Oxide-Clay Mineral as Photoactive Material for Dye Discoloration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photoactive Materials
2.2.1. TiO2–Palygorskite Composite (TiO2–Pal)
2.2.2. ZrO2–TiO2–Palygorskite Composite (ZrO2–TiO2–Pal)
2.3. Characterization
2.4. Photodegradation Assays
2.4.1. Effects of Concentration and Time
2.4.2. Influence of the Sulfate Ion
3. Results
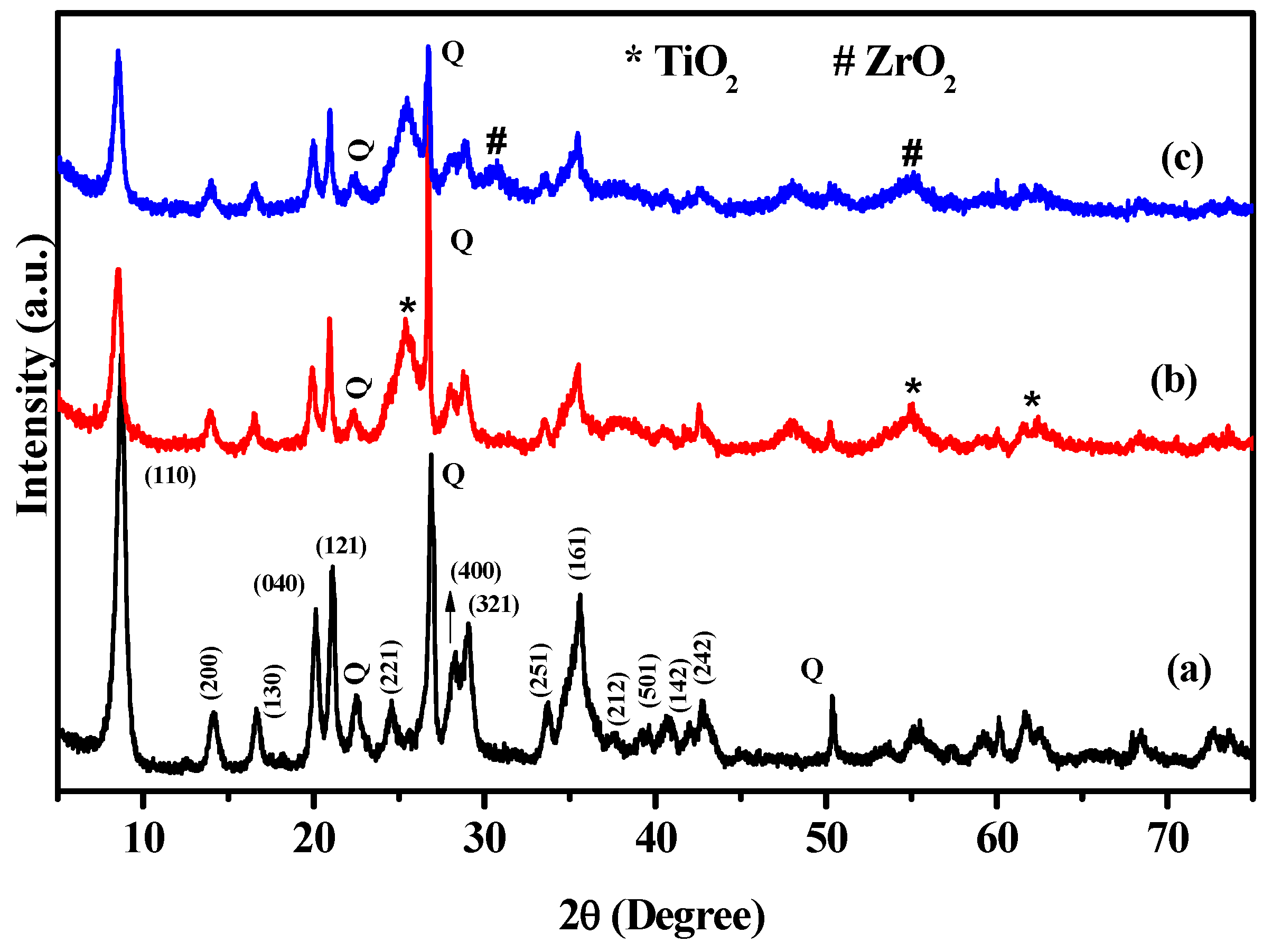
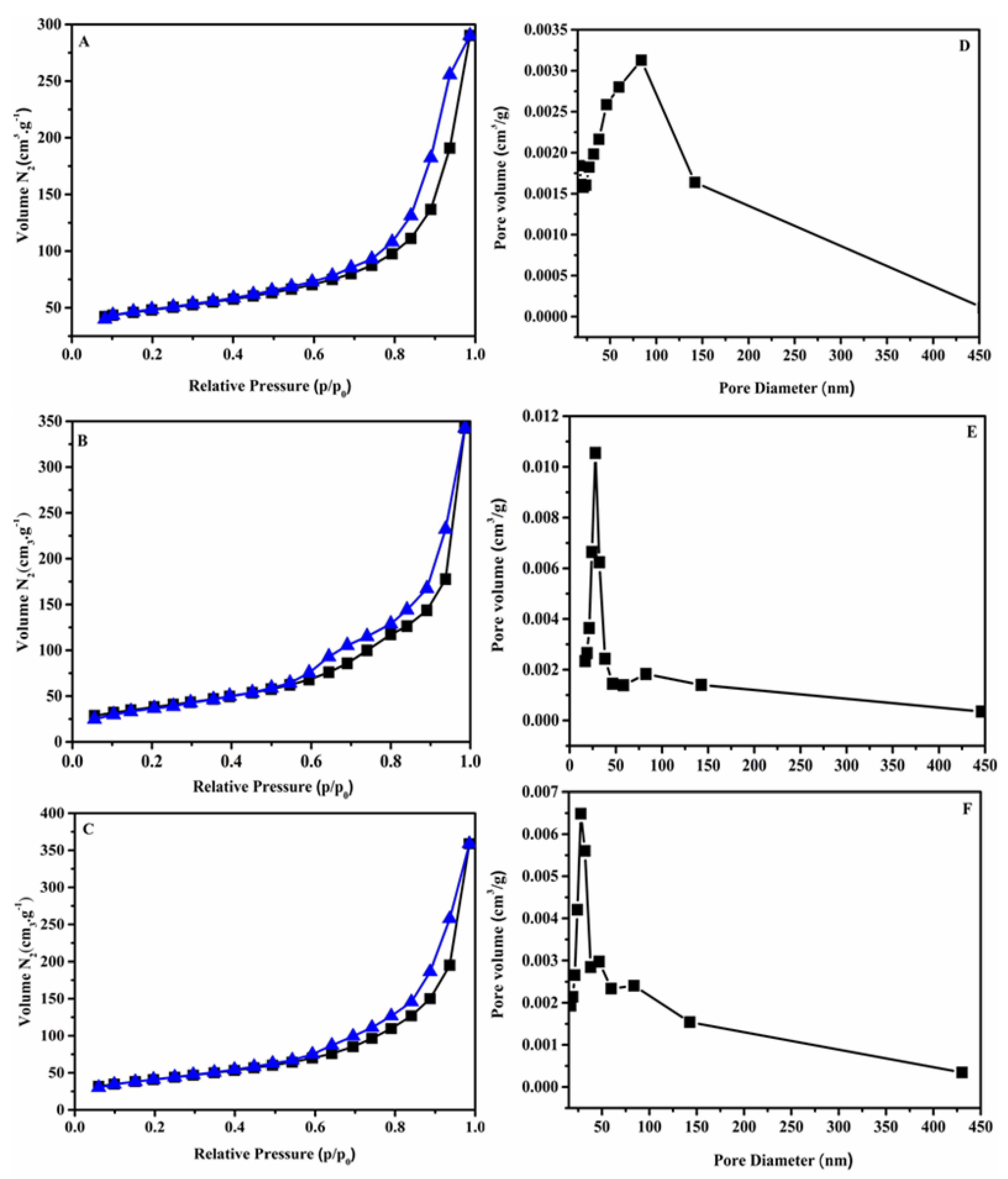
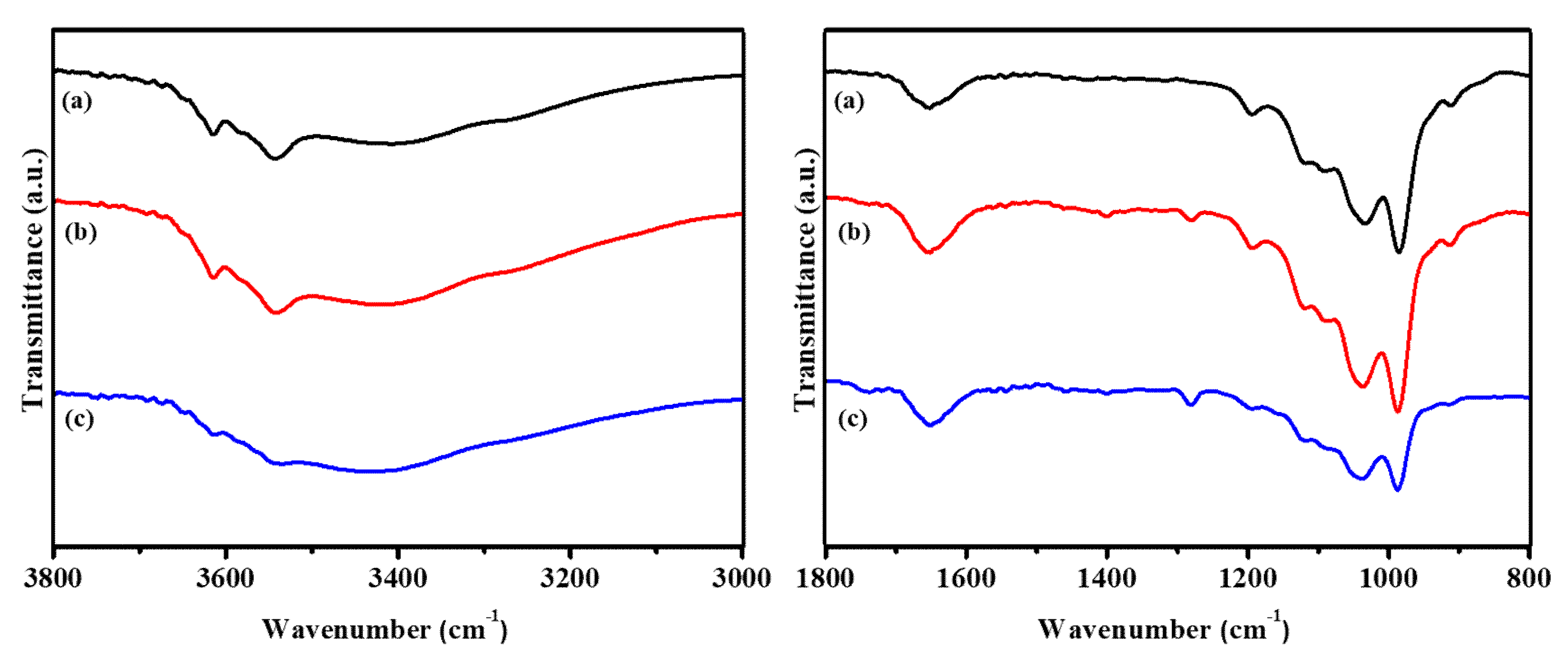
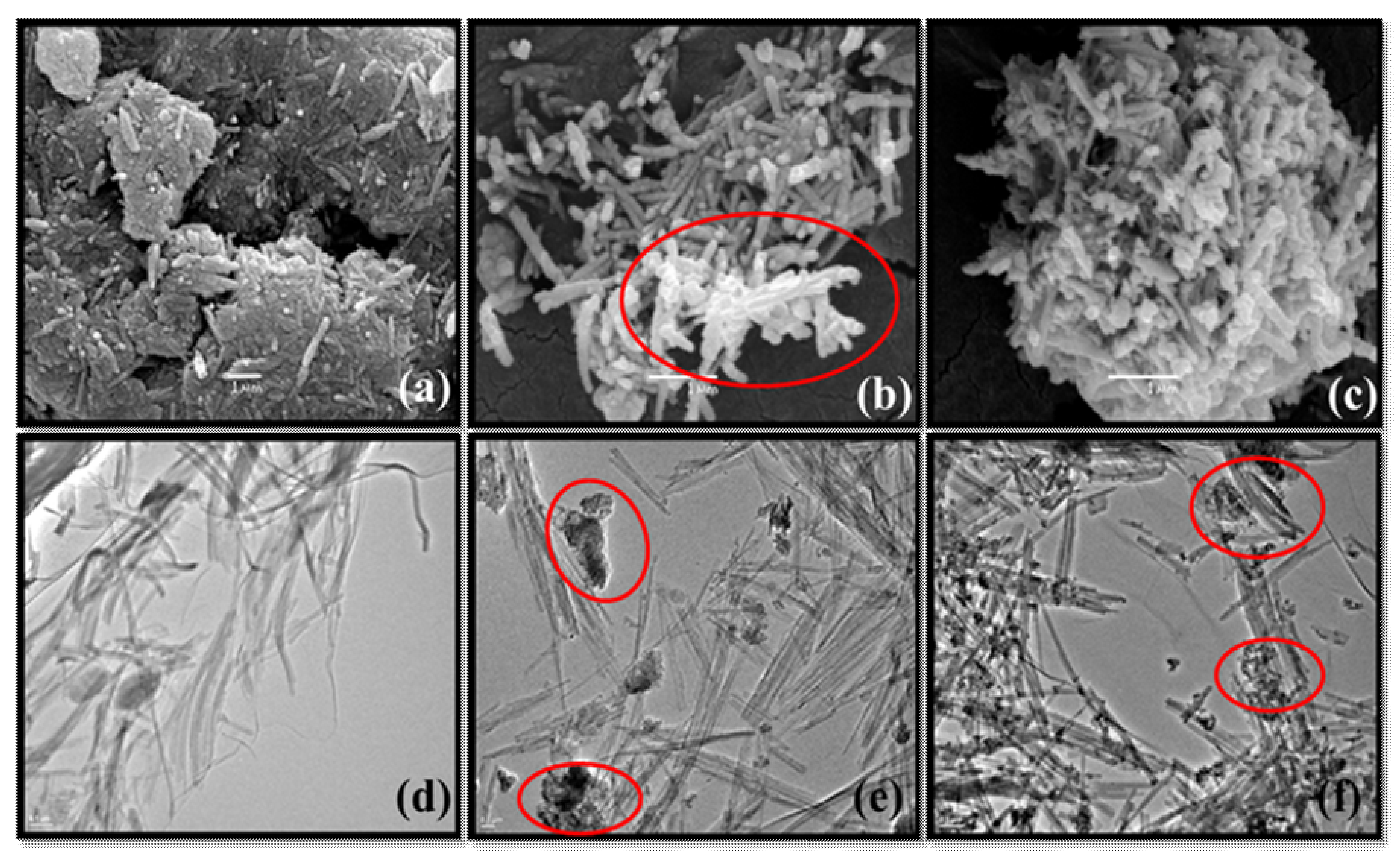
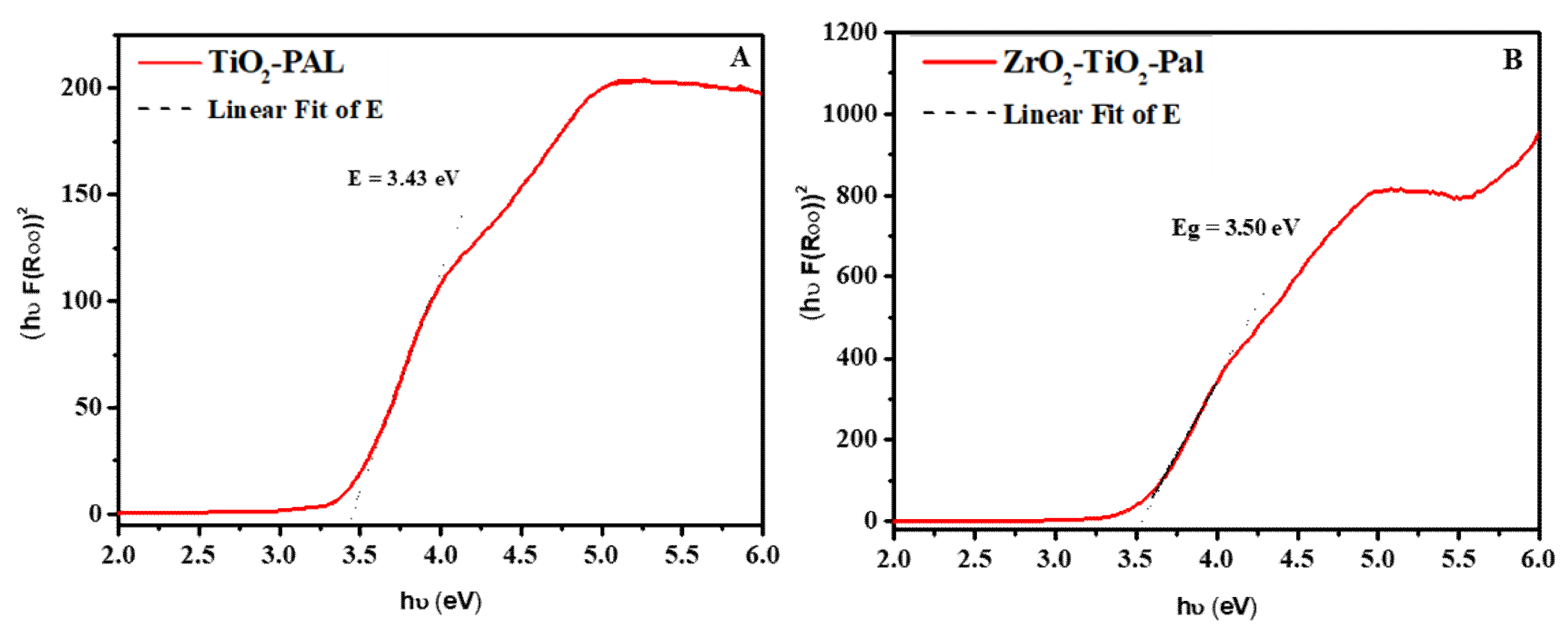
3.1. Characterizations
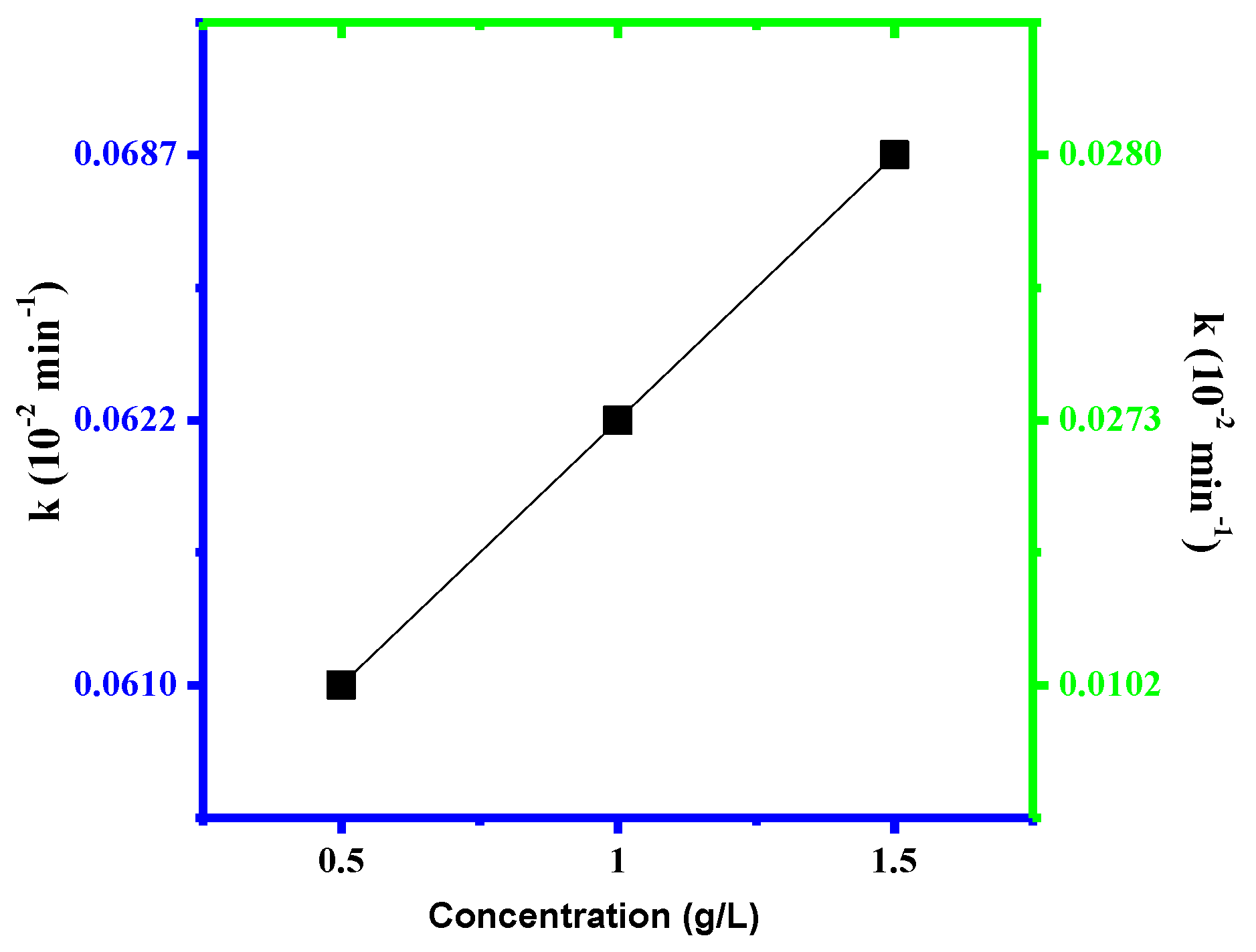
3.2. Photocatalytic Assays
