Geochemical and Geochronological Constraints on the Genesis of Ion-Adsorption-Type REE Mineralization in the Lincang Pluton, SW China
Abstract
1. Introduction
2. Study Areas and Sampling
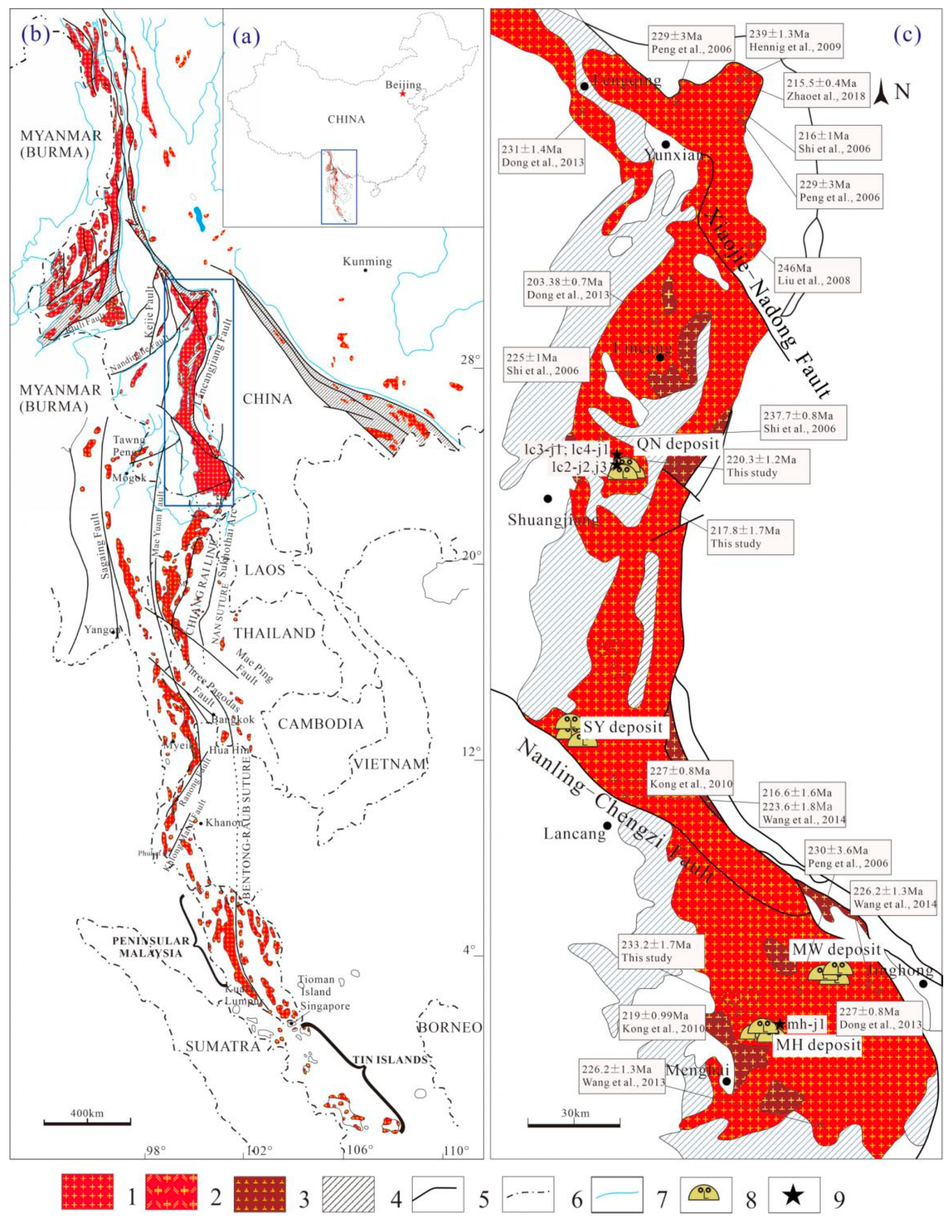
2.1. Geological Background
2.2. The Lincang Granitic Batholith
2.3. Sampling and Methods
2.3.1. Sampling
2.3.2. Methods
3. Petrography
3.1. Petrographic Characteristics of LC Granite
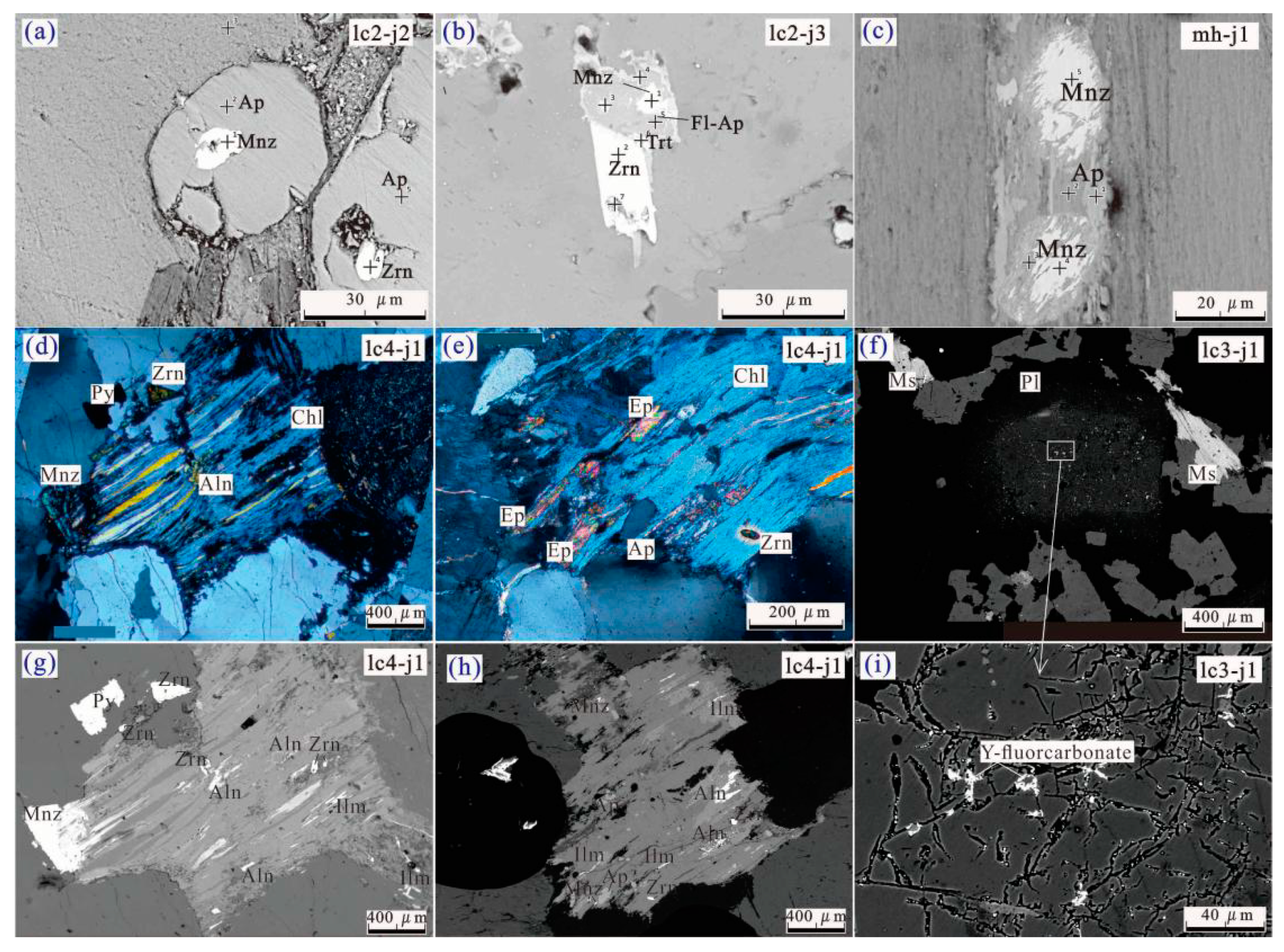
3.2. REE Minerals
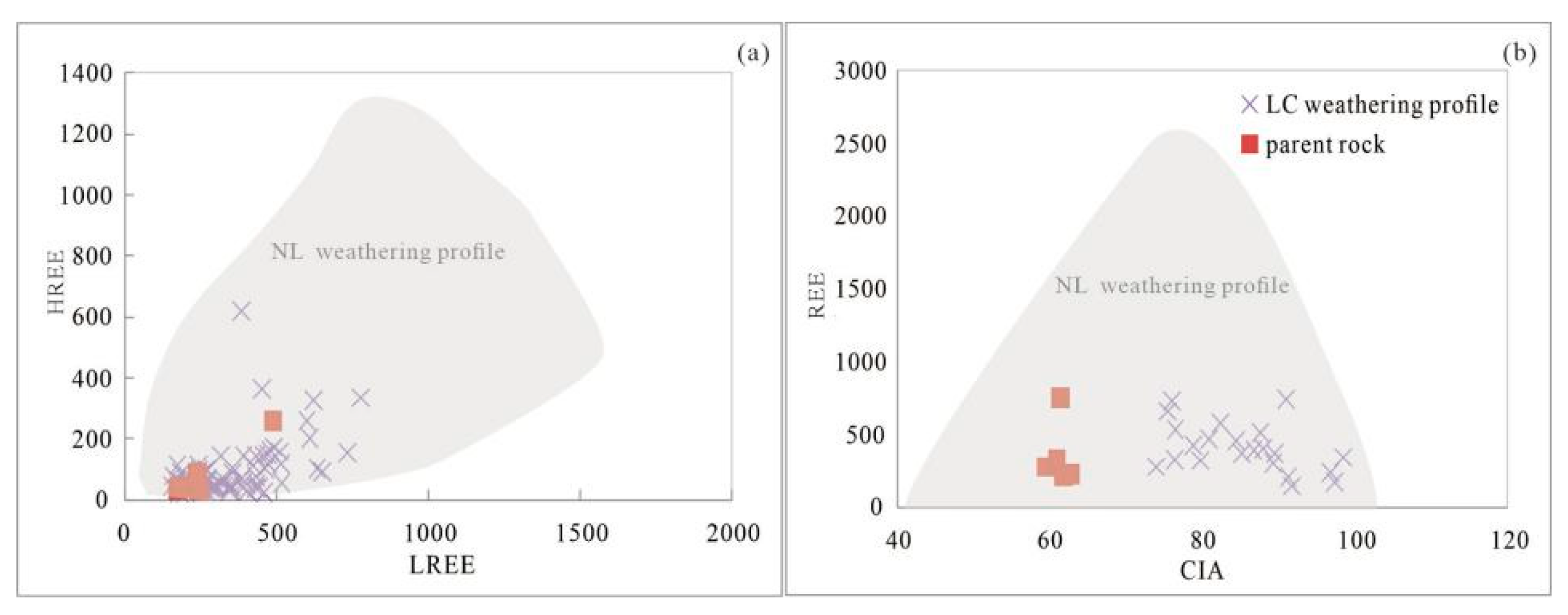
3.3. Weathering Profile of the LC Granite
4. Results
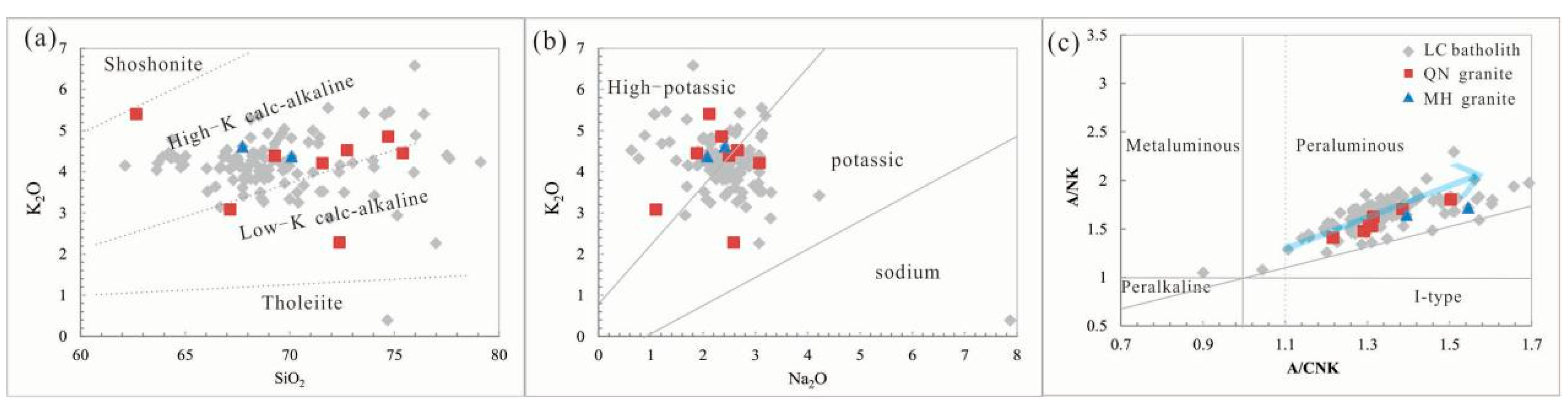
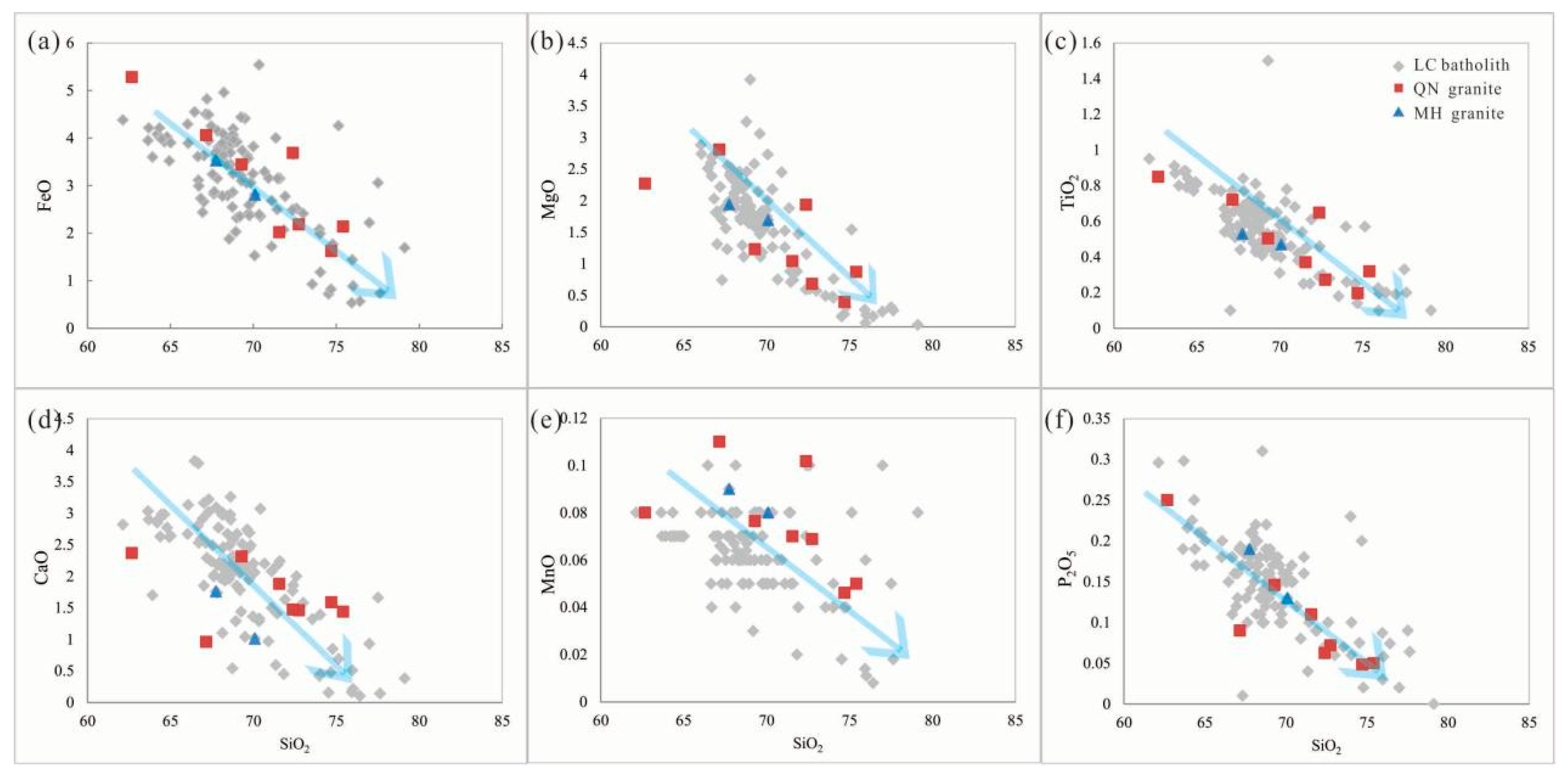
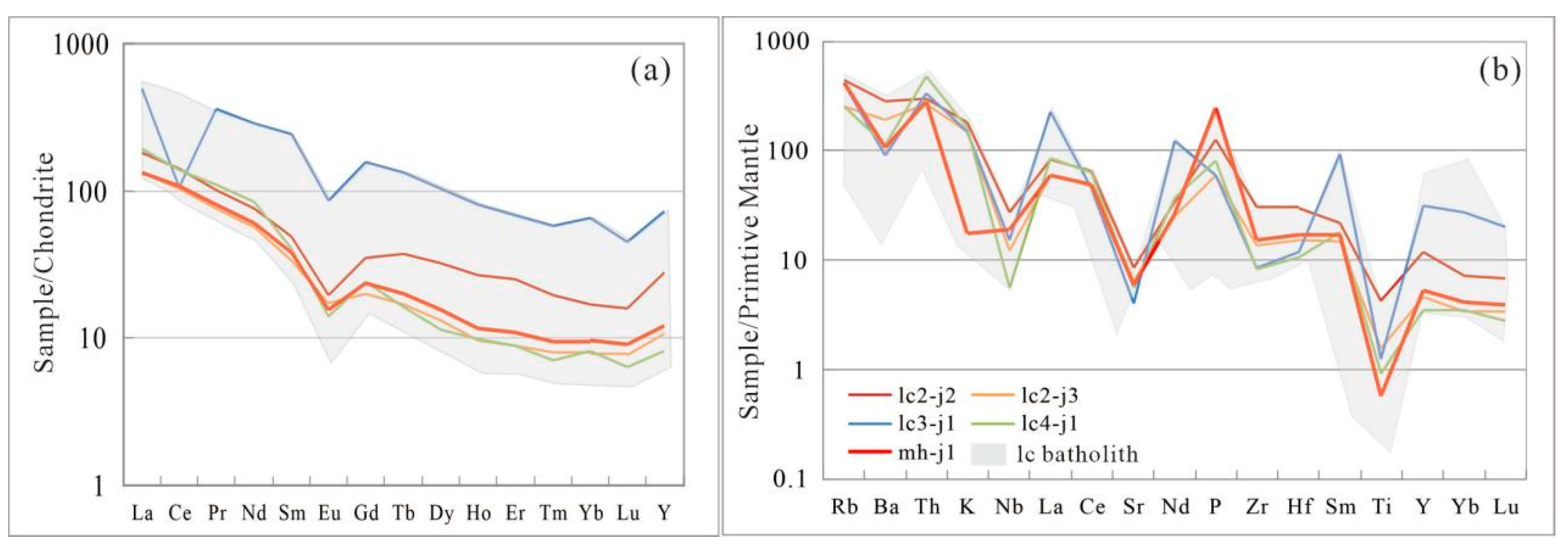
4.1. Geochemistry of the QN Granite
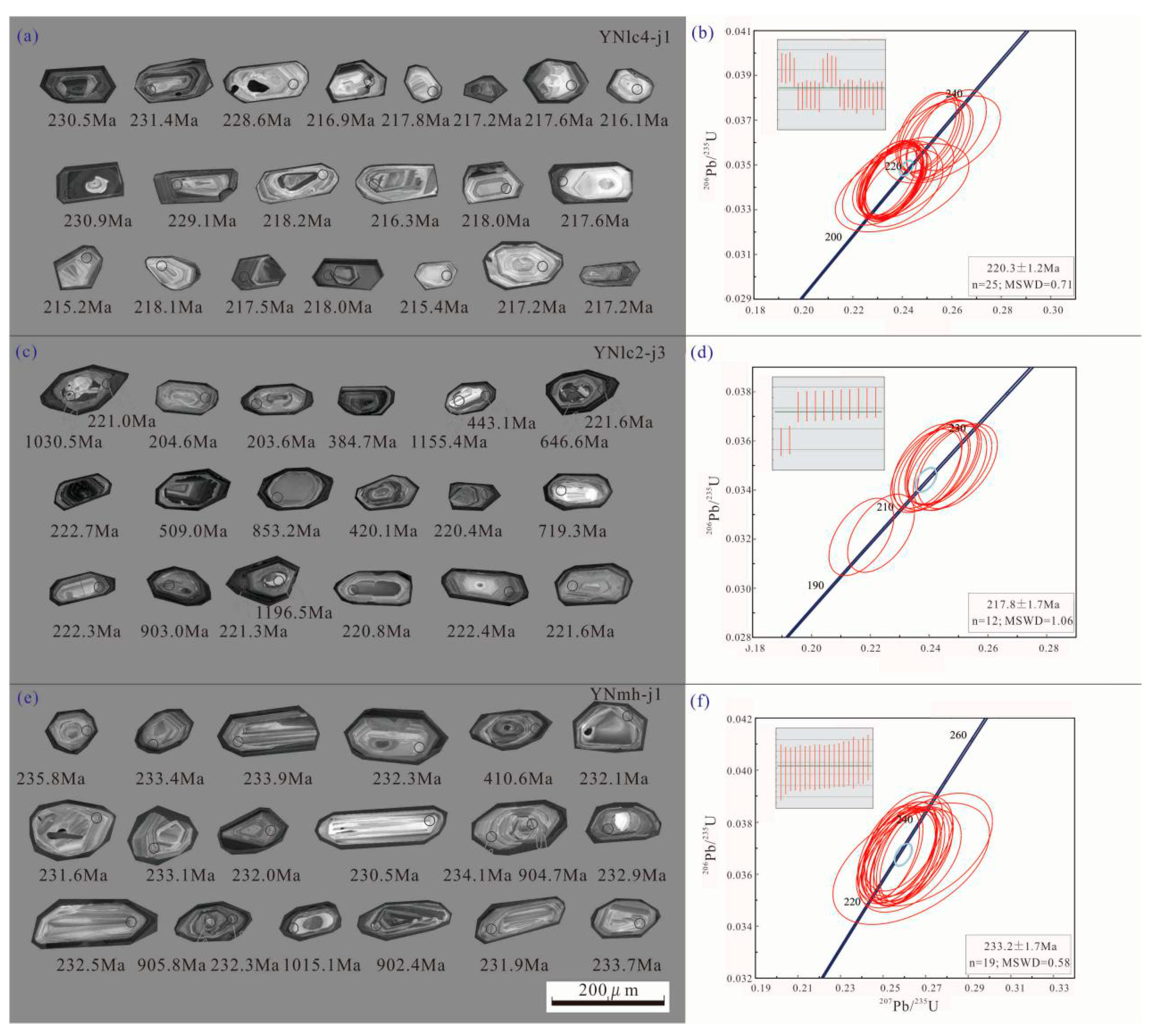
4.2. Zircon U–Pb Dating
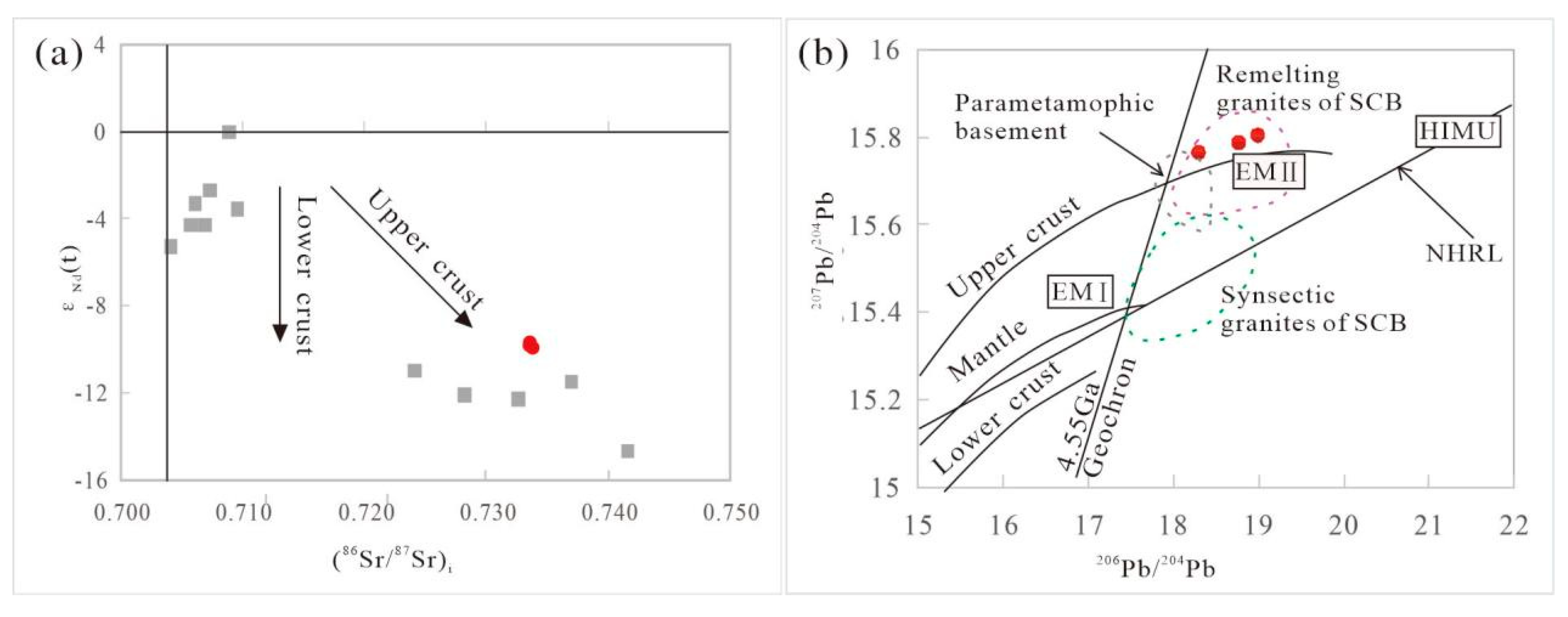
4.3. Sr–Nd–Pb Isotopic Compositions
5. Discussion
5.1. The Timing of REE Mineralization in the LC Granite
5.2. Petrogenesis and Tectonic Setting of the LC Pluton
5.3. Key Factors in REE Mineralization
5.3.1. Rare-Earth Element Minerals
5.3.2. Secondary Minerals
5.3.3. Intensity of Granite Weathering
- Feldspar→kaolinite→gibbsite;
- Muscovite→illite→montmorillonite→kaolinite→gibbsite;
- Biotite→chlorite→vermiculite→montmorillonite→beidellite→kaolinite→gibbsite.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chakhmouradian, A.R.; Wall, F. Rare Earth Elements: Minerals, Mines, Magnets (and More). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Faris, N.; Ram, R.; Tardio, J.; Bhargava, S.; McMaster, S.A.; Pownceby, M.I. Application of ferrous pyrometallurgy to the beneficiation of rare earth bearing iron ores—A review. Miner. Eng. 2017, 110, 20–30. [Google Scholar] [CrossRef]
- Faris, N.; Ram, R.; Tardio, J.; Bhargava, S.; Pownceby, M.I. Characterisation of a ferruginous rare earth bearing lateritic ore and implications for rare earth mineral processing. Miner. Eng. 2019, 134, 23–36. [Google Scholar] [CrossRef]
- Soltani, F.; Abdollahy, M.; Petersen, J.; Ram, R.; Becker, M.; Koleini, S.J.; Moradkhani, D. Leaching and recovery of phosphate and rare earth elements from an iron-rich fluorapatite concentrate: Part I: Direct baking of the concentrate. Hydrometallurgy 2018, 177, 66–78. [Google Scholar] [CrossRef]
- Soltani, F.; Abdollahy, M.; Petersen, J.; Ram, R.; Koleini, S.J.; Moradkhani, D. Leaching and recovery of phosphate and rare earth elements from an iron-rich fluorapatite concentrate: Part II: Selective leaching of calcium and phosphate and acid baking of the residue. Hydrometallurgy 2019, 184, 29–38. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloy. Compd. 2006, 37, 1339–1343. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Hu, C.S.; Luo, Z.M. Geological characteristic of mineralization of rare earth deposit of the ion–absorption type and their prospecting direction. Bull. Chin. Acad. Geol. Sci. 1981, 2, 102–118. [Google Scholar]
- Sanematsu, K.; Murakami, H.; Watanabe, Y.; Duangsurigna, S.; Siphandone, V. Enrichment of rare earth elements (REE) in granitic rocks and their weathered crusts in central and southern Laos. Bull. Geol. Surv. Jpn. 2009, 60, 527–558. [Google Scholar] [CrossRef]
- Sanematsu, K.; Kon, Y.; Imai, A.; Watanabe, K.; Watanabe, Y. Geochemical and mineralogical characteristics of ion-adsorption type REE mineralization in Phuket, Thailand. Miner. Depos. 2013, 48, 437–451. [Google Scholar] [CrossRef]
- Yuan, Z.X.; He, H.H.; Liu, L.J.; Wang, D.H.; Zhao, Z. Rare Metal and Rare Earth Deposit Abroad; Beijing Science Press: Beijing, China, 2016; pp. 1–110. [Google Scholar]
