Downhole Lithological Profile Reconstruction Based on Chemical Composition of Core Samples and Drill Cuttings Measured with Portable X-ray Fluorescence Spectrometer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Versus Chemical Rock Composition
2.2. Methodology
2.2.1. General Description of the Methodological Approach
2.2.2. Chemical Composition Analysis
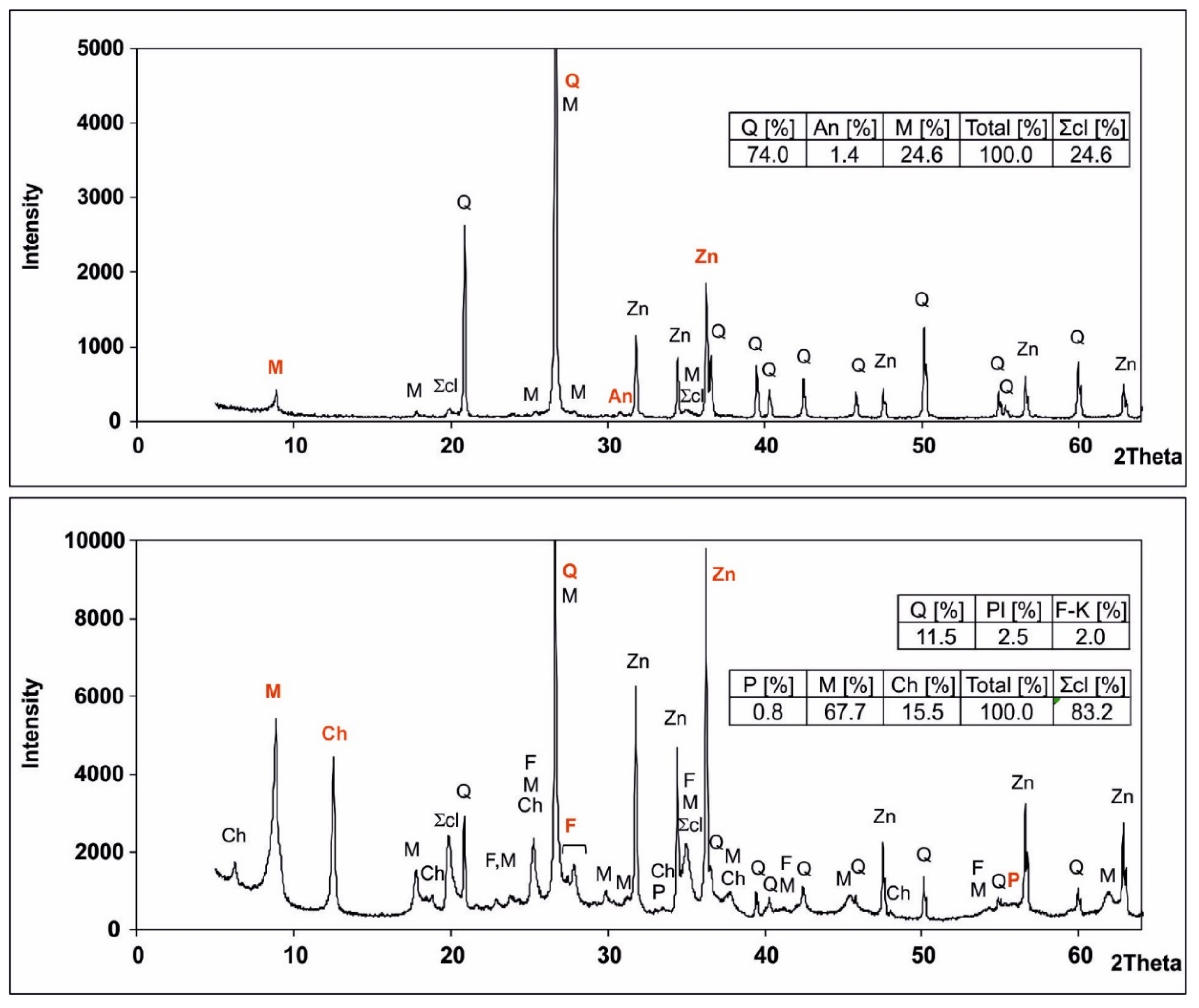
2.2.3. XRD Methodology
2.2.4. Description of Lithological Profile Calculation Procedure
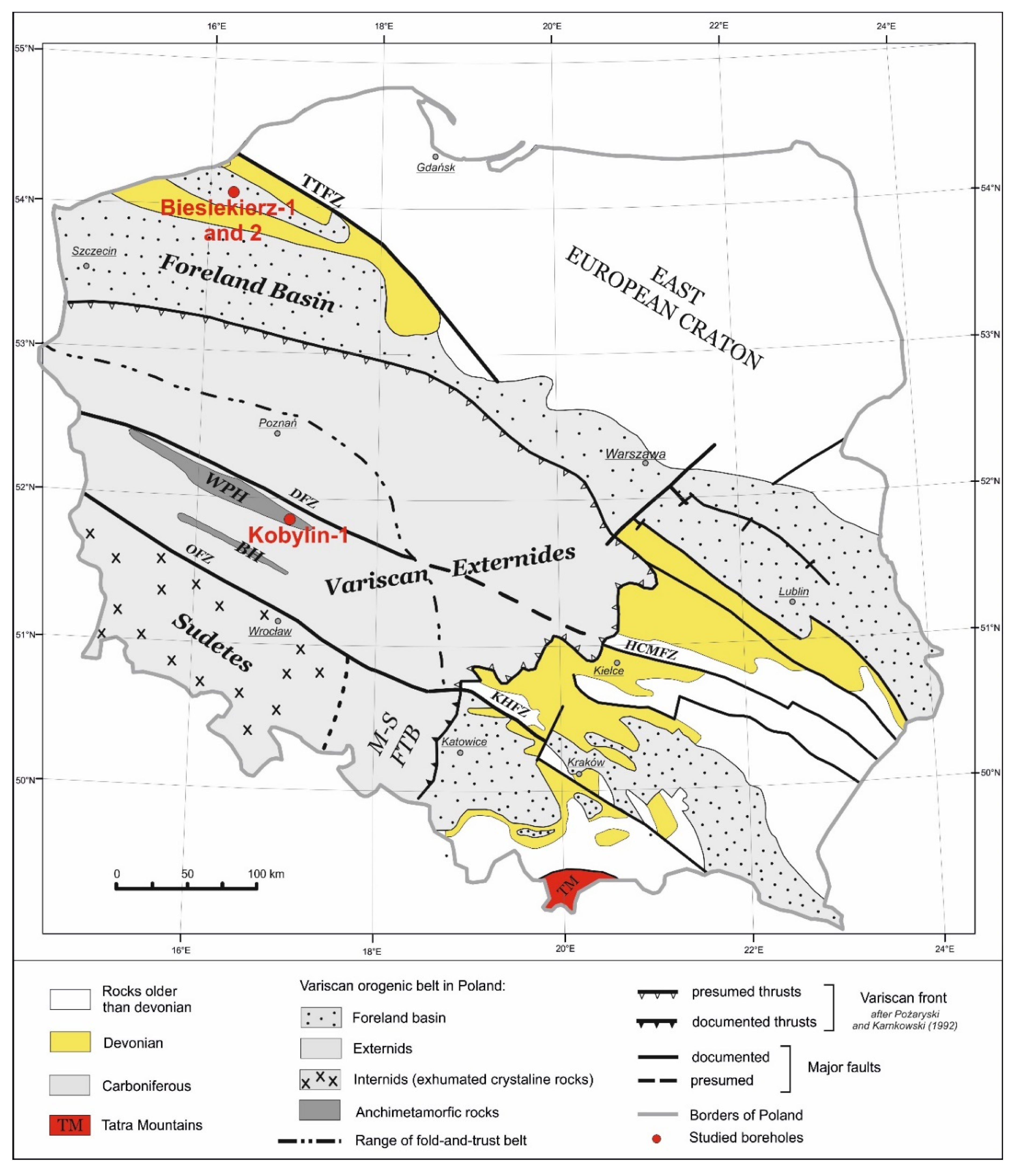
2.3. Research Material
3. Results
3.1. Comparison of Results Obtained from Measurements on Cores and Drill Cuttings
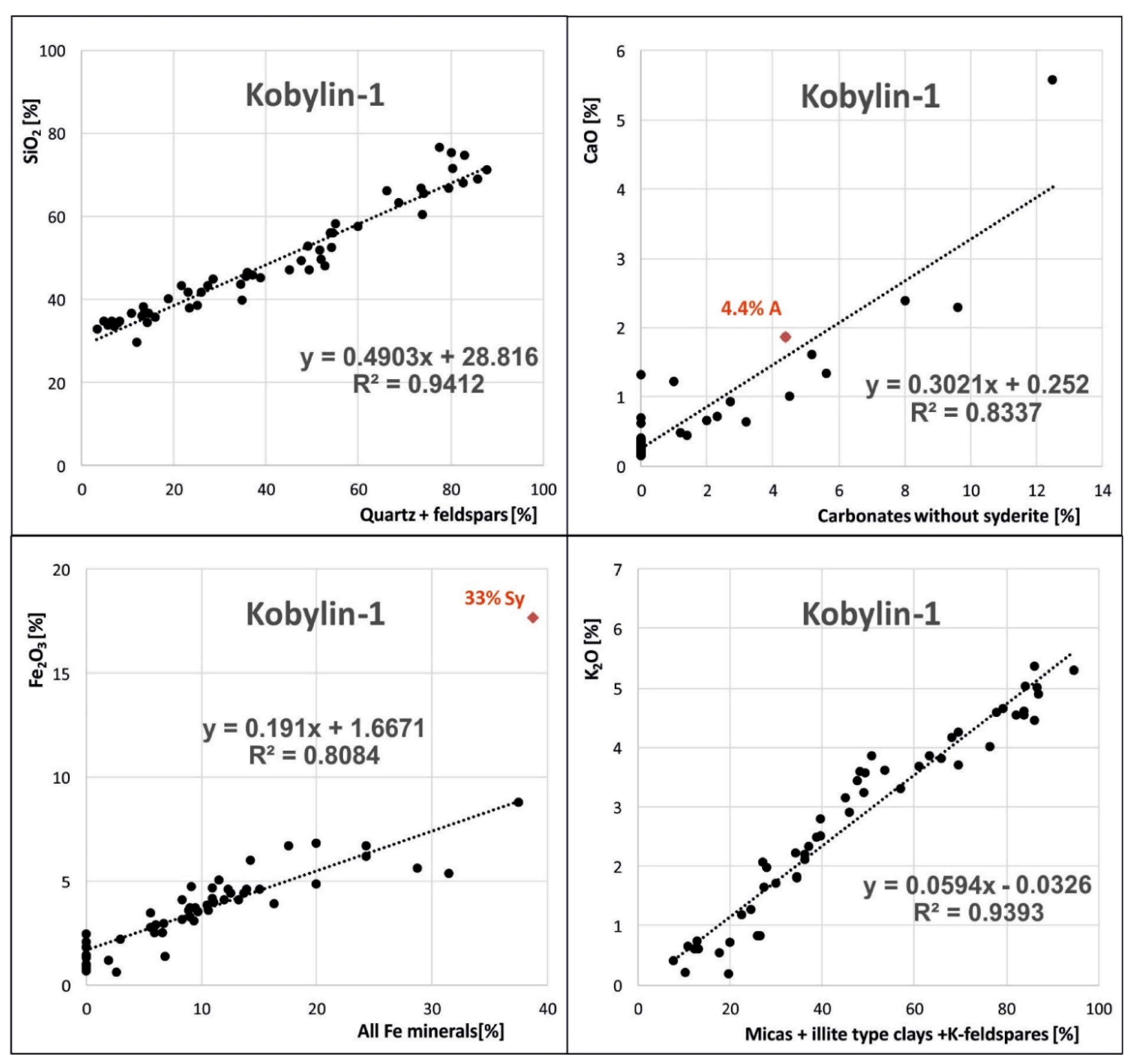
3.2. Mineralogical Model for Carboniferous Rocks from the Kobylin-1 Borehole
3.3. Mineralogical Model for Carboniferous Rocks from the Biesiekierz-2 Borehole
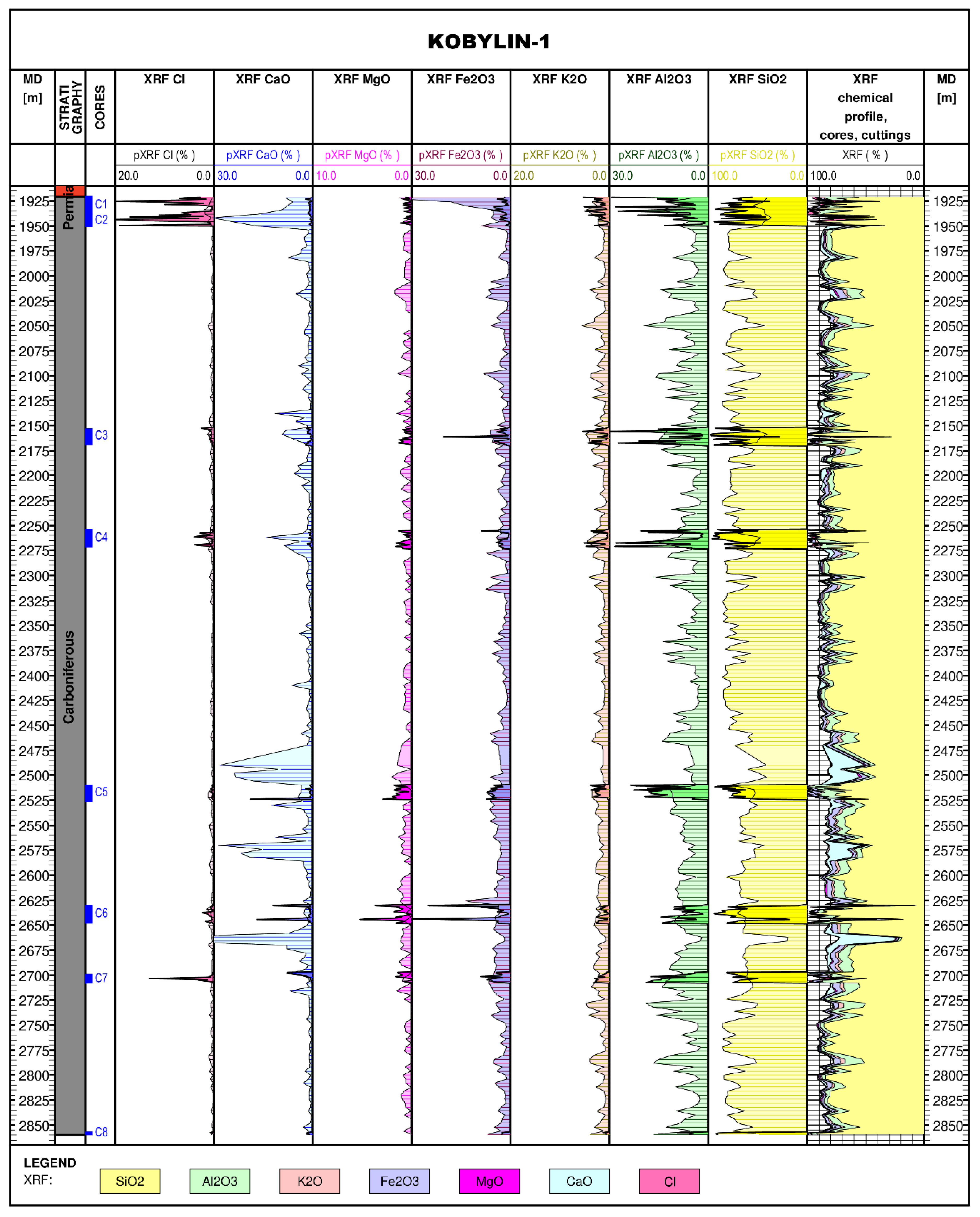
3.4. XRF Measurement Results for the Kobylin-1 and Biesiekierz-2 Boreholes
3.5. Reconstruction of Lithological Profile for the Kobylin-1 Borehole
| Quartz (Q) | |
| Feldspars (F) | |
| Calcite (C), Dolomite (D) | —carbonates |
| Micas and illite group clay minerals (M), Chlorite (Ch) | —clay content |
| Porosity (PHI) | |
| The equation describing the adopted mineralogical composition model: | |
| Q + F + C + D + I + Ch + ∑cl + PHI = 1 |