Effect of the Presence of SO42− Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abhilash, P.C.; Singh, N. Pesticide use and application: An Indian scenario. J. Hazard. Mater. 2009, 165, 1–12. [Google Scholar] [CrossRef]
- Sen Kavurmaci, S.; Bekbolet, M. Photocatalytic degradation of humic acid in the presence of montmorillonite. Appl. Clay Sci. 2013, 75–76, 60–66. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Tan, Y.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Rapid decolorization of anthraquinone and triphenylmethane dye using chloroperoxidase: Catalytic mechanism, analysis of products and degradation route. Chem. Eng. J. 2014, 244, 9–18. [Google Scholar] [CrossRef]
- Pielesz, A.; Baranowska, I.; Rybak, A.; Włochowicz, A. Detection and determination of aromatic amines as products of reductive splitting from selected azo dyes. Ecotoxicol. Environ. Saf. 2002, 53, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.; Lima, L.C.B.; Silva, F.C.; Matos, J.M.E.; Santos, M.R.M.C.; Santos Júnior, L.S.; Sousa, K.S.; da Silva Filho, E.C. Dye anionic sorption in aqueous solution onto a cellulose surface chemically modified with aminoethanethiol. Chem. Eng. J. 2013, 218, 89–98. [Google Scholar] [CrossRef]
- Vidal, J.; Huiliñir, C.; Santander, R.; Silva-Agredo, J.; Torres-Palma, R.A.; Salazar, R. Effective removal of the antibiotic Nafcillin from water by combining the Photoelectro-Fenton process and Anaerobic Biological Digestion. Sci. Total Environ. 2018, 624, 1095–1105. [Google Scholar] [CrossRef]