- Padrones, J.T.; Imai, A.; Takahashi, R. Geochemical Behavior of Rare Earth Elements in Weathered Granitic Rocks in Northern Palawan, Philippines. Resour. Geol. 2017, 67, 231–253. [Google Scholar] [CrossRef]
- Ram, R.; Becker, M.; Brugger, J.; Etschmann, B.; Burcher-Jones, C.; Howard, D.; Kooyman, P.J.; Petersen, J. Characterisation of a rare earth element- and zirconium-bearing ion-adsorption clay deposit in Madagascar. Chem. Geol. 2019, 522, 93–107. [Google Scholar] [CrossRef]
- Wang, D.H.; Wang, R.J.; Li, J.K.; Zhao, Z.; Yu, Y.; Dai, J.J.; Chen, Z.H.; Li, D.X.; Qu, W.J.; Deng, M.C.; et al. The progress in the strategic research and survey of rare earth, rare metal and rare–scattered elements mineral resources. Geol. China 2013, 40, 361–370. [Google Scholar]
- Wang, D.H.; Zhao, Z.; Yu, Y.; Zhao, T.; Li, J.K.; Dai, J.J.; Liu, X.X. Progress, problems and research orientation of ion–adsorpotion type rare earth resources. Rock Miner. Anal. 2013, 32, 796–802. [Google Scholar]
- Wang, F.; Liu, F.L.; Liu, P.H.; Shi, J.R.; Cai, J. Petrogenesis of Lincang granites in the south of Lancangjiang area: Constrain from geochemistry and zircon U–Pb geochronology. Acta Petrol. Sinica 2014, 30, 3034–3050. [Google Scholar]
- Wang, D.H.; Zhao, Z.; Yu, Y.; Wang, C.H.; Dai, J.J.; Sun, Y.; Zhao, T.; Li, J.K.; Huang, F.; Chen, Z.Y.; et al. A Review of the Achievements in the Survey and Study of Ion-absorption Type REE Deposits in China. Acta Geosci. Sinica 2017, 38, 317–325. [Google Scholar]
- Zhao, Z.; Wang, D.H.; Chen, Z.Y.; Guo, N.X.; Liu, X.X.; He, H.H. Metallogenic specialization of rare earth mineralized igneous rocks in the Eastern Nanling Region. Geotecton. Metallog. 2014, 38, 255–263. [Google Scholar]
- Zhao, Z.; Wang, D.H.; Liu, X.X.; Zhang, Q.W.; Yao, M.; Gu, W.N. Geochemical features of rare earth elements in different weathering stage of the Guangxi Huashan granite and its influence factors. Chin. Rare Earth 2015, 3, 14–20. [Google Scholar]
- Zhao, Z.; Wang, D.H.; Chen, Z.H.; Chen, Z.Y. Progress of research on metallogenic regularity of ion-adsorption type REE deposit in the Nanling Range. Acta Geol. Sinica 2017, 91, 2814–2827. [Google Scholar]
- Liu, Y.; Chakhmouradian, A.R.; Hou, Z.; Song, W.; Kynický, J. Development of REE mineralization in the giant Maoniuping deposit (Sichuan, China): Insights from mineralogy, fluid inclusions, and trace-element geochemistry. Miner. Depos. 2018, 54, 701–718. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Z. A synthesis of mineralization styles with an integrated genetic model of carbonatite-syenite-hosted REE deposits in the Cenozoic Mianning-Dechang REE metallogenic belt, the eastern Tibetan Plateau, southwestern China. J. Asian Earth Sci. 2017, 137, 35–79. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.Y.; Yang, Z.S.; Sun, X.; Zhu, Z.M.; Zhang, Q.C. Mineralogical and geochemical studies of brecciated ores in the Dalucao REE deposit, Sichuan Province, southwestern China. Ore Geol. Rev. 2015, 70, 613–636. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.; Chen, C.; Zhang, S.; Sun, X.; Yang, Z.; Liang, W. Geochemical and mineralogical characteristics of weathered ore in the Dalucao REE deposit, Mianning—Dechang REE Belt, western Sichuan Province, southwestern China. Ore Geol. Rev. 2015, 71, 437–456. [Google Scholar] [CrossRef]
- Wu, C.Y.; Huang, D.H.; Bai, G.; Ding, X.S. Differentiation of rare earth elements and origin of granitic rocks,Nanling Mountain area. Acta Petrol. Mineral. 1990, 9, 106–116. [Google Scholar]