| 1. Carbonates: 2. Dolomite: 3. Calcite: 4. Brittleness (quartz + feldspars): 5. Quartz: 6. Feldspars: 7. Clay content (sum of clay minerals): 8. Chlorite: 9. Micas and illite group clay minerals: 10. Porosity: | Crb = f(CaO) D = f(MgO) C = Węg − Dol Q + F = f(SiO2) Q = f(SiO2) F = Q_Sk − Q ∑cl = f(Al2O3) Ch = f(Fe2O3) M = ∑cl − Ch PHI—provided porosity logs were applied |
| Carbonates: | C + D = f(CaO) | y = 2.495x − 0.5648 |
| Dolomite: | D = f(MgO) | y = 0.1238x + 1.8183 |
| Quartz: | Q = f(SiO2) | y = 1.8995x − 55.303 |
| Brittleness: | Q + F = f(SiO2) | y = 1.9195x − 52.915 |
| Clay content: | ∑cl = f(Al2O3) | y = 4.3271x + 5.2454 |
| Chlorite: | Ch = f(Fe2O3) | y = 4.1544x − 5.1799 |
3.6. Reconstruction of Lithological Profiles for the Biesiekierz-2 and 1 Boreholes
| Quartz (Q) | |
| Plagioclases (Pl), Potassium feldspars (F—K) | —feldspars |
| Calcite (C), Dolomite (D) | —carbonates |
| Anhydrite (A), Pyrite (P) | —sulphur compounds |
| Clay content (∑cl) | |
| Porosity (PHI) |
- Q + F—K+ Pl + C + D + A + P + ∑cl + PHI = 1 (1)
| 1. Carbonates: 2. Dolomite: 3. Calcite: 4. Sulphur compounds: 5. Anhydrite: 6. Pyrite: 7. Clay content + potassium feldspars: 8. Clay content + feldspars: 9. Brittleness: 10. Plagioclases: (2) → (3) 11. Quartz: (3) → (1) 12. Clay content: (4) → (1) 13. Potassium feldspars: (5) → (2) 14. Porosity: | Crb = f(CaO) D = f(MgO) C = Crb-D S = f(S) A = S* index—adopted coefficient P = S* (1 − index) ∑cl + F—K = f(K2O) (2) ∑cl + F—K + Pl = f(Al2O3) (3) Q + F—K + Pl = f(SiO2) (4) Pl = f(Al2O3) − f(K2O) Q = 1 − PHI − Crb − S − f(Al2O3) ∑cl = 1 − PHI − Crb − S − f(SiO2) (5) F—K = f(K2O) − 1 + PHI + Crb + S + f(SiO2) PHI—the applied porosity logs are based on samples from the final report |
