5. Discussion
When a database consisting of 487 samples of the EGM from rocks of the Lovozero massif was formed, the problem of calculating the EGM formulas appeared. It turned out that the calculation method based on Si + Al + Ti + Zr + Nb + Hf + W + Ta = 29 [
34] is not always suitable for the EGM from the Lovozero massif. In 30% of the analyses, the amount of zirconium does not exceed 3 Zr pfu, but, in the remaining 70%, there is excess zirconium (
Table 2 and
Table 3), which is included in the
M(2B) site [
35,
47,
48]. The basis Si + Al + Ti + Zr + Nb + Hf + W + Ta = 29 does not provide for the possibility of zirconium entering the
M(2) position and cannot be used for the hyperzirconium samples. The apfu values obtained by this calculation method can differ significantly from the apfu calculated based on structural data (
Table 3). Other methods for calculating the EGM formula are not suitable for large number of samples, since they require solving the crystal structure or other additional studies [
35]. The rocks of the Lovozero massif were formed under narrow range of physical and chemical conditions, and even minor changes in the chemical composition of rock-forming minerals are important for establishing their genesis. That is why the best approach to studying the evolution of the Lovozero massif using indicator minerals is a statistical analysis of a large number of data [
51,
54].
The coefficients in the formula of any mineral are normalized values of atomic amounts of elements. To trace the patterns of changes in the composition of a mineral, e.g., the eudialyte group, it is possible to analyze changes in the ratios of atomic amounts. We suggest that this approach can be used for other minerals of complex composition.
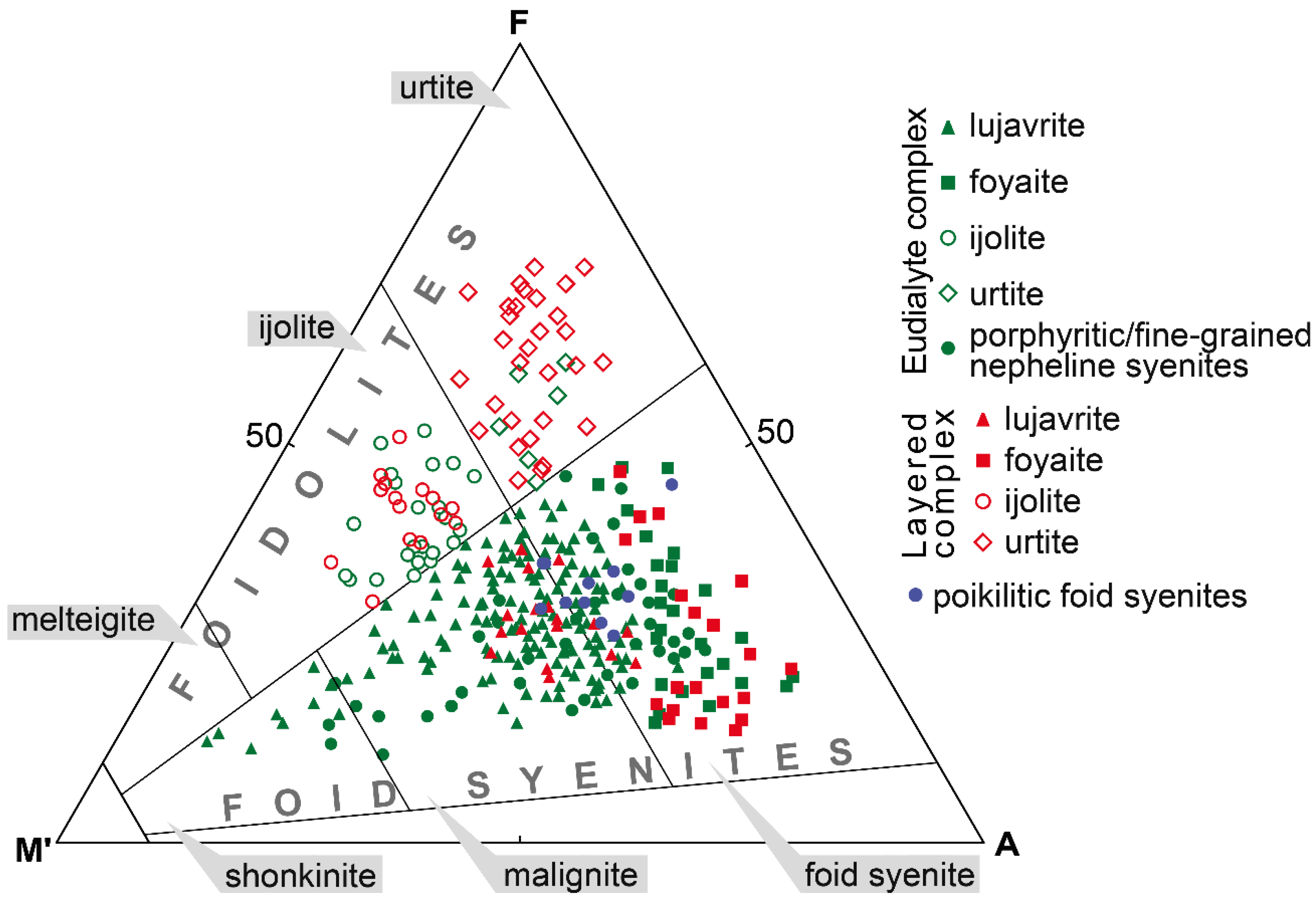
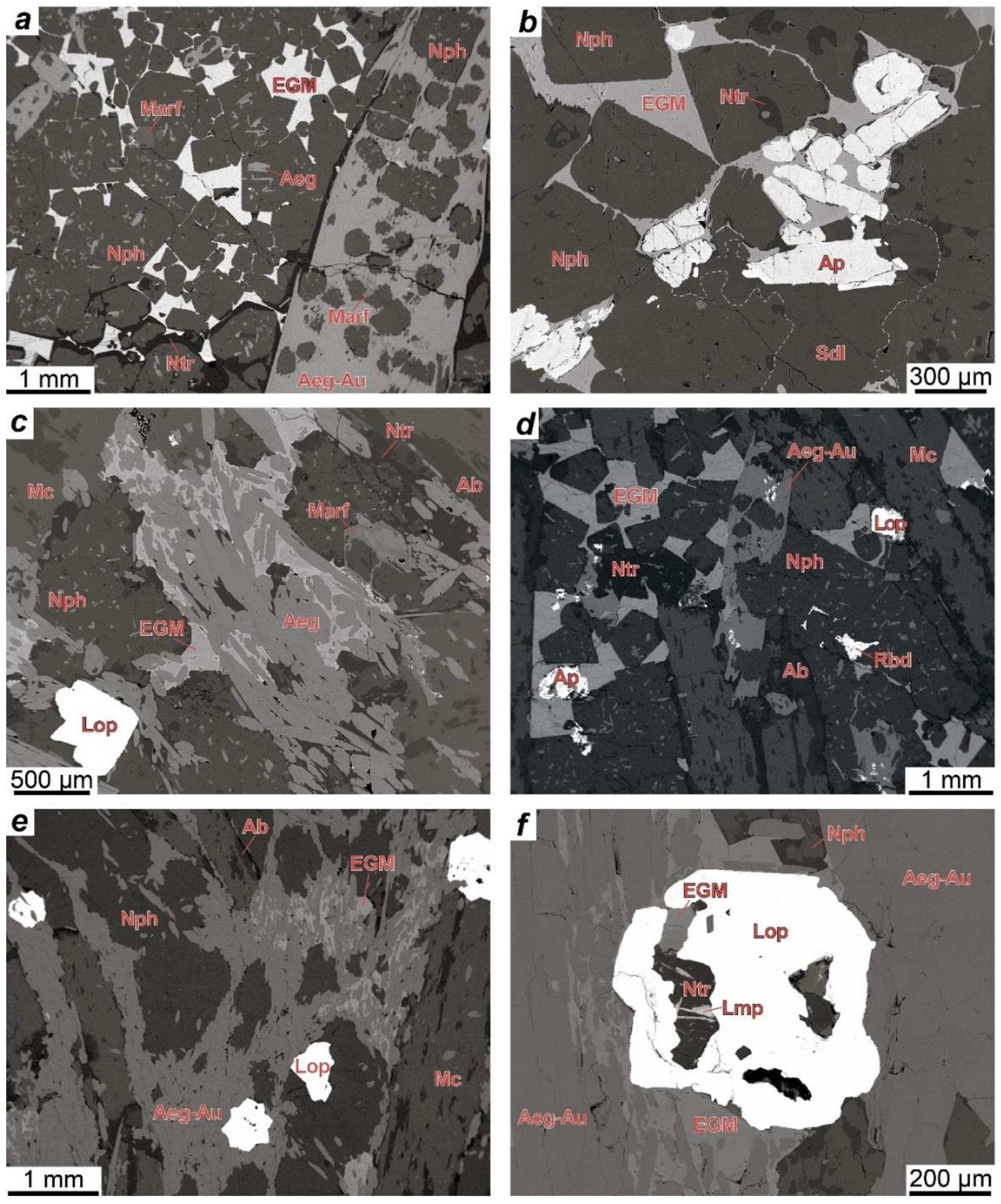
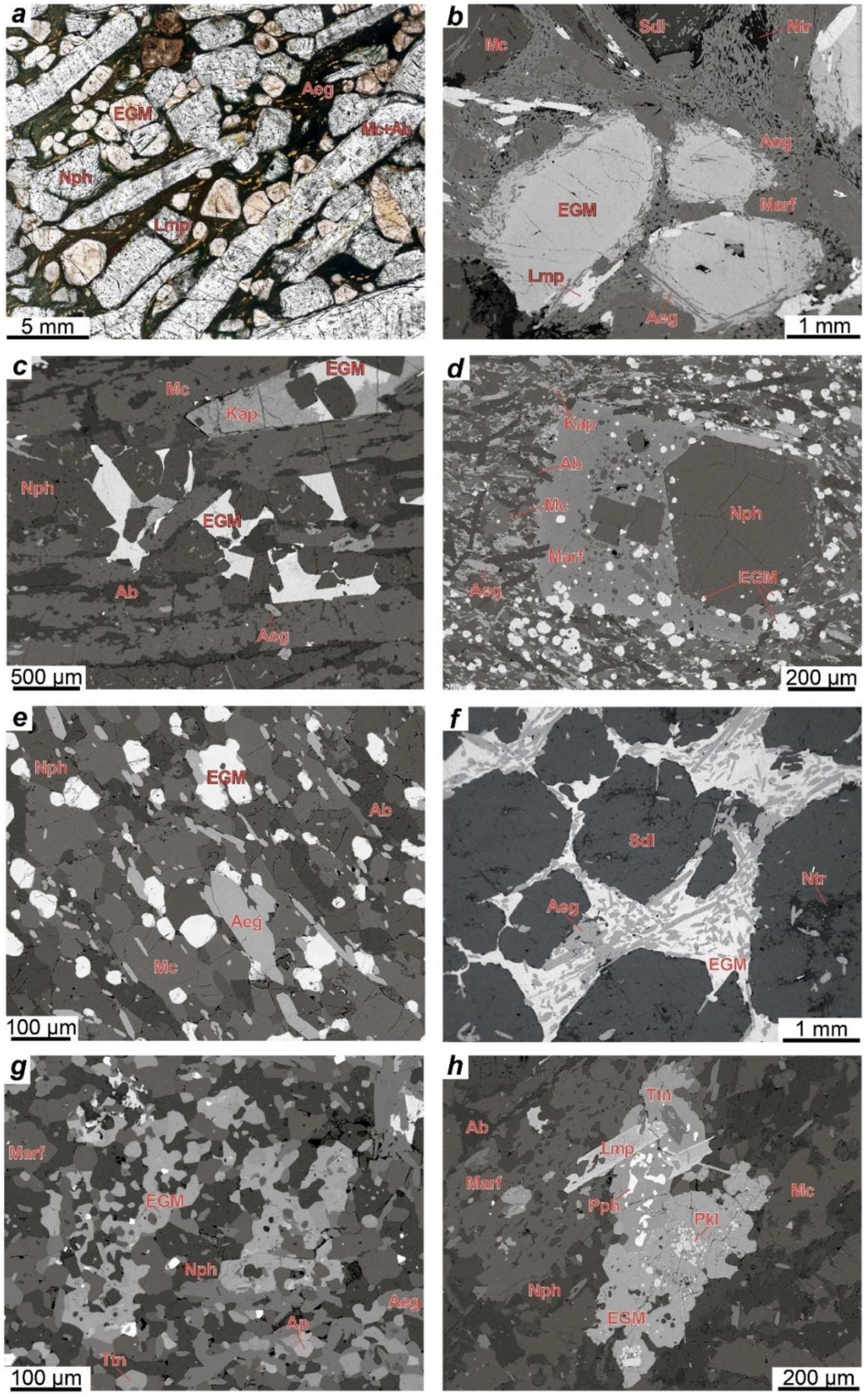
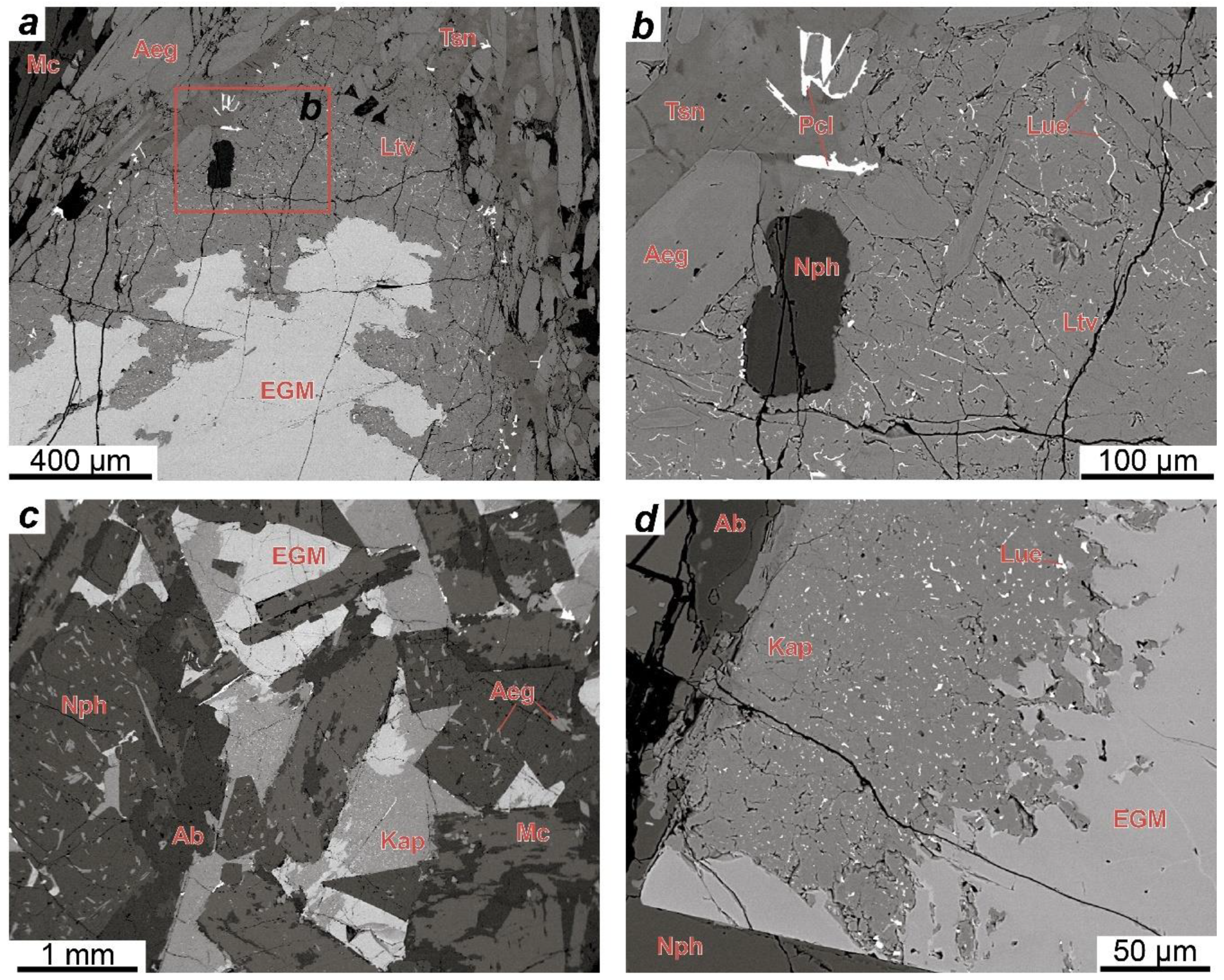
The EGM are a characteristic accessory and sometimes rock-forming mineral in most types of rocks of the Lovozero alkaline massif. Petrographic data indicate that the EGM in lujavrite of the Layered complex crystallized in the late magmatic stage after alkaline clinopyroxenes and amphiboles and filled the interstices in the aggregate of their prismatic crystals (
Figure 3e,f). It is important to note that Kogarko [
55] proposed a gradual transition from melt to hydrothermal fluid in agpaitic systems. Therefore, in the studied rocks, it is impossible to accurately separate the late magmatic and hydrothermal mineral associations.
