1. Introduction
The geochemistry of salt has been developing for over half a century (e.g., [
1], with references). Analytical results proved that halite’s bromine content might also indicate the origin of primary brines (marine/non-marine brines). The isotope composition of sulfates and chemistry of fluid inclusion from primary halite has led to a shift in our understanding of the Phanerozoic oceans’ ionic stability ([
2], with references).
During the last two decades, numerous geochemical studies of the Badenian salts from the Carpathian Foredeep in Poland have been carried out. These studies concerned the chemical composition of main and trace elements in halite [
3,
4,
5,
6,
7], the chemical composition of fluid inclusions in halite [
8,
9,
10], the isotopes of chlorine [
11], oxygen, and hydrogen (aqueous phase) in fluid inclusions in halite [
12], and the isotopic composition of sulfur and oxygen in anhydrite collected from a saline series [
8,
10]. The data, derived from outcrops in salt mines and boreholes, showed that the salt deposits were formed in the deepest parts of the evaporite basin. In sea bottom depressions, evaporites were deposited due to mass movements and density currents [
13,
14,
15].
Most of these data and results have been obtained by studying the halite samples collected from depths not exceeding 1000 m (i.e., from salt mine outcrops or boreholes). However, the results presented in this paper are based on new core samples obtained from a deep well where Badenian salt occurs at a depth of ~5000 m. Initial microscopic observations showed, quite unexpectedly, that not all of the encountered deep salts have recrystallized—some halite crystals retained their original structures—and their geochemical record can thus be used to reconstruct the environment of the salt sedimentation. The geochemical studies of salt samples and the research results on the chemical composition of primary fluid inclusions, presented in this paper, significantly complement previously available information on the Miocene (Badenian) salt sedimentation Carpathian area.
2. Geological Setting
The Outer Carpathians of Poland were thrust over the foreland plate in Mid-Miocene (Badenian-Sarmatian)—e.g., [
16,
17,
18] for detailed overviews and further references. The Carpathian Foredeep developed in front of the advancing Carpathian thrust belt [
19,
20]. Its most external, relatively non-deformed segment is located to the north of the Outer Carpathian thrust sheets. The southern parts of the basin were either overridden by the Carpathians or incorporated into the orogenic belt. They presently form the most external Boryslav, Stebnik, and Zgłobice thrust sheets (
Figure 1).
The Eastern segment of the Carpathian Foredeep in Poland, together with its counterpart of Western Ukraine, underwent substantial normal Miocene faulting due to Miocene flexural extension in front of the advancing Carpathians and reactivation of the deeply-rooted older faults within the broadly-defined Teisseyre-Tornquist Zone, located between the East European Craton and the West European Platform, cf. [
19,
21,
22,
23]. The foreland extension was accompanied by localized reverse faulting and strike-slip movements [
19,
24].
In the Miocene, the Carpathian Foredeep and segments of the emerging Carpathian fold-and-thrust belt occupied the sea of the Central Paratethys. The Paratethys sea existed from the early Oligocene to the latest Pliocene and consisted of an interrelated chain of basins of diverse tectonic origins. At the time of the Badenian salinity crisis (early Serravalian ~13.8–13.4 Ma) [
25], the sea lost its connection with the Mediterranean domain and was transformed into semi-isolated evaporitic basins [
26].
The Badenian evaporite sediments occur in the Carpathian Foredeep in two facies: sulfate (gypsum, anhydrite: Krzyżanowice Formation) and chloride (rock salts, anhydrite, gypsum, and clays: Wieliczka Formation). The sulfate facies, 10–30 m thick, occur within the almost entire area of the Carpathian Foredeep (
Figure 2). Evaporite deposits are locally missing north of Rzeszów, where they are preserved only in axial parts of deep erosional paleovalleys incised into the pre-Miocene basement [
27]. The chloride facies sediments (up to 100 m thick) are present only within the narrow band, along the Carpathian overthrust, from Kraków to Pilzno. They were also found under the Carpathian nappes [
19,
28].
The Huwniki-1 well is located south of Przemyśl, close to the Polish–Ukraine border. The general setting of the Miocene evaporates encountered by the Huwniki-1 well is shown in
Figure 3. This well was drilled through the frontal Carpathian orogenic wedge, built of the Miocene Stebnik unit. At a depth of 3730 m, the well reached the autochthonous Miocene Foredeep infill. The Miocene evaporites were intersected from 4991 m until the end of the hole at 5001.7 m. The Huwniki-1 well penetrated only the topmost part of the evaporitic succession. The sub-evaporitic seismic imaging is relatively poor, so the presence of the Miocene sub-evaporitic clastics beneath the evaporites could not be excluded, similarly to the northern part of the basin, where evaporites are underlain by the Miocene Skawina Beds. Their existence or thicknesses in the study area remain, however, unknown.
The evaporites discovered in the Huwniki-1 well seem to have been deposited within the local tectonic graben, formed within the hanging wall of the Kniażyce fault zone during the older stage of flexural extension when the orogenic front was located farther to the south (cf. [
21,
22]). During the next phase of thrusting, the Kniażyce fault zone was overridden by the frontal Carpathian thrust. The flexural extension was shifted towards the foreland, into the Wielkie Oczy fault zone (
Figure 3).
3. General Characteristics of Evaporites Drilled by the Huwniki-1 Well
Rock salt was encountered by the Huwniki-1 well at the depth range of 4991–5001.7 m. Different types of rock salt could be observed in the borehole profile. The upper part of the core (4991–4995 m) contained colorless and partly transparent crystal-texture halite, as well as medium-grained, white, and grey halite. The sizes of halite crystals varied from 5 mm to even 10 cm. They formed layers, up to 50 cm thick, and their bed dipping was ~30° (
Figure 4A,B).
Below (4995–5001.7 m), the salt deposits are lying almost horizontally. They are fine- and medium-grained, laminated rock salts, containing various clay admixtures (
Figure 4C,D,F). Particular layers were 1–30 cm thick. Salt had an equi-granular texture, with crystals ranging from 5 to 10 mm in size. The terrigenous admixtures (clay/mud), visible as either dark bands or thin laminated layers of mudstones (up to 1 cm thick), could be observed within halite (
Figure 4D). At the depth interval of 4998.1–4998.7 m, the medium-grained white salt bed was found to crumble into spindly grains (granoblastic texture) (
Figure 4E). Such evidence of salt tectonics’ influence caused partial recrystallization of halite and was also found in other profile sections.