| Sulphur minerals (anhydrite + pyrite): | A + P = f(S) | y = 4.8548x − 0.2192 |
| Carbonates: | C + D = f(CaO) | y = 2.3867x + 7.016 |
| Dolomite: | D = f(MgO) | y = 14.11x − 19.676 |
| Brittleness (quartz + feldspars): | Q + F = f(SiO2) | y = 1.9883x − 15.982 |
| Clay content + feldspars: | ∑cl + F = f(Al2O3) | y = 7.8624x + 5.3036 |
| Clay content + potassium feldspars: | ∑cl + F—K = f(K2O) | y = 9.4461x + 18.23 |
3.7. Comparison of Lithological (Composition) Reconstruction Results with XRD Mineral Composition Benchmark Results
4. Discussion of the Obtained Results
4.1. Comparison of pXRF Results with GEM Probe Measurements for the Kobylin-1 Borehole
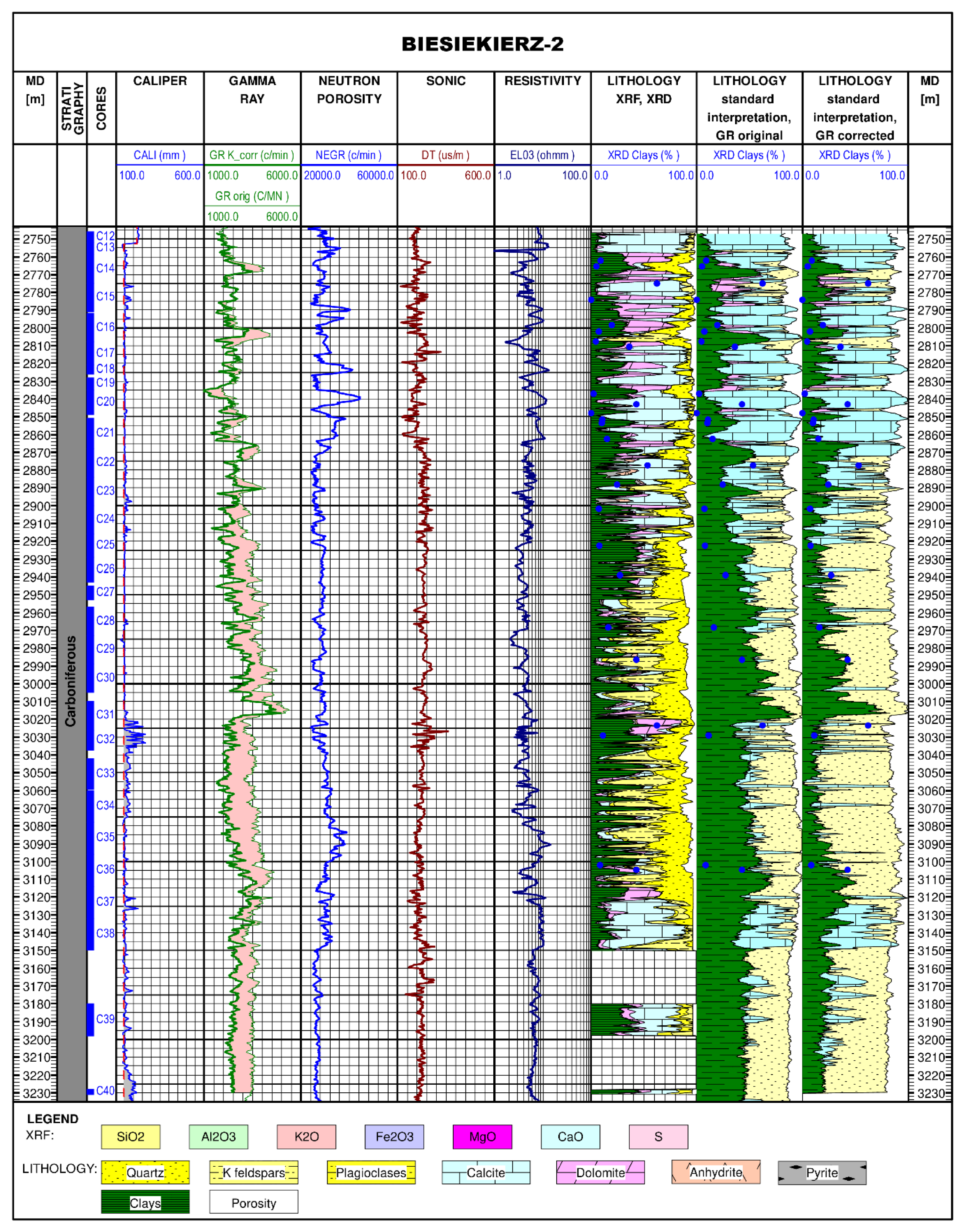
4.2. Comparison of Lithological Profile Reconstruction Results with a Standard Interpretation of Geophysical Logging in the Biesiekierz-2 Borehole
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rider, M. The Geological Interpretation of Well Logs, 2nd ed.; Whittles Publishing: Dunbeath Mill, UK, 2000; ISBN 10:1870325362. [Google Scholar]
- Elis, D.V.; Singer, J.M. Well Logging for Earth Scientists, 2nd ed.; Springer: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-3738-2. [Google Scholar]