In foyaite and urtite, the content of mafic minerals decreases, clinopyroxenes, and amphiboles form large poikilitic crystals (
Figure 3a), and the spatial connection between the EGM and mafic minerals disappears. During the transition from lujavrite to foyaite and urtite, the modal content of the EGM increases [
29]. In foyaite and urtite, the EGM also crystallized at a late magmatic stage in the interstices of rock-forming minerals (
Figure 3a,b,d). For example, in urtite, the EGM crystallized later than fluorapatite (
Figure 3b), which is a late magmatic mineral [
29].
Data on the composition of clinopyroxenes allowed us to additionally confirm petrographic conclusions about the relative crystallization time of the EGM. We suggested that, in rocks of the Layered complex, the eudialyte-group minerals crystallized later than clinopyroxenes because clinopyroxenes contain a significant amount of zirconium [
56]. Following [
56], zirconium is incorporated into the clinopyroxene structure, as an Na(Fe
2+,Mg)
0.5Zr
0.5Si
2O
6 end-member, only when no other zirconium-bearing phase such as eudialyte is formed.
The question about the relative time of the EGM crystallization in meso- and melanocratic coarse-grained rocks (eudialyte lujavrite and ijolite) of the Eudialyte complex is difficult to solve only by petrographic observations. The EGM in these rocks form round or oval grains that are situated in the axial parts of aggregates (“streams”) of mafic minerals (
Figure 4b). The rock-forming aegirine-(augite) in these rocks is also enriched in zirconium [
49,
51], but its content is lower than in the rocks of the Layered complex [
49]. Larsen [
57] suggested that “As long as eudialyte does not precipitate, Zr in the melt (and in the pyroxene) must rise with differentiation, but the onset of crystallization of eudialyte (with 12–15% ZrO
2) in sufficient amounts will probably cause a fall in the Zr-content of both melt and pyroxenes”. However, rocks of the Eudialyte complex are more evolved then rocks of the Layered complex, and contain clinopyroxenes extremely enriched in the aegirine component [
51]. After [
58], Zr abundances are mainly controlled by the major element composition of the host clinopyroxene crystal, which, in turn, determines the crystal site parameters. Thus, the zirconium content in the clinopyroxenes of the Eudialyte complex is decreased due to the crystal-chemical effect. This decrease is apparently not related to the crystallization of EGM.