However, not all the layers underwent transformation or metamorphism related to depth and high temperature. In the upper section of the profile, the presence of redeposited halite–rudite layers (salt conglomerates and breccias) was observed (
Figure 4C). Those poorly sorted layers, 10–30 cm thick, preserved sedimentary structures—e.g., normal gradation. They consisted of halite grains (crystal fragments), as well as light-color mudstone clasts. Sedimentary zonation was identified in halite crystals (
Figure 4B) and confirmed later by microscopic observations. The presence of sedimentary bottom-growth chevron texture in halite crystals was rather unusual at a depth of ~5 km. Additionally, the chemical composition of sedimentary brines locked in fluid inclusions could be studied.
4. Methodology of Geochemical Studies
Geochemical studies were carried out for 15 samples of halite, collected within the core from the depth interval of 4991–5000 m. The bromine content was measured in all 15 samples by an X-ray spectrometer, using a Rigaku Denki Co., Ltd., Tokyo, Japan, fluorescence spectrometer. Strontium and rubidium were determined by the ICP method in the same samples. Our analyses were conducted in the laboratory of the Polish Geological Institute—National Research Institute.
Microscopic observations, designed to determine fluid inclusions’ presence and distribution, were performed under a transmitted-light optical microscope. Four core samples were selected for inclusion studies (III-70, IV-0, IV-80, and V-70). The chemical composition of inclusion brines was determined by the ultramicrochemical method (UMCA) developed by Petrychenko [
29], which applies a traditional chemical analysis to microscopic samples [
30]. The contents of K
+, Mg
+2, Ca
+2, and SO
4−2 ions could be determined by that method. The analytical error of the approach applied was 15–23% (for Mg
2+ and K
+) and 31–43% (for SO
4−2 and Ca
2+) when a single measurement was taken. In the present study, 2–3 parallel analysis courses were performed for each of the ions, and the analytical error dropped down to 10–17%. The brine’s chemical composition was analyzed, involving primary and secondary (early-diagenetic) fluid inclusions in halite.
5. Results and Interpretation
5.1. Bromine Contents
The bromine method is commonly used to analyze the saline sedimentation environment of all chlorides [
2,
31,
32]. Bromine concentrations in halite are closely related to the advanced degree of evaporite cycle—i.e., the degree of brine evaporation (D.E.) [
33]. In the initial seawater, the bromine content was 65–67 ppm. As evaporation progressed, the content of bromide ions in brine increased, and, at the beginning of the halite crystallization, it already amounted to about 510 ppm [
31].
Bromide did not form its own minerals in the evaporation process, but it only diadochically replaced chlorine in chlorides (ion radius Br− = 1.94 Å, Cl− = 1.81 Å). Therefore, crystallizing halite would contain a certain amount of bromine, being typical for the degree of brine concentration and corresponding to the fractionation coefficient: b = wt.% Br (in halite)/wt.% Br (in solution).
The fractionation coefficient was practically constant at the given temperature. For halite precipitated from modern seawater at 25 °C, the coefficient value was 0.14 +/− 0.02 [
31,
33,
34]. Since brines contained ~510 ppm Br in the initial stage of halite crystallization, primary halite should be precipitated as follows: 510 × (0.14 +/− 0.01) = 65–75 ppm Br content. In later generations of chlorides, the bromine content would increase, and, for example, when the first potassium minerals appeared, the salts already contained 270 ppm Br [
31].
The bromine contents showed relatively little variability in the analyzed 15 pure halite samples, and they ranged from 36 to 49 ppm (
Table 1,
Figure 5); the average Br content was 43 ppm. Previously performed analyses of two halite samples collected from the Huwniki-1 well (using the ion chromatography method) showed similar Br contents of 34 and 35 ppm [
35].
The results of bromine content determination in halite (36–49 ppm) were slightly below the minimum values (65–75 ppm) that are typical for primary halite precipitated as a result of normal (gradual) seawater evaporation [
31,
33,
36]. There was no variation or regularity of bromine contents in halite, depending on the sample location within the core profile (
Figure 5). However, in pure and white coarse-crystalline varieties of salt, the bromine contents were slightly higher than those in fine-crystalline and laminated halites. Our bromine content analysis results did not differ significantly from the data obtained from the studies of other profiles of the Carpathian Foredeep Badenian salt cores [
4,
6,
7,
9,
10,
14,
37,
38,
39].
5.2. Strontium and Rubidium Contents
As one of the trace elements, strontium plays an important role in the study of evaporites. It can be a useful indicator of the sedimentation environment, as well as of the origins and transformations occurring during the diagenesis of the carbonate and sulfate facies [
2]. The occurrence of strontium in evaporites is mainly related to the isovalent substitution by calcium. In rock salt, strontium is accompanied by the admixtures of calcium sulfates: anhydrite and gypsum. Strontium minerals can also precipitate, in the form of celestine (SrSO
4) and rare strontianite (SrCO
3), in the evaporation cycle.
In the initial phase of seawater evaporation, increases in strontium concentrations are observed in brine. Calcite crystallization absorbs strontium to a small extent at that stage. In the next step of evaporation, the Sr concentrations decrease in brine. This is related to the crystallization of calcium sulfates, where the majority of strontium is incorporated into the crystal lattice. The highest strontium concentrations, in the order of thousands of ppm, is observed in anhydrite. The depletion of strontium in brine is recorded in the further stages of halite and potassium–magnesium chloride precipitation. In chloride minerals, however, strontium content is low and fluctuates within a few ppm. The potassium–magnesium sulfate salts, with calcium (e.g., polyhalite, glauberite, or syngenite), reach much higher Sr contents of up to 1000 ppm.
Like bromine contents, the content of strontium and rubidium was also studied in macroscopically pure halite crystals. The Sr content was unevenly distributed in the core profile (
Table 1,
Figure 5). There were some samples in which Sr was not detected (<3 ppm) and those with slightly higher Sr contents (max. 281 ppm). Such results slightly differed from those obtained by [
35]: four samples with similar Sr contents (50–280 ppm) and two samples with higher strontium contents (1060 and 2060 ppm, respectively). Those relatively high strontium contents might be due to higher sulfate concentrations and the presence of anhydrite locally.
Rubidium is a typical diffuse element. This was associated with potassium in igneous rocks, and, therefore, rubidium occurred in higher quantities in potassium feldspars and mica. The rubidium was previously found in the potassium salts, brine of crude oil deposits, and some lignite deposits, especially in ashes [
40]. Rubidium minerals were not identified in this study. The rubidium analyses performed by the ICP method showed that the contents of that element were almost constant (8–10 ppm) in the entire salt core profile of the Huwniki-1 well. One could assume that those relatively low rubidium contents showed no significant potassium salt admixtures (
Figure 5).