- Herron, M.M.; Herron, S.L. Geological application of geochemical well logging. In Geological Applications of Wireline Logs; Hurst, A., Lovell, M.A., Morton, A.C., Eds.; Geological Society of London Special Publication: London, UK, 1990; Volume 48, pp. 165–175. ISBN 10:0903317966. [Google Scholar]
- Lofts, J.; Harvey, P.; Lovell, M. Reservoir characterization from downhole mineralogy. Mar. Pet. Geol. 1995, 12, 233–246. [Google Scholar] [CrossRef]
- Herron, M.M. Mineralogy from geochemical well logging. Clays Clay Miner. 1986, 34, 204–213. [Google Scholar] [CrossRef]
- Anderson, R.N.; Dove, R.E.; Broglia, C.; Silver, L.T.; James, E.W.; Chappell, B.W. Elemental and mineralogical analyses using geochemical logs from the Cajon Pass Scientific Drillhole, California, and their preliminary comparison with core analyses. Geophys. Res. Lett. 1988, 15, 969–972. [Google Scholar] [CrossRef] [Green Version]
- Colson, J.L.; Grau, J.A.; Herron, M.M.; Herron, S.L.; Schweitzer, J.S.; Hertzog, R.C.; Johnston, J. Applications Using Geochemical Logs. In Proceedings of the Middle East Oil Show (Society of Petroleum Engineers), Manama, Bahrain, 11–14 March 1989. [Google Scholar]
- Hertzog, R.; Colson, L.; Seeman, B.; O’Brien, M.; Scott, H.; McKeon, D.; Herron, M.; Grau, J.; Ellis, D.; Schweitzer, J. Geochemical logging with spectrometry tools. SPE Form. Eval. 1989, 4, 153–162. [Google Scholar] [CrossRef]
- Yarbrough, L.D.; Carr, R.; Lentz, N. X-ray fluorescence analysis of the Bakken and Three Forks Formations and logging applications. J. Pet. Sci. Eng. 2019, 172, 764–775. [Google Scholar] [CrossRef]