- Wu, C.Y.; Bai, G.; Huang, D.H.; Zhu, Z.S. Characteristics and significance of HREE-rich granitoids of the Nanling mountain area. Bull. Chin. Acad. Geol. Sci. 1992, 25, 43–58. [Google Scholar]
- Wu, C.Y.; Huang, D.H.; Guo, Z.X. REE geochemistry in the weathering process of granites in Longnan County, Jiangxi Province. Acta Geol. Sinica 1992, 63, 349–362. [Google Scholar]
- Bao, Z.; Zhao, Z. Geochemistry of mineralization with exchangeable REY in the weathering crusts of granitic rocks in South China. Ore Geol. Rev. 2008, 33, 519–535. [Google Scholar] [CrossRef]
- Lu, L.; Wang, D.H.; Wang, C.H.; Zhao, Z.; Feng, W.J.; Xu, X.C.; Yu, F. Mineralization regularity of ion–adsorption type REE deposits on Lincang granite in Yunnan Province. Acta Geol. 2019, 96, 1466–1478. [Google Scholar]
- Lu, L.; Wang, D.H.; Wang, C.H.; Zhao, Z.; Feng, W.J.; Xu, X.C.; Chen, C.; Zhong, H.R. The metallogenic regularity of ion-adsorption type REE deposit in Yunnan Province. Acta Geol. 2020, 94, 179–191. [Google Scholar]
- Migdisov, A.; Williams-Jones, A.; Brugger, J.; Caporuscio, F. Hydrothermal transport, deposition, and fractionation of the REE: Experimental data and thermodynamic calculations. Chem. Geol. 2016, 439, 13–42. [Google Scholar] [CrossRef]
- Yusoff, Z.M.; Ngwenya, B.T.; Parsons, I. Mobility and fractionation of REEs during deep weathering of geochemically contrasting granites in a tropical setting, Malaysia. Chem. Geol. 2013, 349, 71–86. [Google Scholar] [CrossRef]
- Yang, M.; Liang, X.; Ma, L.; Huang, J.; He, H.; Zhu, J. Adsorption of REEs on kaolinite and halloysite: A link to the REE distribution on clays in the weathering crust of granite. Chem. Geol. 2019, 525, 210–217. [Google Scholar] [CrossRef]
- YNBGMR (Yunnan Bureau Geological Mineral Resource). Regional Geology of Guizhou Province; Geology Publish House: Beijing, China, 1990; pp. 1–729. [Google Scholar]
- Zhang, Y.F. An approach to the characeristics of the Indo–China movement in western Yunnan area. Yunnan Geol. 1985, 1, 59–68. [Google Scholar]
- Heppe, K.; Helmcke, D.; Wemmer, K. The Lancang River zone of southwestern Yunnan, China: A questionable location for the active continental margin of Paleo-Tethys. J. Asian Earth Sci. 2007, 30, 706–720. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Fan, W.; Zi, J.; Cai, Y.; Yang, G. Petrogenesis and tectonic implications of Late-Triassic high ɛ Nd(t)-ɛ Hf(t) granites in the Ailaoshan tectonic zone (SW China). Sci. China Earth Sci. 2014, 57, 2181–2194. [Google Scholar] [CrossRef]
- Turner, S.; Foden, J.; Morrison, R. Derivation of some A-type magmas by fractionation of basaltic magma: An example from the Padthaway Ridge, South Australia. Lithos 1992, 28, 151–179. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of eocene calc-alkaline volcanic rocks from the Kastamonu area, Northern Turkey. Contrib. Miner. Pet. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Kemp, A.; Hawkesworth, C. Granitic Perspectives on the Generation and Secular Evolution of the Continental Crust. Treatise Geochem. 2003, 3, 349–410. [Google Scholar] [CrossRef]
- Peng, T.P.; Wang, Y.J.; Fan, W.M.; Liu, D.Y.; Shi, Y.R.; Miao, L.C. SHRIMP zircon U–Pb geochronology of early Mesozoic felsic igneous rocks from the southern Lancangjiang and its tectonic implications. Sci. China Ser. D Earth Sci. 2006, 49, 1032–1042. [Google Scholar] [CrossRef]
- Peng, T.; Wilde, S.A.; Wang, Y.; Fan, W.; Peng, B. Mid-Triassic felsic igneous rocks from the southern Lancangjiang Zone, SW China: Petrogenesis and implications for the evolution of Paleo-Tethys. Lithos 2013, 15–32. [Google Scholar] [CrossRef]