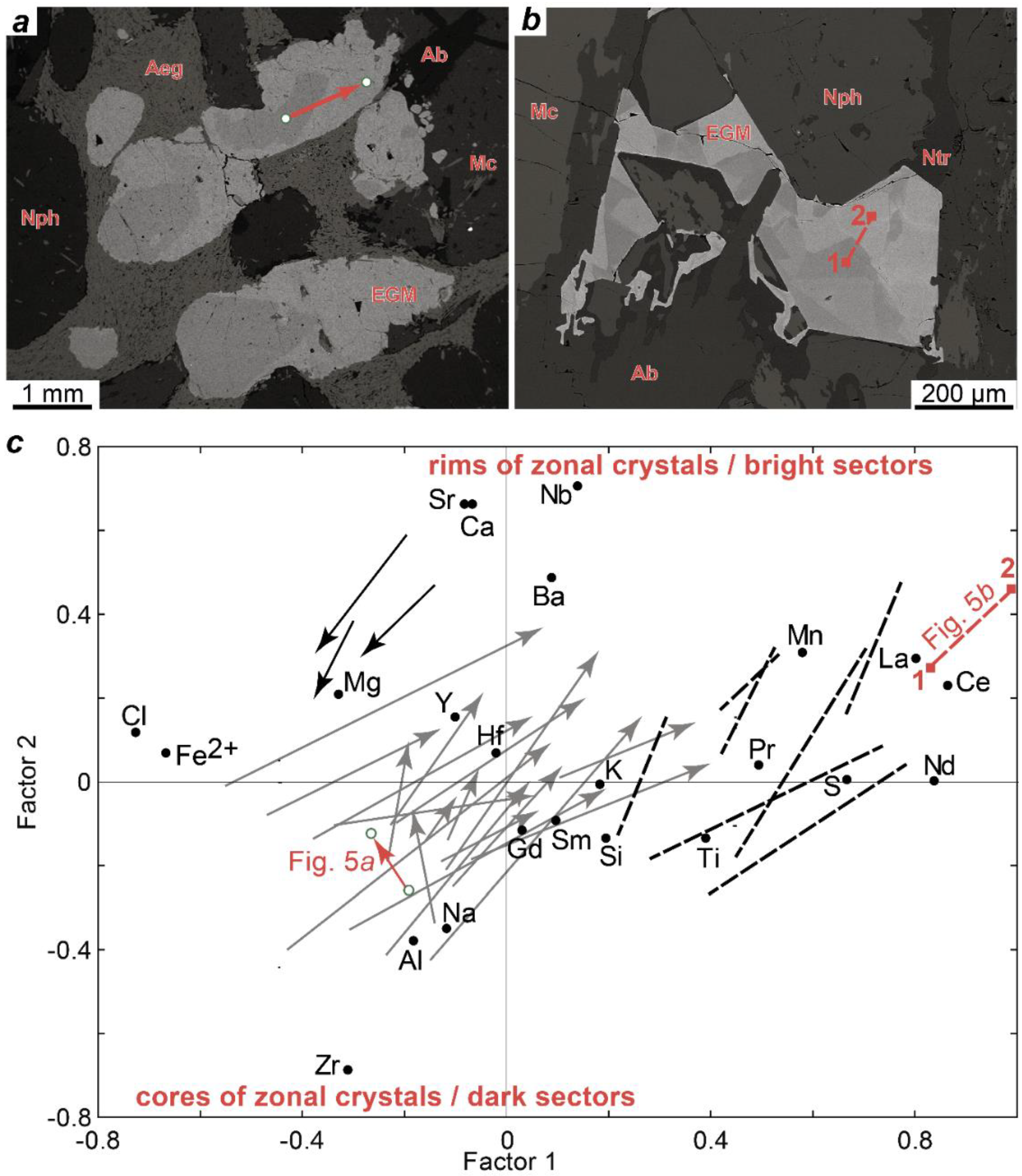
Yet, data on the composition of clinopyroxenes can still help to establish the time of eudialyte crystallization. In the eudialyte lujavrite and ijolite of the Eudialyte complex, the compositions of clinopyroxenes and EGM correlate with each other (
Figure 11), and we assume that this relation is the result of simultaneous crystallization. The crystallization of EGM in these rocks was completed after the formation of clinopyroxenes. In the leucocratic rocks (foyaite and urtite) of the Eudialyte complex, there is no such relationship between the compositions of minerals.
In foyaite and urtite of the Eudialyte complex, the EGM crystallized at a late magmatic stage along with other zirconosilicates (minerals of the lovozerite group) in the interstices of rock-forming minerals. The texture of porphyritic/fine-grained nepheline syenites (
Figure 4d,e) suggests that, in these rocks, the minerals of the eudialyte group crystallized simultaneously with clinopyroxenes. The composition of these rocks corresponds to the phonolitic eutectic, and they are one of the late and low-temperature igneous rocks of the Eudialyte complex [
53]. Thus, in igneous rocks of different composition and texture, the minerals of the eudialyte group crystallized at a late magmatic stage, either immediately after clinopyroxenes (aegirine and aegirine-augite) or almost simultaneously with them.
