01 | 21.78 | 24.88 | 24.63 | 23.08 | 23.86 | 23.92 | 23.42 |
| MnO | 0.60 | 0.54 | 0.51 | 0.43 | 0.40 | 0.48 | 0.48 | 0.45 |
| Na2O | 11.17 | 11.82 | 11.97 | 11.89 | 11.47 | 11.02 | 13.55 | 12.47 |
| sum | 98.84 | 99.96 | 98.41 | 94.78 | 98.23 | 98.18 | 99.56 | 97.96 |
| Formula based on 4 cations and 6 oxygen atoms, apfu | ||||||||
| Si | 1.96 | 1.94 | 1.97 | 1.99 | 1.99 | 1.96 | 1.95 | 1.96 |
| Ti | 0.07 | 0.05 | 0.07 | 0.06 | 0.05 | 0.05 | 0.12 | 0.06 |
| Zr | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Al | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.03 | 0.05 |
| Ca | 0.21 | 0.26 | 0.12 | 0.09 | 0.18 | 0.23 | 0.08 | 0.14 |
| Mg | 0.16 | 0.17 | 0.11 | 0.09 | 0.14 | 0.13 | 0.07 | 0.12 |
| Fe2+ | 0.02 | ‒ | 0.05 | 0.05 | 0.05 | 0.03 | ‒ | ‒ |
| Fe3+ | 0.70 | 0.67 | 0.73 | 0.76 | 0.68 | 0.73 | 0.74 | 0.74 |
| Mn | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 |
| Na | 0.81 | 0.84 | 0.88 | 0.90 | 0.84 | 0.81 | 0.97 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, J.A.; Pakhomovsky, Y.A.; Panikorovskii, T.L.; Bazai, A.V.; Yakovenchuk, V.N. Eudialyte Group Minerals from the Lovozero Alkaline Massif, Russia: Occurrence, Chemical Composition, and Petrogenetic Significance. Minerals 2020, 10, 1070. https://doi.org/10.3390/min10121070
Mikhailova JA, Pakhomovsky YA, Panikorovskii TL, Bazai AV, Yakovenchuk VN. Eudialyte Group Minerals from the Lovozero Alkaline Massif, Russia: Occurrence, Chemical Composition, and Petrogenetic Significance. Minerals. 2020; 10(12):1070. https://doi.org/10.3390/min10121070
Chicago/Turabian StyleMikhailova, Julia A., Yakov A. Pakhomovsky, Taras L. Panikorovskii, Ayya V. Bazai, and Victor N. Yakovenchuk. 2020. "Eudialyte Group Minerals from the Lovozero Alkaline Massif, Russia: Occurrence, Chemical Composition, and Petrogenetic Significance" Minerals 10, no. 12: 1070. https://doi.org/10.3390/min10121070
APA StyleMikhailova, J. A., Pakhomovsky, Y. A., Panikorovskii, T. L., Bazai, A. V., & Yakovenchuk, V. N. (2020). Eudialyte Group Minerals from the Lovozero Alkaline Massif, Russia: Occurrence, Chemical Composition, and Petrogenetic Significance. Minerals, 10(12), 1070. https://doi.org/10.3390/min10121070