- Lemière, B. A review of pXRF (field portable X-ray fluorescence) applications for applied geochemistry. J. Geochem. Explor. 2018. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.N.; Malicse, A. Chemostratigraphic studies of the Marcellus Shale by handheld X-ray fluorescence (HHXRF) analysis. In Proceedings of the AAPG Annual Conference, Houston, TX, USA, 10–13 April 2011. [Google Scholar]
- Rowe, H.; Hughes, N.; Robinson, N. The quantification and application of handheld energy-dispersive X-ray fluorescence (ED-XRF) in mudrock chemostratigraphy and geochemistry. Chem. Geol. 2012, 324, 122–131. [Google Scholar] [CrossRef]
- Spencer, R.; Weedmark, T. Application of X-Ray fluorescence (XRF) analyses to the characterization of tight reservoirs. Conference Paper. In Proceedings of the GasMexico Congress and Exhibition, Villahermosa, Mexico, 29–30 September 2015. [Google Scholar]
- Eberl, D. User’s Guide to RockJock–A Program for Determining Quantitative Mineralogy from Powder X-ray Diffraction Data; Open–File Report 03-78; U.S. Geological Survey: Boulder, Colorado, 2003.
- Środoń, J.; Drits, V.A.; McCarty, D.K.; Hsieh, J.C.C.; Eberl, D.D. Quantitative X-ray diffraction analysis of clay-bearing rocks from random preparations. Clays Clay Miner. 2001, 49, 514–528. [Google Scholar] [CrossRef]