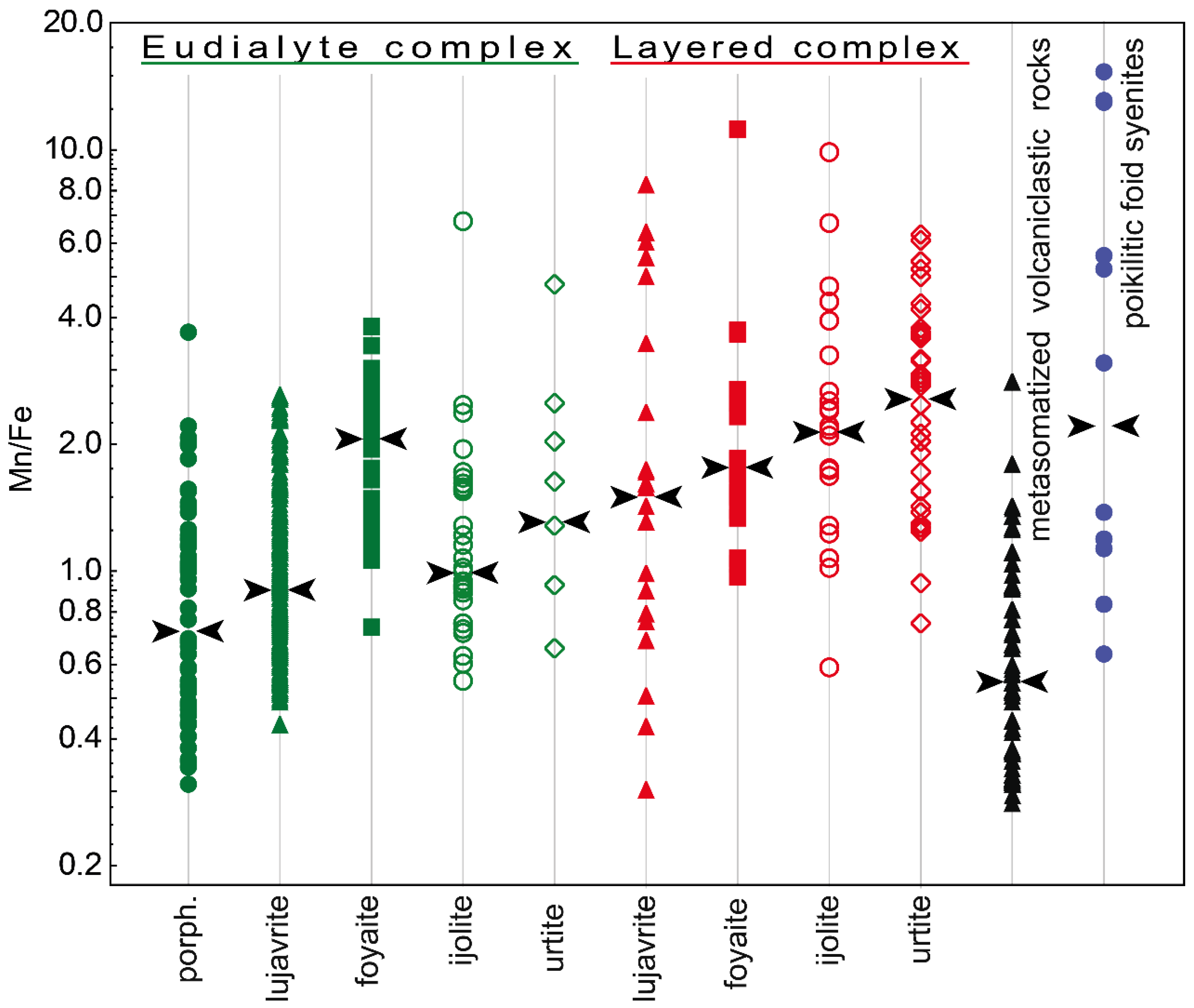
The Mn/Fe ratio of eudialytes has been suggested as a monitor for magmatic evolution by various authors [
13,
59,
60,
61]. In general, the Mn/Fe ratio of both melt and EGM increase with fractionation. This ratio is controlled by (1) the fractionation stage and composition of the coexisting melt, (2) the partition coefficients of Fe and Mn for eudialyte-melt, and (3) co-crystallizing minerals [
57]. The latter point implies that the EGM incorporate elements that are left after the crystallization of simultaneously and previously crystallizing Mn- and Fe-incorporating minerals. The Mn/Fe ratio in the EGM from all types of rocks varies very widely. In general, this ratio increases from lujavrite to urtite (i.e., from mela- and mesocratic to leucocratic rock) within both Eudialyte and Layered complexes. Moreover, the Mn/Fe ratio increases from the Eudialyte complex to the Layered complex (
Figure 7), and this change occurs due to a decrease in the iron content (
Figure 8).