5.3. Description of Fluid Inclusions in Halite
In the studied halite samples, the primary chevron textures were well-preserved. They were composed of alternating inclusion-rich and inclusion-poor growth bands (
Figure 6A,B). The primary fluid inclusions had cubic or close to cubic shapes. They ranged from a few microns to 700 μm in size. Numerous two-phase (gas-liquid) inclusions were found (
Figure 6C). In some textures, inclusions also contained anisotropic mineral crystals (anhydrite). The pressure in the inclusions was close to normal atmospheric pressure. The gas saturation was low, and the gas phase consisted mainly of water vapor.
Early diagenetic secondary fluid inclusions were also observed. They represented two-phase (gas–liquid) and three-phase (gas–liquid–anhydrite) types. The differences between the primary and early diagenetic inclusions were not always noticeable visually. Still, the majority of early diagenetic inclusions were placed between the chevron and the transparent bands, and the sizes of inclusions exceeded 200 µm (
Figure 6D). The pressure in primary and in secondary inclusions was close to atmospheric.
Additionally, secondary one-phase (liquid), two-phase (gas-liquid), and multi-phase inclusions were found in recrystallized transparent halite (
Figure 6C). Together with brine and gas bubbles, they contained descendant crystals, most often anhydrite (
Figure 6E). Some multi-phase inclusions had more than one anisotropic mineral. Inclusions of that type were usually larger than others: ~250 μm. Some inclusions were shaped irregularly, with their walls sometimes deformed and presenting complex forms. The pressure inside inclusions significantly exceeded normal atmospheric pressure. In two samples, a different type of secondary inclusion was observed. In addition to the liquid–gas–solid phase, they contained a “bushy” light-yellow to brown to black substance, usually located in the center of inclusions (
Figure 6E,F). Most likely, it was a low-carbon organic substance. The shapes of those inclusions were quite complex. In one sample, it suggested the presence of combined two smaller inclusions. Anhydrite crystals were also found in large quantities in the halite mass. They appeared in various forms and sizes, from several dozen µm to large aggregates exceeding 1 mm in length (
Figure 6E).
Depending on deposition depth, stresses caused two salt deformation types: brittle or plastic [
41]. The halite samples showed the effects of fragile strains in cracks, microcracks, and fissures. They often contain the so-called stylolite seams—i.e., the places through which not fully saturated brine was squeezed out under stress, causing surface dissolution effects [
42].
Another brittle deformation effect was visible in some samples, where the deformation features were filled with liquid. Brine was trapped between the deformed surfaces either during the deposition or simultaneous during dissolution and deposition. Microcracks change their shape with time to achieve the lowest surface energy state. This process is called “necking down” [
43], and it consists of the rearrangement of liquid inclusions along with the former fracture positions.
5.4. Chemical Composition of Brines Collected from Fluid Inclusions
The chemical composition of brines originating from the primary fluid inclusions enclosed in halite crystals was used to reconstruct Badenian seawater chemistry. Such studies of Middle Miocene seawater have been previously published by [
10,
44,
45,
46,
47,
48,
49,
50,
51,
52]. Only primary inclusions could be used for that type of research. Such inclusions were usually found either in sedimentary halite, formed at the bottom of the salt basin (chevron halite), or on the brine surface (cumulate halite), or within the zone of mixing brines of various compositions (salting-out halite) [
30].
Four halite samples were selected for our study after preserved sedimentary chevron textures were identified under microscopic examinations. The results of ultramicrochemical analyses of those samples are summarized in
Table 2 below.
The brines originating from the primary inclusions of halites under examination were classified as the chemical type Na-K-Mg-Cl-SO
4 (SO
4-rich). Generally, the compositions of brines and the ratios of components were similar to those recorded in the Badenian time of the Carpathian Foredeep and the entire Carpathian region [
53].
The chemical compositions of brines originating from large (exceeding 200 µm) early diagenetic inclusions were different than those of primary inclusions (
Table 3). The contents of the main components in brines were reduced, and ratios of the components were preserved, predominantly in the same way as in the brines of primary inclusions (
Table 2,
Figure 7). The chemical compositions of early diagenetic brine participating in halite recrystallization might result from the effect of water delivered by gypsum dehydration.
6. Discussion
The geochemical study of halite crystals (bromine, rubidium, and strontium contents, with fluid inclusion compositions) from the Huwniki-1 well demonstrated that seawater was the brine source. However, the salts were formed from seawater’s brines mixed with low-salinity or freshwater inflows. This was indicated by lower than expected bromine contents in halite, and it marked the dissolution of previously deposited salts (
Figure 5).
Evaporation is not a regular process. The fluctuations in concentration, density, temperature, and Eh-pH relationships in brine were commonly observed in the salt basin, especially close to the front of the advancing orogenic thrust belt. When brine was saturated with dissolved salts, the bromine content was lower than that of primary brine, formed in the normal evaporation cycle. Presently, completely secondary salts are considered to be those with a bromine content of <20 ppm, while secondary salts of non-marine origin are those that contain <10 ppm Br [
31].
Not all bromine was primary in halite since a significant proportion of bromides could be absorbed by brines that penetrated burial salt deposits [
32]. The recrystallization processes that caused halite deformations could also cause bromide migration outside of the halite crystal lattice [
39]. Still, that process operated only when halite was recrystallized in the presence of brines whose bromide contents were lower than those of the original brine [
61].
The study of strontium contents showed its uneven distribution. There were places in the core profile where strontium was not detected and samples with strontium content exceeding 200 ppm. This was caused by the irregular distribution of sulfate minerals (anhydrite), in which strontium was present within their crystal lattices. Secondary transformations and recrystallization of evaporites played important roles in the distribution of strontium. Those processes often caused significant changes in the Sr concentrations. The most common secondary alteration occurring in evaporite deposits involved the conversion of anhydrite into gypsum. This process strongly influenced the migration of strontium because primary anhydrite generally contained more strontium than gypsum. The reverse process—i.e., the conversion of gypsum into anhydrite—might a lead to strontium deficiency in secondary anhydrite [
5]. One could assume that anhydrite, present in the salts from the Huwniki-1 well, is of secondary origin, owing to the transformation of gypsum, explaining the low strontium contents in anhydrite. Our analysis of the rubidium contents implies the absence of potassium–magnesium salts (<10 ppm).