- Chipera, S.J.; Bish, D.L. Fitting Full X-Ray diffraction patterns for quantitative analysis: A method for readily quantifying crystalline and disordered phases. Adv. Mater. Phys. Chem. 2013, 3, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Taut, T.; Kleeberg, R.; Bergmann, J. The new Seifert Rietveld program BGMN and its application to quantitative phase analysis. Mater. Struct. 1998, 5, 57–64. [Google Scholar]
- Taylor, C.J.; Hińczak, I. Rietveld Made Easy: A Practical Guide to the Understanding of the Method and Successful Phase Quantifications; Sietronics Pty Limited: Mitchell, Australia, 2006. [Google Scholar]
- Skupio, R. The use of portable XRF spectrometer for measuring the chemical composition of rocks. Nafta-Gaz 2014, 11, 771–777. (In Polish) [Google Scholar]
- Kowalska, S. Quantitative analysis of the mineral composition of rocks containing clay minerals by Rietveld method. Nafta I Gaz 2013, 12, 887–893. (In Polish) [Google Scholar]
- Omotoso, O.; McCarty, D.K.; Kleeberg, R.; Hillier, S. Some successful approaches to quantitative mineral analysis as revealed by the 3rd Reynolds cup contest. Clays Clay Miner. 2006, 54, 748–760. [Google Scholar] [CrossRef]
- Żelaźniewicz, A.; Aleksandrowski, P.; Buła, Z.; Karnkowski, P.; Konon, A.; Oszczypko, N.; Ślączka, A.; Żaba, J.; Żytko, K. Regionalizacja Tektoniczna Polski; Komitet Nauk Geologicznych PAN: Wrocław, Poland, 2011; p. 60. ISBN 978-83-63377-01-4. [Google Scholar]