A change in the Mn/Fe ratio from lujavrite to urtite of the Layered complex (
Figure 7) indicates that lujavrite is the earliest rock of each rhythm, and urtite is the more evolved and latest one. The composition of clinopyroxenes can confirm it. Aegirine and aegirine-augite are found in all rocks of the Lovozero massif [
51] and, therefore, are most suitable for tracing the physico-chemical evolution of the Lovozero magmas. The evolution from diopside-rich pyroxene compositions towards aegirine-rich pyroxene is typical of alkaline massifs worldwide [
57]. The major difference between various massifs is the amount of Fe
2+ enrichment relative to Na and Fe
3+ enrichment during their evolution. Three evolutionary paths were documented: (1) from diopside to aegirine without significant Fe
2+ enrichment (e.g., Katzenbuckel, Germany [
62]), (2) from diopside via hedenbergite to aegirine (e.g., Ilímaussaq, Greenland [
57,
63]), and (3) intermediate paths (e.g., Lovozero [
64]).
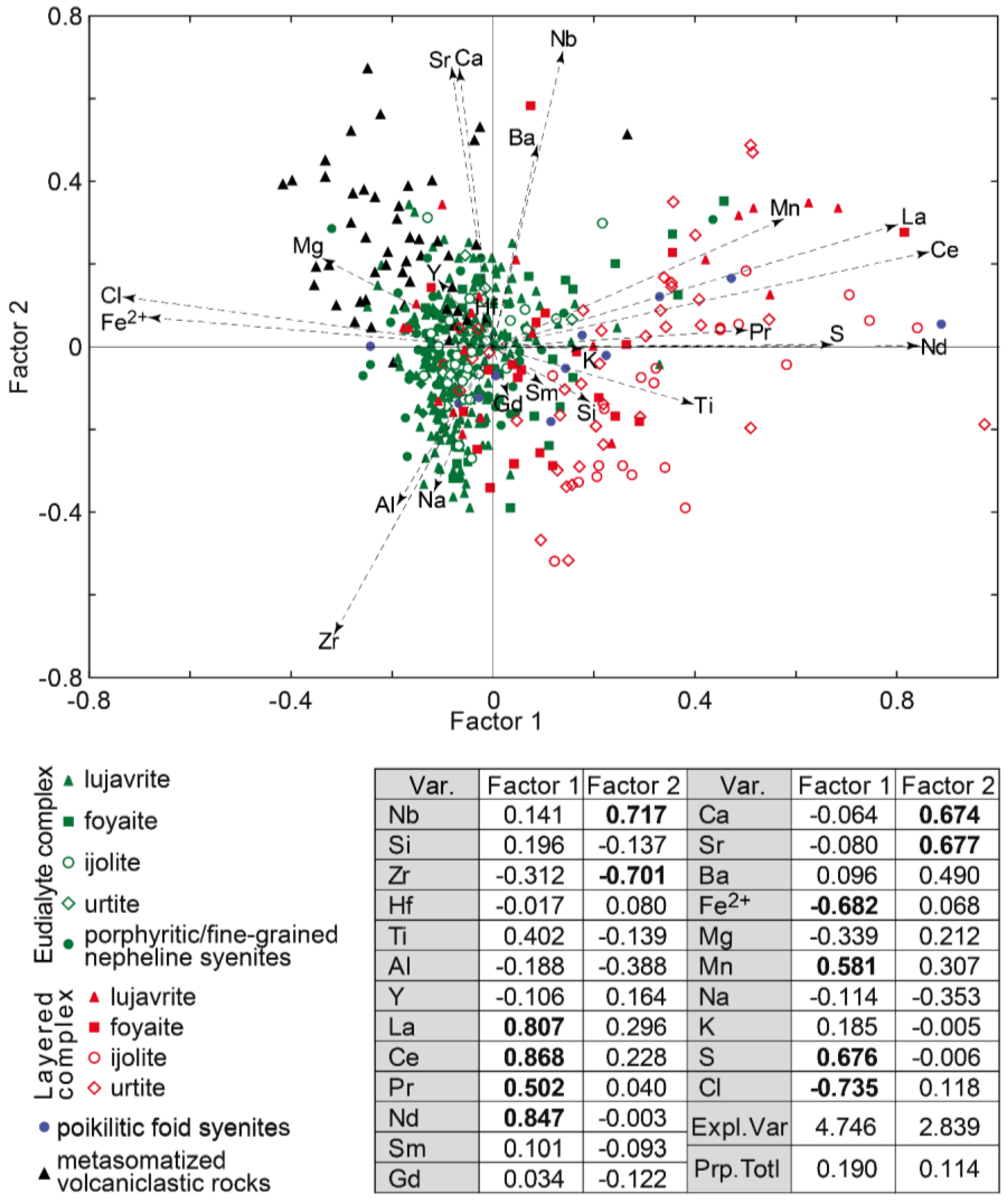
The clinopyroxenes from rocks of the Layered complex differ by the content of Ca, Mg (diopside end-member), and Na, Fe
3+ (aegirine end-member). According to the results of factor analysis (
Figure 10), the higher concentration of Ca, Mg (and Zr) is in clinopyroxenes from lujavrite. The content of sodium and ferric iron is greater in clinopyroxenes from urtite, and foyaite and ijolite are in an intermediate position. Thus, changes in the compositions of clinopyroxenes and EGM indicate that each rhythm of the Layered complex consists of less- (lujavrite) and more-evolved (urtite) rocks.