The chemical composition of primary brine, originating fluid brines of the Huwniki-1 well, corresponded more closely to the geochemical characteristics of brines collected in the eastern segment of the Carpathian Foredeep—e.g., at Hrynivka [
62] or Slanic-Prahova [
46]. Upon comparison of these results with the results of the chemical composition of modern seawater (
Table 1,
Figure 7), it was concluded that the former contained slightly fewer sulfate ions. This was associated with the evolutionary changes of the sulfate ion contents during the Cenozoic [
52]. At the beginning of the Cenozoic, the chemical composition approached a transitional type (from chloride to sulfate), and later, it was subjected to gradual changes. In the Messinian, the sulfate ion ratios and contents were already close to those of modern seawater (
Figure 7).
The SO
4−2 ion concentrations in seawater during the Pliocene–Pleistocene periods were lower than those of modern seawater. The difference was caused by global changes in seawater chemistry [
45,
51,
52]. The changes in the chemical composition of seawater in the Cenozoic were significant and consisted primarily of increasing SO
4−2 ion contents between the Eocene and the Holocene. The deviations from the strictly regular increases in that ion’s contents were recorded in the Eocene and the Oligocene, which may have been caused by influence of local factors. Otherwise, we may be missing adequate quantities of data.
7. Conclusions
The results of our petrological studies of the salt core, collected from the depth of ~5000 m, showed the presence of the chevron structure relics, typical for primary halite. New geochemical results and the studies of fluid inclusions found in such structures indicated that primary brines were of marine origin. The bromine, strontium, and rubidium content values suggested that the marine origin of salts may have undergone partial dissolution and redeposition under lower salinity water inflows. The ratios of the main ions (K, Mg, SO4) in the fluid inclusions were typical for those of the Badenian brines collected from the eastern segment of the Carpathian Foredeep. Upon comparison of those results with the chemical composition of modern seawater, it was found that the former contained slightly lower sulfate ion quantities. This was associated with evolutionary changes occurring in the contents of sulfate ions during the Cenozoic.
The current research has only considered the context of the petrology and geochemistry of rock salts. Further research is required to the stratigraphy of evaporites based on microfauna study and provides a detailed description of the front of the advancing Carpathians in the study area based on seismic lines.
Author Contributions
Conceptualization: K.B., A.G., P.K. and A.M., methodology and formal analysis: K.B. and A.G.; sample collection: K.B. and A.M.; writing—original draft preparation: K.B. and P.K.; funding acquisition: K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant no 16.16.140.315 from the AGH University of Science and Technology.
Acknowledgments
We sincerely thank the Polish Gas and Oil Company (PGNiG) for making available their rock-salt core samples selected for this study. We wish to thank Hanna Tomassi-Morawiec for laboratory analyses and Bartłomiej Hajnold for help to prepare the text. We thank two anonymous reviewers for their constructive comments, which helped us to improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Babel, M.; Schreiber, B.C. Geochemistry of evaporites and evolution of seawater. In Treatise on Geochemistry, 2nd ed.; Turekian, K., Holland, H., Eds.; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Warren, J.K. Evaporites. A Compendium; Springer: Berlin, Germany, 2016; p. 1854. [Google Scholar]
- Toboła, T. Badania koncentracji potasu i magnezu w solach kamiennych złoża Bochni. Przegląd Geol. 2000, 48, 1163–1168, (In Polish with English summary). [Google Scholar]
- Toboła, T. Study of bromine contents in salt deposit of Bochnia (Badenian, southern Poland). Przegląd Geol. 2000, 48, 688–693, (In Polish with English summary). [Google Scholar]
- Toboła, T. Occurrence of strontium in Badenian rock salts—An example of Bochnia salt deposit. Kwart. AGH Geol. 2001, 27, 363–381, (In Polish with English summary). [Google Scholar]
- Toboła, T. Study of bromine contents in salt deposit Siedlec-Moszczenica. Kwart. AGH Geol. 2004, 30, 5–22, (In Polish with English summary). [Google Scholar]
- Bukowski, K. Badenian Saline Sedimentation between Rybnik and Dębica Based on Geochemical, Isotopic, and Radiometric Research; Dissertation Monographs 236; AGH: Kraków, Poland, 2011; p. 184, (In Polish with English summary). [Google Scholar]
- Bukowski, K.; Galamay, A.R.; Goralski, M. Inclusion brine chemistry of the Badenian salt from Wieliczka. J. Geochem. Explor. 2000, 69–70, 87–90. [Google Scholar] [CrossRef]