- Zagórska, U.; Kowalska, S.; Sláma, J.; Dziubińska, B.; Wolański, K. Detrital zircon provenance of Carboniferous sandstones of the Variscan Externides (SW Poland)–record of the eastern Variscides exhumation. Int. J. Earth Sci. 2020, 109, 2169–2187. [Google Scholar] [CrossRef]













| Minerals | Chemical Formula | Oxide Form |
|---|---|---|
| Quartz | SiO2 | SiO2 |
| Cristobalite | SiO2 | SiO2 |
| Opal | SiO2·n(H2O) | SiO2 + nH2O |
| Feldspars | ||
| Orthoclase | KAlSi3O8 | 1/2K2O + 1/2Al2O3 + 3SiO2 |
| Albite | NaAlSi3O8 | 1/2Na2O + 1/2Al2O3 + 3SiO2 |
| Anorthite | CaAl2Si2O8 | CaO + Al2O3 + 2SiO2 |
| Carbonates | ||
| Calcite | CaCO3 | CaO + CO2 |
| Aragonite | CaCO3 | CaO + CO2 |
| Dolomite | CaMg(CO3)2 | CaO + MgO + 2CO2 |
| Siderite | Fe(CO3)2 | FeO + 2CO2 |
| Ankerite | Ca(Fe, Mg, Mn) (CO3)2 | CaO + (Fe, Mg, Mn)O + 2CO2 |
| Magnesite | MgCO3 | MgO + CO2 |
| Sulphate | ||
| Anhydrite | CaSO4 | CaO + SO3 |
| Gipsum | CaSO4·2H2O | CaO + SO3 + 2H2O |
| Barite | BaSO4 | BaO + SO3 |
| Celestine | SrSO4 | SrO + SO3 |
| Sulfide | ||
| Pyrite | FeS2 | Fe + 2S |
| Marcasite | FeS2 | Fe + 2S |
| Galena | PbS | Pb + S |
| Sphalerite | ZnS | Zn + S |
| Oxides | ||
| Hematite | Fe2O3 | Fe2O3 |
| Goethite | FeO(OH) | 1/2Fe2O3 + 1/2H2O |
| Rutile | TiO2 | TiO2 |
| Anatase | TiO2 | TiO2 |
| Chlorides/Fluorides | ||
| Halite | NaCl | Na + Cl |
| Sylvite | KCl | K + Cl |
| Fluorite | CaF2 | Ca + 2F |
| Clays | Approximate chemical formula | |
| Kaolinite | Al4(Si4O10)(OH)8 | |
| Muscovite | KAl2Si3AlO10(OH,F)2 | |