- Jian, P.; Liu, D.; Kröner, A.; Zhang, Q.; Wang, Y.; Sun, X.; Zhang, W. Devonian to Permian plate tectonic cycle of the Paleo-Tethys Orogen in southwest China (I): Geochemistry of ophiolites, arc/back-arc assemblages and within-plate igneous rocks. Lithos 2009, 113, 748–766. [Google Scholar] [CrossRef]
- Jian, P.; Liu, D.; Kröner, A.; Zhang, Q.; Wang, Y.; Sun, X.; Zhang, W. Devonian to Permian plate tectonic cycle of the Paleo-Tethys Orogen in southwest China (II): Insights from zircon ages of ophiolites, arc/back-arc assemblages and within-plate igneous rocks and generation of the Emeishan CFB province. Lithos 2009, 113, 767–784. [Google Scholar] [CrossRef]
- Kong, H.L. Genchemistty, Geochronology and Petrogenisis of Lincang Granites in Southern Lancangjiang Zone of Sanjiang Area. Master’s Thesis, China University of Geosciences, Beijing, China, 2011. [Google Scholar]
- Kong, H.L.; Dong, G.C.; Mo, X.X.; Zhao, Z.D.; Zhu, D.C.; Wang, S.; Li, R.; Wang, Q.L. Petrogenesis of Lincang granites in Sanjiang area of western Yunnan Province: Constraints from geochemistry, zircon U-Pb geochronology and Hf isotope. Acta Petrol. Sinica 2012, 28, 1438–1452. [Google Scholar]
- Whalen, J.B.; Currie, K.L.; Chappell, B.W. A-type granites: Geochemical characteristics, discrimination and petrogenesis. Contrib. Miner. Pet. 1987, 95, 407–419. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and Isotopic Systematics of Oceanic Basalts: Implications for Mantle Composition and Processes. In Magmatism in the Ocean Basins; Geological Society: London, UK, 1989; Volume 42, pp. 313–345. [Google Scholar]
- Cong, B.L.; Wu, G.Y.; Zhang, Q. Petrotectonic evolution of the Tethys zone in western Yunnan, China. Chin. Sci. Bull. 1993, 23, 1201–1207. [Google Scholar]
- Qiu, Y.M.; Gao, S.; McNaughton, N.J.; Groves, D.I.; Ling, W.L. First evidence of >3.2 Ga continental crust in the Yangtze craton of south China and its implications for Archean crustal evolution and Phanerozoic tectonics. Geology 2000, 33, 309–314. [Google Scholar]
- Shi, X.B.; Qiu, X.L.; Liu, H.L.; Chu, Z.Y.; Xia, B. Thermochronological analyses on the cooling history of the Lincang granitoid batholith, Western Yunnan. Acta Petrol. Sinica 2006, 22, 465–479. [Google Scholar]
- Li, X.L. Basic characteristic and formation structural environment of Lincang composite granite batholith. Yunnan Geol. 1996, 1, 1–18. [Google Scholar]
- Pupin, J.P. Zircon and granite petrology. Contrib. Miner. Pet. 1980, 73, 207–220. [Google Scholar] [CrossRef]
- Zapata, A.; Botelho, N.F. Mineralogical and geochemical characterization of rare-earth occurrences in the Serra do Mendes massif, Goiás, Brazil. J. Geochem. Explor. 2018, 188, 398–412. [Google Scholar] [CrossRef]
- Sylvester, P.J. Post-collisional strongly peraluminous granites. Lithos 1998, 45, 29–44. [Google Scholar] [CrossRef]
- Altherr, R.; Holl, A.; Hegner, E.; Langer, C.; Kreuzer, H. High-potassium, calc-alkaline I-type plutonism in the European Variscides: Northern Vosges (France) and northern Schwarzwald (Germany). Lithos 2000, 50, 51–73. [Google Scholar] [CrossRef]
- Chappell, B.W.; White, A.J.R. I- and S-type granites in the Lachlan Fold Belt. Earth Environ. Sci. Trans. R. Soc. Edinb. 1992, 83, 1–26. [Google Scholar] [CrossRef]
- Chappell, B.W.; White, A.J.R. Two contrasting granite types: 25 years later. Aust. J. Earth Sci. 2001, 48, 488–489. [Google Scholar] [CrossRef]
- Chappell, B.W.; White, A.J.R. Two contrasting granite types. Pacific Geol. 1974, 8, 173–174. [Google Scholar]
- Harris, N.B.W.; Pearce, J.A.; Tindle, A.G. Geochemical Characteristics of Collision-Zone Magmatism. In Collision Tectonics; Geological Society: London, UK, 1986; pp. 67–81. [Google Scholar]