- Galamay, A.R.; Bukowski, K.; Czapowski, G. Chemical composition of brine inclusions in halite from clayey salt (zuber) facies from the upper tertiary ( miocene) evaporite basin (Poland). J. Geochem. Explor. 2003, 79, 509–511. [Google Scholar] [CrossRef]
- Cendón, D.I.; Peryt, T.M.; Ayora, C.; Pueyo, J.J.; Taberner, C. The importance of recycling processes in the middle miocene badenian evaporite basin (carpathian foredeep): Palaeoenvironmental implications. Palaeogeography 2004, 212, 141–158. [Google Scholar] [CrossRef]
- Eastoe, C.J.; Peryt, T. Stable chlorine isotope evidence for non-marine chloride in Badenian evaporites, Carpathian mountain region. Terra Nova 1999, 11, 118–123. [Google Scholar] [CrossRef]
- Bukowski, K.; Duliński, M.; Różański, K. Origin of Badenian salts from Wieliczka as indicated by stable isotope composition of fluid inclusion. Biul. Państw. Inst. Geol. 2001, 396, 27–29. [Google Scholar]
- Ślączka, A.; Kolasa, K. Resedimented salt in the Northern Carpathians Foredeep (Wieliczka, Poland). Slovak Geol. Mag. 1997, 3, 135–155. [Google Scholar]
- Bukowski, K.; Czapowski, G.; Karoli, S.; Bąbel, M. Sedimentology and geochemistry of the Middle Miocene (Badenian) salt-bearing succession from East Slovakian Basin (Zbudza Formation). In Evaporites through Space and Time; Special Publications; Schreiber, B.C., Lugli, S., Bąbel, M., Eds.; Geological Society: London, UK, 2007; Volume 285, pp. 247–264. [Google Scholar]
- Gonera, M.; Bukowski, K.; D’Obryn, K.; Wiewiórka, J. Foraminifera in slump deposits of the Badenian (middle Miocene) Green Stratified Salt in Wieliczka, Poland. Geol. Q. 2012, 56, 869–880. [Google Scholar] [CrossRef] [Green Version]
- Nemčok, M.; Krzywiec, P.; Wojtaszek, M.; Ludhová, L.; Klecker, R.A.; Sercombe, W.J.; Coward, M.P. Tertiary development of the Polish and eastern Slovak parts of the Carpathian accretionary wedge: Insights from balanced cross-sections. Geol. Carpathica 2006, 57, 355–370. [Google Scholar]
- Ślączka, A.; Kruglov, S.; Golonka, J.; Oszczypko, N.; Popadyuk, I. Geology and hydrocarbon resources of the Outer Carpathians, Poland, Slovakia, and Ukraine: General Geology. AAPG Mem. 2006, 84, 221–258. [Google Scholar]
- Gągała, Ł.; Vergés, J.; Saura, E.; Malata, T.; Ringenbach, J.-C.; Werner, P.; Krzywiec, P. Architecture and orogenic evolution of the northeastern Outer Carpathians from cross-section balancing and forward modelling. Tectonophysics 2012, 532–535, 223–241. [Google Scholar] [CrossRef]
- Oszczypko, N.; Krzywiec, P.; Popadyuk, I.; Peryt, T. Carpathian Foredeep Basin (Poland and Ukraine)—Its sedimentary, structural and geodynamic evolution. AAPG Mem. 2006, 84, 293–350. [Google Scholar]
- Oszczypko, N.; Oszczypko-Clowes, M. Stages of development in the Polish Carpathian Foredeep Basin. Cent. Eur. J. Geosci. 2012, 4, 138–162. [Google Scholar] [CrossRef]
- Krzywiec, P. Miocene tectonic evolution of the Eastern Carpathian foredeep Basin (Przemyśl—Lubaczów) in light of seismic data interpretation. Prace Państw. Inst. Geol. 1999, 168, 249–276, (In Polish with English summary). [Google Scholar]
- Krzywiec, P. Contrasting tectonic and sedimentary history of the central and eastern parts of the Polish Carpathian Foredeep Basin—Results of seismic data interpretation. Mar. Pet. Geol. 2001, 18, 13–38. [Google Scholar] [CrossRef]
- Krzywiec, P. Geodynamic and tectonic control on evolution of foreland basins, with references to the Carpathian foredeep. Przegląd Geol. 2006, 54, 404–412, (In Polish with English summary). [Google Scholar]
- Krzywiec, P.; Aleksandrowski, P.; Ryzner-Siupik, B.; Papiernik, B.; Siupik, J.; Mastalerz, K.; Wysocka, A.; Kasiński, J. Geological structure and origin of the Miocene Ryszkowa Wola Horst (Sieniawa–Rudka area, eastern part of the Carpathian Foredeep Basin)—Results of 3D seismic data interpretation. Przegląd Geol. 2005, 53, 656–663, (In Polish with English summary). [Google Scholar]
- de Leeuw, A.; Bukowski, K.; Krijgsman, W.; Kuiper, K.F. Age of the Badenian salinity crisis; impact of Miocene climate variability on the circum-Mediterranean region. Geology 2010, 38, 715–718. [Google Scholar] [CrossRef]
- Peryt, T.M. The beginning, development and termination of the Middle Miocene Badenian salinity crisis in Central Paratethys. Sediment. Geol. 2006, 188–189, 379–396. [Google Scholar] [CrossRef]
- Krzywiec, P.; Wysocka, P.; Oszczypko, N.; Mastalerz, K.; Papiernik, P.; Wróbel, G.; Oszczypko-Clowes, M.; Aleksandrowski, P.; Madej, K.; Kijewska, S. Evolution of the Miocene deposits of the Carpathian foredeep in vicinity of Rzeszów (area of 3D seismic survey „Sokołów—Smolarzyny”). Przegląd Geol. 2008, 56, 232–244, (In Polish with English summary). [Google Scholar]
- Garlicki, A. Sedimentation of Miocene Salts in Poland; Prace Geologiczne PAN: Kraków, Poland, 1979; Volume 119, p. 66, (In Polish with English summary). [Google Scholar]