| Illite | (K, Na, NH4)0.90 (Al1.85Fe3+0.05Mg0.10) (Si3.20Al0.80) O10(OH)2 | |
| Montmorillonite | R+0.33 (Al1.67Mg0.33) Si4 O10(OH)2, where R - exchangeable cations | |
| Fe-Chlorite | (Fe++,Mg,Fe+++)5Al(Si3Al)O10(OH,O)8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, S.; Kubik, B.; Skupio, R.; Wolański, K. Downhole Lithological Profile Reconstruction Based on Chemical Composition of Core Samples and Drill Cuttings Measured with Portable X-ray Fluorescence Spectrometer. Minerals 2020, 10, 1101. https://doi.org/10.3390/min10121101
Kowalska S, Kubik B, Skupio R, Wolański K. Downhole Lithological Profile Reconstruction Based on Chemical Composition of Core Samples and Drill Cuttings Measured with Portable X-ray Fluorescence Spectrometer. Minerals. 2020; 10(12):1101. https://doi.org/10.3390/min10121101
Chicago/Turabian StyleKowalska, Sylwia, Benedykt Kubik, Rafał Skupio, and Krzysztof Wolański. 2020. "Downhole Lithological Profile Reconstruction Based on Chemical Composition of Core Samples and Drill Cuttings Measured with Portable X-ray Fluorescence Spectrometer" Minerals 10, no. 12: 1101. https://doi.org/10.3390/min10121101
APA StyleKowalska, S., Kubik, B., Skupio, R., & Wolański, K. (2020). Downhole Lithological Profile Reconstruction Based on Chemical Composition of Core Samples and Drill Cuttings Measured with Portable X-ray Fluorescence Spectrometer. Minerals, 10(12), 1101. https://doi.org/10.3390/min10121101