- Brown, M. Granite: From genesis to emplacement. Bull. Geol. Soc. Am. 2013, 125, 1079–1113. [Google Scholar] [CrossRef]
- Harris, N.B.W.; Inger, S. Trace element modelling of pelite-derived granites. Contrib. Miner. Pet. 1992, 110, 46–56. [Google Scholar] [CrossRef]
- Zindler, A.; Hart, S. Chemical geodynamics. Annu. Rev. Earth Planet. Sci. 1986, 14, 493–571. [Google Scholar] [CrossRef]
- Mo, X.X.; Shen, S.Y.; Zhu, Q.W. Volcanics-Ophiolite and Mineralization of Middle and Southern Part. In Sanjiang, Southern China; Geological Publishing House: Beijing, China, 1998; pp. 1–128. [Google Scholar]
- Sone, M.; Metcalfe, I. Parallel Tethyan sutures in mainland Southeast Asia: New insights for Palaeo-Tethys closure and implications for the Indosinian orogeny. Comptes Rendus Geosci. 2008, 340, 166–179. [Google Scholar] [CrossRef]
- Fan, W.M.; Wang, Y.J.; Zhang, A.M.; Zhang, F.F.; Zhang, Y.Z. Permian arc-backarc basin development along the Ailaoshan tectonic zone: Geochemical, isotopic and geochronological evidence from the Mojiang volcanic rocks, Southwest China. Lithos 2010, 119, 553–568. [Google Scholar] [CrossRef]
- Bonin, B. A-type granites and related rocks: Evolution of a concept, problems and prospects. Lithos 2007, 97, 1–29. [Google Scholar] [CrossRef]
- Pearce, J.A.; Harris, N.B.W.; Tindle, A.G. Trace Element Discrimination Diagrams for the Tectonic Interpretation of Granitic Rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- Pearce, J.A. Sources and settings of granitic rocks. Episodes 1996, 19, 120–125. [Google Scholar] [CrossRef]
- Li, M.Y.H.; Zhou, M.-F.; Williams-Jones, A.E. The Genesis of Regolith-Hosted Heavy Rare Earth Element Deposits: Insights from the World-Class Zudong Deposit in Jiangxi Province, South China. Econ. Geol. 2019, 114, 541–568. [Google Scholar] [CrossRef]
- Henderson, P. Rare Earth Element Geochemistry; Elsevier: Amsterdam, The Netherlands, 1984; pp. 180–213. [Google Scholar]
- Chen, D.Q.; Wu, J.S. The mineralization mechanism of ion-adsorbed REE deposit. J. Chin. Rare Earth Soc. 1990, 8, 175–179. [Google Scholar]
- Qi, L.; Hu, J.; Gregoire, D.C. Determination of trace elements in granites by inductively coupled plasma mass spectrometry. Talanta 2000, 51, 507–513. [Google Scholar]
- Liu, Y.S.; Gao, S.; Hu, Z.C.; Gao, C.G.; Zong, K.Q.; Wang, D.B. Continental and oceanic crust recycling-induced melt-peridotite interactions in the Trans-North China Orogen: U-Pb dating, Hf isotopes and trace elements in zircons from mantle xenoliths. J. Petrol. 2010, 51, 537–571. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, Y.; Li, Q.L.; Guo, C.H.; Chamberlain, K.R. Precise determination of Phanerozoic zircon Pb/Pb age by multi-collector SIMS without external standardi- zation. Geochem. Geophys. Geosyst. 2009, 10. [Google Scholar] [CrossRef]
- Sláma, J.; Košler, J.; Condon, D.J. Plešovice zircon—A new natural reference material for U-Pb and Hf isotopic microanalysis. Chem. Geol. 2008, 249, 1–353. [Google Scholar] [CrossRef]
- Wiedenbeck, M.; Alle, P.; Corfu, F.; Griffin, W.L.; Meier, M.; Oberli, F.; Vonquadt, A.; Roddick, J.C.; Speigel, W. Three natural zircon standards for U-Th–Pb, Lu–Hf, trace–element and REE analyses. Geostandard Newslett. 1995, 19, 1–23. [Google Scholar] [CrossRef]
- Ludwig, K.R. Users Manual for Isoplot/Ex rev. 2.49. Berkeley Geochronology Centre Special Publication. 2001. Available online: http://www.geo.cornell.edu/geology/classes/Geo656/Isoplot%20Manual.pdf (accessed on 21 February 2020).