- Petrichenko, O.I. Methods of Study of Inclusions in Minerals of Saline Deposits; Naukova dumka: Kyiv, Ukraine, 1973; p. 90. [Google Scholar]
- Galamay, A.R.; Bukowski, K.; Sydor, D.V.; Meng, F. The Ultramicrochemical Analyses (UMCA) of fluid inclusions in halite and experimental research to improve the accuracy of measurement. Minerals 2020, 10, 823. [Google Scholar] [CrossRef]
- Holser, W.T. Trace elements and isotopes in evaporites. In Reviews in Mineralogy; Burns, R.G., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1979; Volume 6, pp. 295–346. [Google Scholar]
- Sonnenfeld, P. Brines and Evaporites; Academic Press Inc.: Orlando, FL, USA, 1984; p. 613. [Google Scholar]
- McCaffrey, M.A.; Lazar, B.; Holland, H.D. The evaporation path of seawater and the coprecipitation of Br and K with halite. J. Sediment. Petrol. 1987, 57, 928–937. [Google Scholar]
- Hermann, A.G. Bromine distribution between halite and NaCl-saturated seawater. Chem. Geol. 1980, 28, 171–177. [Google Scholar] [CrossRef]
- Cebulski, D.; Urbaniec, A.; Łukaszewski, P. Possibilities of Adaptation of the Selected Research Methods to Determine the Properties and Structural Features of Salt Rocks; Prace Naukowe Instytutu Nafty i Gazu: Kraków, Poland, 2017; Volume 217, pp. 1–191, (In Polish with English summary). [Google Scholar]
- Valiashko, M.G. The Principle of Forming of Salt Deposits; M.G.U.: Moscow, Russia, 1962; 396p. (In Russian) [Google Scholar]
- Garlicki, A.; Wiewiórka, J. The distribution of bromine in some halite rock salts of the Wieliczka salt deposit (Poland). Ann. Soc. Geol. Pol. 1981, 51, 353–359. [Google Scholar]
- Galamay, A.R.; Karoli, S. Geochemical peculiarities of Badenian salts from East-Slovakian basin. Slovak Geol. Mag. 1997, 3, 187–192. [Google Scholar]
- Bukowski, K. Bromine concentration in Badenian age salts from Bochnia. Przegląd Geol. 1997, 45, 819–821. (In Polish) [Google Scholar]
- Morawiecki, A. Inorganic Chemical Raw Materials and Their Economic Use; Wydawnictwo Geologiczne: Warszawa, Poland, 1975; p. 316. (In Polish) [Google Scholar]
- Jackson, M.P.A.; Hudec, M.R. Salt Tectonics. Principles and Practice; Cambridge University Press: Cambridge, UK, 2017; p. 498. [Google Scholar]
- Cyran, K. Inclusions in salts from Bochnia and Wieliczka mines, South Poland. Proof of tectonic deformation. Miner. Resour. Manag. 2008, 24, 241–250, (In Polish with English summary). [Google Scholar]
- Roedder, E. Ancient fluids in crystals. Sci. Am. 1962, 207, 38–47. [Google Scholar] [CrossRef]
- Petrichenko, O.I. Physico-Chemical Conditions of Sedimentation in Ancient Salt-Bearing Basins; Naukova Dumka: Kyiv, Ukraine, 1988; 128p. (In Russian) [Google Scholar]
- Kovalevich, V.M. Halogenesis and Chemical Evolution of Ocean in the Phanerozoic; Naukova Dumka: Kyiv, Ukraine, 1990; p. 154. (In Russian) [Google Scholar]
- Kovalevich, V.M.; Petrichenko, O.I. Chemical composition of brines in Miocene evaporate basins of the Carpathian region. Slovak Geol. Mag. 1997, 3, 173–180. [Google Scholar]
- Kovalevich, V.M.; Peryt, T.M.; Petrichenko, O.I. Secular variation in seawater chemistry during the Phanerozoic as indicated by brine inclusions in halite. J. Geol. 1998, 106, 695–712. [Google Scholar] [CrossRef]
- Garcia-Veigas, J.; Rossel, L.; Garlicki, A. Petrology and geochemistry (fluid inclusions) of Miocene halite rock salts (Badenian, Poland). Slovak Geol. Mag. 1997, 3, 181–186. [Google Scholar]
- Zimmermann, H. Tertiary seawater chemistry—Implications from primary fluid inclusions in marine halite. Am. J. Sci. 2000, 300, 3–45. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Timofeeff, M.N.; Brennan, S.T.; Hardie, L.A.; Demicco, R.V. Oscillations in Phanerozoic seawater chemistry: Evidence from fluid inclusions. Science 2001, 294, 1086–1088. [Google Scholar] [CrossRef] [Green Version]
- Lowenstein, T.K.; Demicco, R.V.; Timofeeff, M.N.; Hardie, L.A.; Brennan, S.T. Ramifications of secular variations in seawater chemistry. Geol. Soc. Am. Abstr. Programs 2003, 35, 203. [Google Scholar]
- Horita, J.; Zimmermann, H.; Holland, H.D. Chemical evolution of seawater during the Phanerozoic: Implications from the record of marine evaporites. Geochim. Cosmochim. Acta 2002, 66, 3733–3756. [Google Scholar] [CrossRef]
- Galamay, A.R.; Bukowski, K. Chemical composition of Badenian brines from primary fluid inclusions in halite (Transcarpathian Basin, Ukraine). Geol. Kwart. AGH 2011, 37, 245–267, (In Polish with English summary). [Google Scholar]
- Eugster, H.P.; Harvie, C.E.; Weare, J.H. Mineral equilibria in six-components water system, Na-K-Mg-Ca-SO4-Cl-H2O, at 25 °C. Geochim. Cosmochim. Acta 1980, 44, 1335–1347. [Google Scholar] [CrossRef]
- Galamay, A.R.; Bukowski, K.; Przybyło, J. Chemical composition of brines in the Badenian evaporite basin of the Carpathian Foredeep: Fluid inclusion data from Wieliczka (Poland). Slovak Geol. Mag. 1997, 3, 165–171. [Google Scholar]