The Eudialyte complex was formed later than the Layered one. It is confirmed by geological observations [
30], geochemical evidence (extremely high concentration of HFSE and volatile components, high (Na+K)/Al ratio [
29]), as well as a change in the composition of rock-forming minerals. Kogarko and co-authors [
51] described the composition of clinopyroxene throughout the whole vertical section (2.5 km thick) of the Lovozero massif. They found that pyroxenes from the rocks of the Eudialyte complex are more enriched in sodium and ferric iron compared to pyroxenes from the Layered complex. However, the Mn/Fe ratio in the EGM from rocks of the Eudialyte complex is significantly lower (
Figure 7) due to the high iron content (
Figure 8). The maximum iron contents and, accordingly, the minimum Mn/Fe values are typical of the EGM, which crystallized simultaneously with clinopyroxenes. These are eudialyte-group minerals from eudialyte lujavrite, ijolite, and porphyritic/fine-grained nepheline syenites. According to Markl and co-authors [
65], crystallization of aegirine or arfvedsonite from a melt must involve the reduction of the melt according to the two following two reactions:
The crystallization of aegirine leads to an increase in the content of ferrous iron in the mineral-forming melt/solution, and this iron enters the EGM, which grow simultaneously with aegirine.
The increase of the EGM content in leucocratic rocks (foyaite, urtite) of the Layered complex and the appearance of primary minerals of the lovozerite group together with the EGM in the leucocratic rocks of the Eudialyte complex can be explained as follows. The lujavrite → foyaite → urtite fractionation path started with potassic alkali feldspar, clinopyroxene, and nepheline, whose removal drove the melts towards
HFSE- and Na-rich compositions, then the EGM started to crystallize. A similar fractionation path occurs in the Na
2O-Al
2O
3-Fe
2O
3-SiO
2 system [
66], where a melt of the “ijolite” type (approximately 50% of aegirine) evolves towards “nepheline syenite” (approximately 10% of aegirine). Following [
66], after the crystallization of lujavrite, the residual foyaitic melt was significantly enriched in sodium disilicate. Thus, in the rhythm of the Layered complex, the higher EGM content in foyaite and urtite is a result of fractional crystallization.
It is important to note that the zirconosilicates of the eudialyte- and the lovozerite groups differ by sodium content and crystallize at different alkalinity. According to Khomyakov [
67], for silicate minerals with the general formula A
xM
ySi
pO
q (A = Na, K, and other strong bases; M = Zr, Nb, Ti, Be, and other Al-substituting elements), the so-called alkalinity modulus can be calculated: K
alk = (x × 100)/(x + y + p). By the value of K
alk, the minerals and rocks, in which they are present, are subdivided into five groups, namely miaskitic (K
alk ≪ 15%), low agpaitic (K
alk = 15–25%), medium agpaitic (K
alk = 25–35%), highly agpaitic (K
alk = 35–40%), and hyperagpaitic (K
alk > 40%). Minerals of the lovozerite group are characterized by the highest alkalinity, and belong to the hyperagpaitic group, and the K
alk of the minerals of the eudialyte group is lower (highly agpaitic group). The melt evolutionary paths during crystallization of the Eudialyte and Layered complexes were similar, i.e., melanocratic melt evolved towards leucocratic (foyaitic) melt extremely enriched with sodium disilicate. However, during the crystallization of the rocks of the Eudialyte complex, the proportion of sodium in the residual melt was greater than during the crystallization of the rhythm (from lujavrite to foyaite and urtite) of the Layered complex. As a result, primary hyperagpaitic minerals (lovozerite, kapustinite, litvinskite) crystallized in the leucocratic rocks of the Eudialyte complex.
The EGM decomposition products are indicative of an increase in sodium concentration from early melanocratic rocks (lujavrite) to late foyaite and urtite. Substitution of the EGM by the lovozerite-group minerals occurs at the post-magmatic stage under the influence of residual solutions. The alkali concentration in these solutions determines which of the lovoserite-group minerals will be formed. In lujavrite and ijolite, eudialyte is replaced by litvinskite, and in foyaite and other leucocratic rocks, it is replaced by kapustinite. Late hydrothermal solutions may not affect eudialyte. Under their influence, only previously formed rims of the secondary lovozerite-group minerals are destroyed.
The eudialyte-group minerals are formed during the metasomatic alteration of volcanoclastic rocks. Unchanged, these rocks contain extremely low concentrations of ZrO
2 (maximum 0.01 wt.% for basalt) and
REE (Σ
REE2O
3 = 300 ppm in average for basalt), but are significantly enriched in CaO (10.18 wt.% in average for basalt), MgO (6.24 wt.% in average for basalt), and FeO (9.43 wt.% in average for basalt) [
68]. The EGM from metasomatites are quite different in composition to the EGM from igneous rocks, since they are formed during the replacement of calcium-enriched rocks. The arrangement of zones in the oscillatory zoned EGM grains from metasomatites is opposite to the arrangement of zones in the magmatic EGM, and indicates an increase in the concentration of Zr, Al, Na, Fe
2+, and Cl during metasomatism.