| Mineral | Formula | REO | LREO | HREO | LREO/HREO | Nd2O3/REO | Ce2O3/REO | Eu2O3/REO | Dy2O3/REO | Y2O3/REO |
|---|---|---|---|---|---|---|---|---|---|---|
| Sphene (n = 2) | CaTi[SiO4]O | 0.37 | 0.21 | 0.39 | 0.78 | 0.12 | 0.25 | 0.00 | 0.13 | 0.19 |
| apatite (n = 3) | Ca5(PO4)3F | 8.46 | 7.59 | 1.56 | 6.49 | 0.21 | 0.50 | 0.00 | 0.09 | 0.41 |
| allanite (n = 17) | (Ce,Ca)2(Al,Fe3+)3(SiO4)3(OH) | 16.92 | 14.53 | 5.78 | 8.25 | 0.22 | 3.42 | 0.01 | 0.03 | 0.25 |
| Monazite (n = 21) | (Ce,La,Nd,Th)PO4 | 61.03 | 57.55 | 17.01 | 16.80 | 0.22 | 12.89 | 0.00 | 0.00 | 1.03 |
| xenotime (n = 5) | YPO4 | 53.05 | 0.77 | 52.28 | 0.01 | 0.01 | 0.00 | 0.00 | 0.07 | 0.74 |
| thorite (n = 2) | (Y,Th,Ca,U)(Ti,Fe3+)3(O,OH)4 | 17.61 | 16.55 | 1.07 | 10.67 | 0.39 | 0.31 | 0.00 | 0.00 | 0.00 |
| zircon (n = 2) | (Zr,Y)(Si,P)O4 | 0.67 | 0.11 | 0.56 | 0.26 | 0.00 | 0.15 | 0.00 | 0.18 | 0.11 |
| allanite-Y (n = 7) | (Ce,Ca,Y)2(Al,Fe3+)3(SiO4)3(OH) | 31.83 | 20.89 | 10.95 | 1.80 | 0.34 | 0.02 | 0.00 | 0.09 | 0.16 |
| flourite-Y (n = 1) | (Ca,Y)F2 | 15.56 | 9.38 | 6.18 | 1.52 | 0.30 | 0.03 | 0.00 | 0.06 | 0.21 |
| REE-flourcarbonate (n = 1) | 59.20 | 31.94 | 27.26 | 1.17 | 0.27 | 0.06 | 0.00 | 0.08 | 0.28 |
| Sample | lc2-j2 | lc2-j3 | lc3-j1 | lc4-j1 | mh1-j1 |
|---|---|---|---|---|---|
| (wt.%) | |||||
| SiO2 | 62.7 | 75.4 | 72.7 | 74.7 | 67.7 |
| Al2O3 | 15.9 | 12.1 | 13.7 | 12.9 | 0.53 |
| CaO | 2.37 | 1.44 | 1.46 | 1.59 | 14.7 |
| Fe2O3 | 6.44 | 2.77 | 0.12 | 0.09 | 4.29 |
| FeO | 5.28 | 2.14 | 2.18 | 1.62 | 3.53 |
| K2O | 2.27 | 0.87 | 0.68 | 0.39 | 0.09 |
| MgO | 0.08 | 0.05 | 0.07 | 0.05 | 1.94 |
| MnO | 2.12 | 1.88 | 2.66 | 2.35 | 1.76 |
| Na2O | 5.40 | 4.45 | 4.52 | 4.85 | 2.42 |
| P2O5 | 0.85 | 0.32 | 0.27 | 0.20 | 4.61 |
| TiO2 | 0.25 | 0.05 | 0.07 | 0.05 | 0.19 |
| CO2 | 0.36 | 0.20 | 0.23 | 0.20 | 0.48 |
| H2O+ | 0.87 | 0.65 | 0.70 | 0.44 | 0.92 |
| LOI | 0.83 | 0.50 | 0.63 | 0.49 | 0.90 |
| (×10−6) | |||||
| Li | 37.8 | 14.8 | 36.0 | 16.2 | 39.1 |
| Be | 2.27 | 1.46 | 5.00 | 1.40 | 6.03 |
| Cr | 41.4 | 41.4 | 19.1 | 17.6 | 64.6 |
| Co | 15.4 | 5.68 | 4.09 | 3.00 | 10.2 |
| Ni | 16.0 | 10.8 | 6.71 | 2.61 | 21.9 |
| Cu | 17.2 | 11.1 | 10.2 | 4.25 | 13.2 |
| Zn | 93.4 | 38.4 | 36.0 | 23.0 | 63.4 |
| Ga | 26.1 | 15.9 | 16.6 | 13.3 | 19.0 |
| Rb | 277 | 163 | 257 | 159 | 268 |
| Sr | 181 | 136 | 85.5 | 119 | 126 |
| Mo | 1.06 | 0.84 | 0.39 | 0.68 | 0.82 |
| Cd | 0.07 | <0.05 | 0.07 | 0.03 | 0.10 |
| In | 0.13 | <0.05 | 0.05 | 0.02 | 0.07 |
| Cs | 8.66 | 3.77 | 9.51 | 4.47 | 16.4 |
| Ba | 1972 | 1322 | 638 | 792 | 754 |
| Tl | 1.47 | 0.82 | 1.38 | 0.90 | 1.50 |
| Pb | 43.3 | 55.2 | 41.1 | 37.6 | 42.4 |
| Bi | 0.19 | 0.17 | 1.13 | 0.11 | 1.10 |
| Th | 25.2 | 22.9 | 28.6 | 40.5 | 24.2 |
| U | 3.45 | 3.53 | 6.03 | 3.53 | 7.90 |
| Nb | 19.7 | 8.66 | 10.9 | 3.96 | 13.6 |
| Ta | 1.70 | 0.91 | 1.47 | 0.48 | 1.54 |
| Zr | 339 | 153 | 96.1 | 92.9 | 172 |
| Hf | 9.21 | 4.74 | 3.71 | 3.27 | 5.21 |
| Sn | 5.06 | 2.73 | 10.5 | 3.89 | 10.9 |
| Sb | 0.16 | 0.09 | 0.09 | 0.07 | 0.07 |
| W | 2.82 | 0.68 | 2.48 | 0.95 | 2.44 |
| As | 2.51 | 1.03 | 1.01 | 1.09 | 11.5 |
| V | 116 | 36.1 | 20.8 | 16.4 | 73.5 |
| Sc | 16.1 | 6.60 | 5.42 | 3.68 | 10.8 |
| La | 56.5 | 41.5 | 154 | 59.5 | 41.5 |
| Ce | 115 | 83.8 | 81.1 | 112 | 87.1 |
| Pr | 12.4 | 9.35 | 41.3 | 13.5 | 9.89 |
| Nd | 45.6 | 34.1 | 166 | 50.1 | 36.3 |
| Sm | 9.65 | 6.52 | 41.7 | 7.93 | 7.52 |
| Eu | 1.45 | 1.27 | 6.18 | 1.03 | 1.14 |
| Gd | 9.11 | 5.17 | 38.9 | 6.28 | 6.11 |
| Tb | 1.79 | 0.80 | 6.38 | 0.78 | 0.95 |
| Dy | 10.5 | 4.24 | 33.5 | 3.68 | 5.07 |
| Ho | 1.92 | 0.70 | 5.78 | 0.70 | 0.83 |
| Er | 5.29 | 1.87 | 14.2 | 1.89 | 2.28 |
| Tm | 0.64 | 0.26 | 1.87 | 0.23 | 0.31 |
| Yb | 3.53 | 1.65 | 13.6 | 1.71 | 2.02 |
| Lu | 0.51 | 0.25 | 1.47 | 0.20 | 0.29 |
| Y | 54.3 | 21.1 | 143 | 16.0 | 24.0 |
| ΣREE | 328 | 212 | 749 | 275 | 225 |
| LREE | 240 | 176 | 490 | 244 | 183 |
| HREE | 87.6 | 36.0 | 258 | 31.5 | 41.8 |
| LREE/HREE | 2.75 | 4.90 | 1.89 | 7.74 | 4.38 |
| δEu | 0.15 | 0.22 | 0.15 | 0.15 | 0.17 |
| Sample/No. | U | Th | Pb(t) | Th/U | 207Pb/235U | 206Pb/238U | 207Pb/235U | 208Pb/232Th | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (×10−6) | Ratio | 1σ | Ratio | 1σ | Ma | 1σ | Ma | 1σ | ||||
| lc4-j1, N = 25 | ||||||||||||
| 1 | 21153 | 28873 | 1703 | 1.36 | 0.2496 | 0.0081 | 0.0365 | 0.0012 | 226.2 | 6.60 | 230.9 | 7.30 |
| 2 | 1341 | 467 | 44.2 | 0.35 | 0.2516 | 0.0082 | 0.0364 | 0.0012 | 227.9 | 6.70 | 230.5 | 7.30 |
| 3 | 285 | 125 | 10.8 | 0.44 | 0.2599 | 0.0093 | 0.0366 | 0.0012 | 234.6 | 7.50 | 231.4 | 7.40 |
| 4 | 326 | 182 | 13.8 | 0.56 | 0.2552 | 0.0088 | 0.0361 | 0.0012 | 230.8 | 7.20 | 228.6 | 7.30 |