- Kovalevich, V.M. Fluid inclusions in Badenian (Miocene) halite of Bochnia. Przeglad Geol. 1997, 45, 822–825. (In Polish) [Google Scholar]
- Galamay, A.R. Physico-chemical conditions of the formation of the Badenian salt deposits of the Ukrainian Forecarpathians (Grynivka area). Geol. Geokhimiya Horyuchykh Kopalyn 2010, 2, 64–77, (In Ukrainian with English summary). [Google Scholar]
- Poberezhskyy, A.V.; Kovalevych, V.M. Chemical composition of seawater in the Cenozoic. Geol. Geochem. Combust. Miner. 2001, 2, 90–109. (In Ukrainian) [Google Scholar]
- Galamay, A.R. Influence of continental run-off on the composition of marine brines of Badenian salt basin central part (Ukrainian Carpathian Foredeep). Mineral. Zbirnyk 2012, 62, 228–235, (In Ukrainian with English summary). [Google Scholar]
- Galamay, A.R.; Bukowski, K.; Poberezhskyy, A.V.; Karoli, S.; Kovalevych, V.M. Origin of the Badenian salts from East Slovakian Basin indicated by the analysis of fluid inclusions. Ann. Soc. Geol. Pol. 2004, 74, 267–276. [Google Scholar]
- Schwerdtner, W.M.; Wardlaw, N.C. Geochemistry of Bromine in Some Salt Rocks of the Prairie Evaporite Formation of Saskatchewan; First Symposium on Salt, Northern Ohio Geol. Soc.: Cleveland, OH, USA, 1963; Volume 1, pp. 240–246. [Google Scholar]
- Galamay, A.R.; Meng, F.; Bukowski, K. Sulphur isotopes in anhydrite from Badenian (Middle Miocene) salts of the Hrynivka area (Ukrainian Carpathian Foredeep). Geol. Q. 2014, 58, 429–438. [Google Scholar]
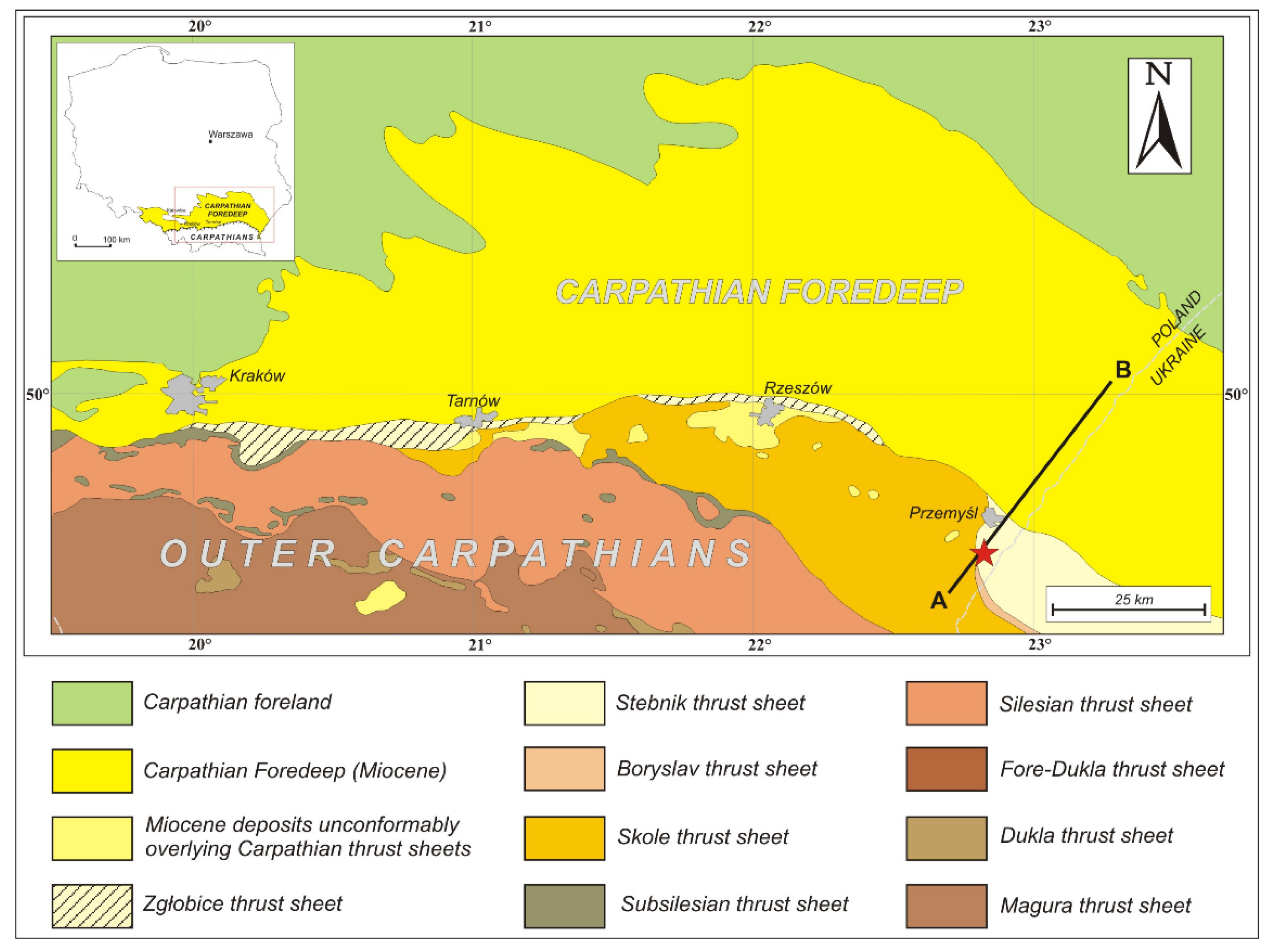
Figure 1.
A simplified geological map of the Outer Carpathians and their foreland between Kraków and Przemyśl. The A–B cross-section is shown in Figure 3. Red star: approximate location of the Huwniki-1 well.
Figure 1.
A simplified geological map of the Outer Carpathians and their foreland between Kraków and Przemyśl. The A–B cross-section is shown in Figure 3. Red star: approximate location of the Huwniki-1 well.
Figure 2.
Distribution of the facies of the Badenian evaporites in the Carpathian Foredeep in Poland, after [
28].
Figure 2.
Distribution of the facies of the Badenian evaporites in the Carpathian Foredeep in Poland, after [
28].
Figure 3.
This seismo-geological cross-section shows the Carpathian orogenic wedge’s frontal part and its foredeep, after [
18,
19,
21,
22,
23], modified. The Huwniki-1 well (see its location in
Figure 1) drilled the Miocene evaporites’ topmost part. The sub-evaporitic structure is not well visible on seismic data. The sub-evaporitic Miocene Skawina Beds may underlie Miocene evaporites.
Figure 3.
This seismo-geological cross-section shows the Carpathian orogenic wedge’s frontal part and its foredeep, after [
18,
19,
21,
22,
23], modified. The Huwniki-1 well (see its location in
Figure 1) drilled the Miocene evaporites’ topmost part. The sub-evaporitic structure is not well visible on seismic data. The sub-evaporitic Miocene Skawina Beds may underlie Miocene evaporites.
Figure 4.
Various types of salt rocks drilled by the Huwniki-1 well. (A) Pure recrystallized halite crystals (up to 50 mm large), within coarse-grained salt; depth: 4991.4 m. (B) Well-preserved chevron (“milky chevron”: inclusion-rich fluid) halite crystals; depth: 4994.7 m. (C) Halite–rudite (or salt breccias): structureless debris of halite crystals, mixed with anhydrite nodules and containing mudstone clasts, located within fine to the pelitic matrix, representing normal grading; depth: 4995.8 m. (D) Laminated mudstone, with anhydrite above medium-grained, porphyritic-textured grey salt; depth: 4998.0 m. (E) Medium-grained white salt, granoblastic texture, with sutured boundaries between fusiform crystals; depth: 4998.3 m. (F) Fine- and medium-grained grey salt, laminated with clay, silt, and fine grains of nodular anhydrite; depth: 4998.7 m.
Figure 4.
Various types of salt rocks drilled by the Huwniki-1 well. (A) Pure recrystallized halite crystals (up to 50 mm large), within coarse-grained salt; depth: 4991.4 m. (B) Well-preserved chevron (“milky chevron”: inclusion-rich fluid) halite crystals; depth: 4994.7 m. (C) Halite–rudite (or salt breccias): structureless debris of halite crystals, mixed with anhydrite nodules and containing mudstone clasts, located within fine to the pelitic matrix, representing normal grading; depth: 4995.8 m. (D) Laminated mudstone, with anhydrite above medium-grained, porphyritic-textured grey salt; depth: 4998.0 m. (E) Medium-grained white salt, granoblastic texture, with sutured boundaries between fusiform crystals; depth: 4998.3 m. (F) Fine- and medium-grained grey salt, laminated with clay, silt, and fine grains of nodular anhydrite; depth: 4998.7 m.
Figure 5.
Distribution of strontium, rubidium, and bromine, with the lithological successions of salt deposits of the Huwniki-1 well.
Figure 5.