| 5 | 285 | 163 | 11.9 | 0.57 | 0.2362 | 0.0083 | 0.0342 | 0.0011 | 215.3 | 6.80 | 216.6 | 6.90 |
| 6 | 967 | 443 | 35.4 | 0.46 | 0.2367 | 0.0078 | 0.0342 | 0.0011 | 215.7 | 6.40 | 216.9 | 6.90 |
| 7 | 475 | 295 | 21.9 | 0.62 | 0.2361 | 0.0079 | 0.0344 | 0.0011 | 215.2 | 6.50 | 217.8 | 6.90 |
| 8 | 1076 | 660 | 48.5 | 0.61 | 0.2366 | 0.0078 | 0.0343 | 0.0011 | 215.6 | 6.40 | 217.2 | 6.90 |
| 9 | 242 | 132 | 10.0 | 0.54 | 0.2376 | 0.0084 | 0.0343 | 0.0011 | 216.4 | 6.90 | 217.6 | 6.90 |
| 10 | 358 | 189 | 14.4 | 0.53 | 0.2337 | 0.0118 | 0.0341 | 0.0012 | 213.3 | 9.70 | 216.1 | 7.30 |
| 11 | 334 | 342 | 21.5 | 1.02 | 0.2519 | 0.0085 | 0.0361 | 0.0012 | 228.1 | 6.90 | 228.3 | 7.20 |
| 12 | 1248 | 232 | 30.2 | 0.19 | 0.2506 | 0.0082 | 0.0365 | 0.0012 | 227.1 | 6.70 | 230.9 | 7.30 |
| 13 | 117 | 72.0 | 5.1 | 0.62 | 0.2563 | 0.0154 | 0.0361 | 0.0013 | 231.7 | 12.50 | 228.9 | 8.10 |
| 14 | 395 | 251 | 18.4 | 0.64 | 0.2545 | 0.0086 | 0.0362 | 0.0012 | 230.2 | 6.90 | 229.1 | 7.20 |
| 15 | 784 | 494 | 36.2 | 0.63 | 0.2375 | 0.0081 | 0.0344 | 0.0011 | 216.4 | 6.70 | 218.2 | 6.90 |
| 16 | 520 | 278 | 20.7 | 0.53 | 0.2356 | 0.0079 | 0.0341 | 0.0011 | 214.8 | 6.50 | 216.3 | 6.80 |
| 17 | 276 | 139 | 10.6 | 0.50 | 0.2348 | 0.0087 | 0.0344 | 0.0011 | 214.2 | 7.20 | 218.0 | 7.00 |
| 18 | 137 | 69.4 | 5.3 | 0.51 | 0.2382 | 0.0106 | 0.0343 | 0.0011 | 217.0 | 8.70 | 217.6 | 7.10 |
| 19 | 165 | 92.6 | 7.2 | 0.56 | 0.2402 | 0.0107 | 0.034 | 0.0011 | 218.6 | 8.80 | 215.2 | 7.10 |
| 20 | 196 | 116 | 9.2 | 0.60 | 0.2403 | 0.0135 | 0.0344 | 0.0012 | 218.7 | 11.00 | 218.1 | 7.50 |
| 21 | 694 | 254 | 21.9 | 0.37 | 0.2364 | 0.0078 | 0.0343 | 0.0011 | 215.5 | 6.40 | 217.5 | 6.90 |
| 22 | 785 | 336 | 28.1 | 0.43 | 0.2341 | 0.0077 | 0.0344 | 0.0011 | 213.5 | 6.30 | 218.0 | 6.90 |
| 23 | 182 | 78.1 | 6.5 | 0.43 | 0.2399 | 0.0178 | 0.034 | 0.0013 | 218.3 | 14.60 | 215.4 | 8.10 |
| 24 | 133 | 74.6 | 5.6 | 0.56 | 0.2364 | 0.0089 | 0.0343 | 0.0011 | 215.5 | 7.30 | 217.2 | 6.90 |
| 25 | 577 | 167 | 16.6 | 0.29 | 0.2352 | 0.0082 | 0.0343 | 0.0011 | 214.5 | 6.80 | 217.2 | 6.90 |
| lc2-j3, N = 25 | ||||||||||||
| 1 | 1027 | 309 | 32.1 | 0.30 | 0.217 | 0.0073 | 0.0321 | 0.0010 | 199 | 6.10 | 204.50 | 4.50 |
| 2 | 974 | 331 | 32.2 | 0.34 | 0.2237 | 0.0075 | 0.0322 | 0.0011 | 205 | 6.20 | 196.60 | 4.30 |
| 3 | 689 | 256 | 22.3 | 0.37 | 0.239 | 0.0080 | 0.0348 | 0.0011 | 218 | 6.50 | 205.40 | 4.50 |
| 4 | 271 | 136 | 10.5 | 0.50 | 0.2407 | 0.0084 | 0.0348 | 0.0011 | 219 | 6.80 | 203.40 | 4.70 |
| 5 | 1159 | 75.0 | 21.4 | 0.06 | 0.2471 | 0.0082 | 0.0349 | 0.0011 | 224 | 6.70 | 213.30 | 5.80 |
| 6 | 597 | 48.2 | 11.1 | 0.08 | 0.2418 | 0.0081 | 0.0349 | 0.0011 | 220 | 6.60 | 226.10 | 6.20 |
| 7 | 449 | 23.4 | 7.9 | 0.05 | 0.247 | 0.0094 | 0.0349 | 0.0012 | 224 | 7.70 | 252.00 | 13.9 |
| 8 | 2237 | 1146 | 104 | 0.51 | 0.2393 | 0.0078 | 0.0350 | 0.0011 | 218 | 6.40 | 212.00 | 4.40 |
| 9 | 171 | 81.0 | 6.2 | 0.47 | 0.2496 | 0.0089 | 0.0350 | 0.0011 | 226 | 7.30 | 192.40 | 4.80 |
| 10 | 425 | 161 | 13.6 | 0.38 | 0.2452 | 0.0083 | 0.0351 | 0.0011 | 223 | 6.70 | 194.60 | 4.40 |
| 11 | 383 | 166 | 13.8 | 0.43 | 0.2444 | 0.0083 | 0.0351 | 0.0011 | 222 | 6.80 | 207.00 | 4.80 |
| 12 | 4335 | 1110 | 142.7 | 0.26 | 0.2443 | 0.0080 | 0.0351 | 0.0011 | 222 | 6.50 | 243.20 | 5.10 |
| 13 | 1931 | 381 | 91.5 | 0.20 | 0.452 | 0.0147 | 0.0615 | 0.0020 | 379 | 10.3 | 368.50 | 7.70 |
| 14 | 1036 | 529 | 79.2 | 0.51 | 0.48 | 0.0157 | 0.0644 | 0.0021 | 398 | 10.8 | 402.80 | 8.40 |
| 15 | 1038 | 94.1 | 51.0 | 0.09 | 0.6739 | 0.0223 | 0.0712 | 0.0023 | 523 | 13.5 | 588.20 | 14.1 |
| 16 | 1808 | 813 | 150 | 0.45 | 0.5983 | 0.0195 | 0.0822 | 0.0027 | 476 | 12.4 | 450.40 | 9.40 |
| 17 | 1153 | 115 | 82.8 | 0.10 | 0.9613 | 0.0319 | 0.1055 | 0.0034 | 684 | 16.5 | 784.80 | 18.8 |
| 18 | 58.0 | 80.0 | 16.5 | 1.37 | 1.0337 | 0.0491 | 0.1180 | 0.0041 | 721 | 24.5 | 692.60 | 19.4 |
| 19 | 517 | 136 | 58.6 | 0.26 | 1.3384 | 0.0438 | 0.1415 | 0.0046 | 863 | 19.0 | 745.60 | 16.0 |
| 20 | 464 | 263 | 84.9 | 0.57 | 1.4514 | 0.0475 | 0.1499 | 0.0048 | 911 | 19.7 | 817.10 | 17.1 |
| 21 | 629 | 109 | 68.2 | 0.17 | 1.45 | 0.0471 | 0.1504 | 0.0048 | 910 | 19.5 | 898.80 | 18.9 |
| 22 | 976 | 169 | 157 | 0.17 | 2.2981 | 0.0751 | 0.1733 | 0.0056 | 1212 | 23.1 | 1240.80 | 26.3 |
| 23 | 356 | 238 | 118 | 0.67 | 2.2211 | 0.0731 | 0.1963 | 0.0064 | 1188 | 23.0 | 1136.30 | 23.5 |
| 24 | 107 | 170 | 52.7 | 1.59 | 2.2998 | 0.0760 | 0.2039 | 0.0066 | 1212 | 23.4 | 1004.40 | 20.8 |
| 25 | 377 | 122 | 97.8 | 0.32 | 3.7222 | 0.1205 | 0.2768 | 0.0089 | 1576 | 25.9 | 1329.70 | 27.1 |
| mh1-j1, N = 25 | ||||||||||||