Distribution of strontium, rubidium, and bromine, with the lithological successions of salt deposits of the Huwniki-1 well.
Figure 6.
Photomicrographs are showing various types of fluid inclusions in halite from the Huwniki-1 well. (A) A fragment of a primary halite crystal, with irregular dissolved surface underlined by sulfates’ presence, sample no. H-III-70. (B) Well-preserved primary chevron texture, sample no. H-III-70. (C) Two-phase (gas–liquid) inclusions, sample no. H-VI-10. (D) Irregular large secondary inclusions in clear halite, sample no. H-IV-0. (E) A secondary three-phase (gas–liquid–anhydrite) inclusion; the high-order interference colors displayed by the euhedral crystal of anhydrite, sample no. H-III-70 crossed nicols. (F) Light-yellow to brown low-carbon organic substance, within a large secondary fluid inclusion, sample no. H-III-70.
Figure 6.
Photomicrographs are showing various types of fluid inclusions in halite from the Huwniki-1 well. (A) A fragment of a primary halite crystal, with irregular dissolved surface underlined by sulfates’ presence, sample no. H-III-70. (B) Well-preserved primary chevron texture, sample no. H-III-70. (C) Two-phase (gas–liquid) inclusions, sample no. H-VI-10. (D) Irregular large secondary inclusions in clear halite, sample no. H-IV-0. (E) A secondary three-phase (gas–liquid–anhydrite) inclusion; the high-order interference colors displayed by the euhedral crystal of anhydrite, sample no. H-III-70 crossed nicols. (F) Light-yellow to brown low-carbon organic substance, within a large secondary fluid inclusion, sample no. H-III-70.
Figure 7.
Brine composition in the primary fluid inclusions of chevron halite (mol %), plotted on the Jänecke diagram, at 25 °C, after [
54]. The numbers and data mean the following on the map: 1—Wieliczka [
8,
46,
55]; 2—Bochnia [
56]; 3—Hrynivka [
57]; 4—Slanic-Prahova [
46]; 5—Huwniki (this paper); 6—Selec-Stupnyca [
46]; 7—Zabolotiv [
58]; 8—Solotvyno [
59]; 9—Mukhachevo [
59]; 10—Victoria [
46]; 11—Ocna Dej [
46]; and 12—Zbudza [
38,
46,
60].
Figure 7.
Brine composition in the primary fluid inclusions of chevron halite (mol %), plotted on the Jänecke diagram, at 25 °C, after [
54]. The numbers and data mean the following on the map: 1—Wieliczka [
8,
46,
55]; 2—Bochnia [
56]; 3—Hrynivka [
57]; 4—Slanic-Prahova [
46]; 5—Huwniki (this paper); 6—Selec-Stupnyca [
46]; 7—Zabolotiv [
58]; 8—Solotvyno [
59]; 9—Mukhachevo [
59]; 10—Victoria [
46]; 11—Ocna Dej [
46]; and 12—Zbudza [
38,
46,
60].
Table 1.
Bromine, rubidium, and strontium contents in pure halite samples collected from the Huwniki-1 well.
Table 1.
Bromine, rubidium, and strontium contents in pure halite samples collected from the Huwniki-1 well.
| No. | Sample No. | Depth [m] | Br [ppm] | Rb [ppm] | Sr [ppm] |
|---|
| 1 | I-10 | 4991.1 | 42 | 8 | 128 |
| 2 | I-50 | 4991.5 | 41 | 9 | 6 |
| 3 | II-0 | 4992.0 | 41 | 10 | 125 |
| 4 | II-40 | 4992.4 | 43 | 10 | 35 |
| 5 | III-70 | 4993.7 | 46 | 9 | 32 |
| 6 | IV-0 | 4994.0 | 37 | 8 | 22 |
| 7 | V-70 | 4995.7 | 42 | 9 | <3 |
| 8 | VI-30 | 4996.3 | 44 | 9 | <3 |
| 9 | VI-60 | 4996.6 | 45 | 9 | 246 |
| 10 | VII-0 | 4997.0 | 42 | 9 | <3 |
| 11 | VII-50 | 4997.5 | 46 | 9 | <3 |
| 12 | VIII-0 | 4998.0 | 49 | 9 | 39 |
| 13 | VIII-60 | 4998.6 | 47 | 9 | <3 |
| 14 | IX-10 | 4999.1 | 36 | 8 | 281 |
| 15 | IX-50 | 4999.5 | 46 | 9 | <3 |
Table 2.
Chemical compositions of brines collected from the primary fluid inclusions of halite. The numbers of fluid inclusions are given in parentheses.
Table 2.
Chemical compositions of brines collected from the primary fluid inclusions of halite. The numbers of fluid inclusions are given in parentheses.
| A Point in Figure 7 | Sample No. | Contents, g/L | Jänecke Unit, mol % |
|---|
| K+ | Mg2+ | SO42− | 2K | Mg | SO4 |
|---|
| 1 | H-VI-10 | 9.4 (3) | 35.9 (2) | 30.7 (2) | 6.3 | 77.0 | 16.7 |
| 2 | H-IV-0 | 7.5 (2) | 28.7 (3) | 24.0 (3) | 6.3 | 77.3 | 16.4 |
| 3 | H-VI-80 | 8.1 (2) | 33.1 (2) | 24.6 (2) | 6.0 | 79.1 | 14.9 |
| 4 | III-70 | 9.9 (3) | 40.6 (3) | 38.0 (2) | 5.8 | 76.2 | 18.0 |
| Modern ocean water, saturated until the beginning of the crystallization of: |
| halite * | | 3.9 | 12.6 | 17.6 | 6.6 | 69.0 | 24.3 |
| epsomite * | | 26.1 | 85.9 | 115.0 | 6.6 | 69.8 | 23.6 |
Table 3.
Chemical composition of brines collected from the early diagenetic fluid inclusions of halite. The numbers of fluid inclusions are given in parentheses.
Table 3.
Chemical composition of brines collected from the early diagenetic fluid inclusions of halite. The numbers of fluid inclusions are given in parentheses.
| A Point in Figure 7 | Sample No. | Contents, g/L | Jänecke Unit, mol % |
|---|
| K+ | Mg2+ | SO42− | 2K | Mg | SO4 |
|---|
| 2a | H-IV-0 | 4.7 (1) | 24.4 (2) | 16.9 (1) | 4.8 | 81.0 | 14.2 |
| 3a | H-VI-80 | 5.8 (1) | 18.1 (1) | 15.5 (1) | 6.0 | 79.1 | 14.9 |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).