| 1 | 286 | 163 | 13.8 | 0.57 | 0.2632 | 0.0243 | 0.0364 | 0.0015 | 237.2 | 19.5 | 230.5 | 9.6 |
| 2 | 283 | 215 | 15.4 | 0.76 | 0.2627 | 0.0158 | 0.0365 | 0.0013 | 236.8 | 12.7 | 231.2 | 8.1 |
| 3 | 403 | 267 | 23.1 | 0.66 | 0.2593 | 0.0101 | 0.0366 | 0.0012 | 234.1 | 8.1 | 231.6 | 7.4 |
| 4 | 136 | 80.8 | 6.20 | 0.60 | 0.2606 | 0.0111 | 0.0366 | 0.0012 | 235.1 | 9.0 | 231.9 | 7.5 |
| 5 | 520 | 271 | 24.7 | 0.52 | 0.2593 | 0.0144 | 0.0366 | 0.0013 | 234.1 | 11.6 | 232.0 | 7.9 |
| 6 | 459 | 294 | 25.4 | 0.64 | 0.2611 | 0.0103 | 0.0367 | 0.0012 | 235.5 | 8.3 | 232.1 | 7.4 |
| 7 | 297 | 196 | 16.0 | 0.66 | 0.2638 | 0.0101 | 0.0367 | 0.0012 | 237.7 | 8.1 | 232.3 | 7.4 |
| 8 | 295 | 15.9 | 5.40 | 0.05 | 0.2622 | 0.0135 | 0.0367 | 0.0013 | 236.5 | 10.8 | 232.3 | 7.8 |
| 9 | 142 | 99.4 | 7.00 | 0.70 | 0.2568 | 0.0122 | 0.0367 | 0.0012 | 232.0 | 9.8 | 232.5 | 7.6 |
| 10 | 297 | 159 | 12.7 | 0.53 | 0.2611 | 0.0094 | 0.0367 | 0.0012 | 235.6 | 7.6 | 232.5 | 7.3 |
| 11 | 98.0 | 55.7 | 4.4 | 0.57 | 0.2577 | 0.0114 | 0.0367 | 0.0012 | 232.8 | 9.2 | 232.6 | 7.5 |
| 12 | 511 | 180 | 19.6 | 0.35 | 0.2535 | 0.0090 | 0.0368 | 0.0012 | 229.4 | 7.3 | 232.9 | 7.3 |
| 13 | 497 | 217 | 20.7 | 0.44 | 0.2533 | 0.0102 | 0.0368 | 0.0012 | 229.3 | 8.2 | 233.1 | 7.5 |
| 14 | 238 | 81.8 | 7.40 | 0.34 | 0.2627 | 0.0134 | 0.0369 | 0.0013 | 236.8 | 10.8 | 233.4 | 7.8 |
| 15 | 108 | 50.7 | 4.10 | 0.47 | 0.2579 | 0.0117 | 0.0369 | 0.0012 | 233.0 | 9.5 | 233.7 | 7.6 |
| 16 | 179 | 49.6 | 6.60 | 0.28 | 0.2705 | 0.0204 | 0.0369 | 0.0014 | 243.1 | 16.3 | 233.9 | 8.9 |
| 17 | 445 | 184 | 18.6 | 0.41 | 0.2604 | 0.0097 | 0.037 | 0.0012 | 235.0 | 7.8 | 234.1 | 7.4 |
| 18 | 510 | 251 | 25.9 | 0.49 | 0.2618 | 0.0137 | 0.0371 | 0.0013 | 236.1 | 11.0 | 234.6 | 7.9 |
| 19 | 181 | 154 | 10.5 | 0.85 | 0.2598 | 0.0122 | 0.0373 | 0.0013 | 234.5 | 9.8 | 235.8 | 7.7 |
| 20 | 284 | 52.5 | 8.80 | 0.18 | 0.2976 | 0.0244 | 0.0405 | 0.0016 | 264.6 | 19.1 | 256.2 | 10.0 |
| 21 | 709 | 127 | 35.5 | 0.18 | 0.5302 | 0.0230 | 0.0658 | 0.0022 | 432.0 | 15.2 | 410.6 | 13.2 |
| 22 | 332 | 265 | 79.7 | 0.80 | 1.4443 | 0.0485 | 0.1503 | 0.0048 | 907.5 | 20.2 | 902.4 | 26.8 |
| 23 | 540 | 253 | 109 | 0.47 | 1.4412 | 0.0462 | 0.1507 | 0.0048 | 906.2 | 19.2 | 904.7 | 26.7 |
| 24 | 167 | 44.0 | 28.4 | 0.26 | 1.9706 | 0.0636 | 0.1509 | 0.0048 | 1105.5 | 21.8 | 905.8 | 26.8 |
| 25 | 136 | 40.6 | 22.8 | 0.30 | 2.1645 | 0.0730 | 0.1706 | 0.0055 | 1169.7 | 23.4 | 1015.1 | 30.0 |
| Sample | t (Ma) | Rb (ppm) | Sr (ppm) | 87Rb/ 86Sr | 87Sr/ 86Sr | 2σ | ISr | εSr (t) | 147Sm/ 144Nd | 143Nd/ 144Nd | 2σ | INd | εNd (0) | εNd (t) | fSm/Nd | T2DM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YNlc3-j1 | 220 | 257 | 85.5 | 3.919 | 0.733514 | 0.000007 | 0.72058 | 232.2 | 0.09349 | 0.511982 | 0.000003 | 0.51184 | −12.8 | −9.75 | −0.52 | 1801 |
| YNlc4-j1 | 220 | 159 | 119 | 3.905 | 0.733459 | 0.000008 | 0.72057 | 232.1 | 0.09267 | 0.51199 | 0.000007 | 0.511849 | −12.64 | −9.57 | −0.53 | 1787 |
| YNmh-j1 | 220 | 246 | 146 | 3.918 | 0.733616 | 0.000006 | 0.72069 | 233.8 | 0.09349 | 0.511982 | 0.000006 | 0.51184 | −12.8 | −9.75 | −0.52 | 1801 |
| Sample | 206Pb/204Pb | 2σ | 207Pb/204Pb | 2σ | 208Pb/204Pb | 2σ | (206Pb/204Pb)i | (207Pb/204Pb)i | (208Pb/204Pb)i |
|---|---|---|---|---|---|---|---|---|---|
| YNlc3-j1 | 19.346 | 0.0000 | 15.823 | 0.00001 | 39.690 | 0.001 | 18.993 | 15.805 | 39.145 |
| YNlc4-j1 | 18.988 | 0.0000 | 15.799 | 0.00001 | 39.819 | 0.001 | 18.763 | 15.788 | 38.979 |
| YNmh-j1 | 19.326 | 0.0000 | 15.817 | 0.00001 | 39.713 | 0.001 | 18.293 | 15.765 | 39.107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Liu, Y.; Liu, H.; Zhao, Z.; Wang, C.; Xu, X. Geochemical and Geochronological Constraints on the Genesis of Ion-Adsorption-Type REE Mineralization in the Lincang Pluton, SW China. Minerals 2020, 10, 1116. https://doi.org/10.3390/min10121116
Lu L, Liu Y, Liu H, Zhao Z, Wang C, Xu X. Geochemical and Geochronological Constraints on the Genesis of Ion-Adsorption-Type REE Mineralization in the Lincang Pluton, SW China. Minerals. 2020; 10(12):1116. https://doi.org/10.3390/min10121116
Chicago/Turabian StyleLu, Lei, Yan Liu, Huichuan Liu, Zhi Zhao, Chenghui Wang, and Xiaochun Xu. 2020. "Geochemical and Geochronological Constraints on the Genesis of Ion-Adsorption-Type REE Mineralization in the Lincang Pluton, SW China" Minerals 10, no. 12: 1116. https://doi.org/10.3390/min10121116
APA StyleLu, L., Liu, Y., Liu, H., Zhao, Z., Wang, C., & Xu, X. (2020). Geochemical and Geochronological Constraints on the Genesis of Ion-Adsorption-Type REE Mineralization in the Lincang Pluton, SW China. Minerals, 10(12), 1116. https://doi.org/10.3390/min10121116