- Osajima, J.A.; Coelho, K. Photolysis and photocatalysis of Bentazon herbicide. Int. J. Agric. Environ. Res. 2016, 2, 843–856. [Google Scholar]
- Clark, K.K.; Mezyk, S.P.; Abbott, A.; Kiddle, J.J. Kinetic studies of the AOP radical-based oxidative and reductive destruction of pesticides and model compounds in water. Chemosphere 2018, 197, 193–199. [Google Scholar] [CrossRef]
- Bouna, L.; Rhouta, B.; Maury, F. Physicochemical study of photocatalytic activity of TiO2 supported palygorskite clay mineral. Int. J. Photoenergy 2013, 2013, 815473. [Google Scholar] [CrossRef]
- Huo, C.; Yang, H. Preparation and enhanced photocatalytic activity of Pd-CuO/palygorskite nanocomposites. Appl. Clay Sci. 2013, 74, 87–94. [Google Scholar] [CrossRef]
- De Morais, J.L.; Zamora, P.P. Use of advanced oxidation processes to improve the biodegradability of mature landfill leachates. J. Hazard. Mater. 2005, 123, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Gu, F.L.; Wu, H. BiOCl-montmorillonite as a photocatalyst for highly efficient removal of Rhodamine B and Orange G: Importance of the acidity and dissolved oxygen. Appl. Clay Sci. 2017, 147, 28–35. [Google Scholar] [CrossRef]
- Quan, G.; Kong, L.; Lan, Y.; Yan, J.; Gao, B. Removal of acid orange 7 by surfactant-modified iron nanoparticle supported on palygorskite: Reactivity and mechanism. Appl. Clay Sci. 2018, 152, 173–182. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, C.; Lu, J.; Liu, H.; Huang, L.; Chen, T.; Chen, D. Catalytic degradation of gaseous benzene by using TiO2/goethite immobilized on palygorskite: Preparation, characterization and mechanism. Solid State Sci. 2015, 49, 1–9. [Google Scholar] [CrossRef]
- Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Photoenergy conversion with TiO2 photocatalysis: New materials and recent applications. Electrochim. Acta 2012, 84, 103–111. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Wang, H.; Zhou, S.; Komarneni, S. Sepiolite-TiO2 nanocomposites for photocatalysis: Synthesis by microwave hydrothermal treatment versus calcination. Appl. Clay Sci. 2017, 146, 246–253. [Google Scholar] [CrossRef]
- Lin, S.; Sun, S.; Shen, K.; Tan, D.; Zhang, H.; Dong, F.; Fu, X. Photocatalytic microreactors based on nano TiO2-containing clay colloidosomes. Appl. Clay Sci. 2018, 159, 42–49. [Google Scholar] [CrossRef]
- Gu, N.; Gao, J.; Li, H.; Wu, Y.; Ma, Y.; Wang, K. Montmorillonite-supported with Cu2O nanoparticles for damage and removal of Microcystis aeruginosa under visible light. Appl. Clay Sci. 2016, 132–133, 79–89. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Chang, P.-H.; Gao, Z.-Y.; Xu, X.-Y.; Chen, Y.-H.; Wang, Z.-H.; Chen, X.-Y.; Yang, Z.-Y.; Wang, T.-H.; Jean, J.-S.; et al. Amitriptyline removal using palygorskite clay. Chemosphere 2016, 155, 292–299. [Google Scholar] [CrossRef]
- Rusmin, R.; Sarkar, B.; Tsuzuki, T.; Kawashima, N.; Naidu, R. Removal of lead from aqueous solution using superparamagnetic palygorskite nanocomposite: Material characterization and regeneration studies. Chemosphere 2017, 186, 1006–1015. [Google Scholar] [CrossRef]
- Li, Z.; Fitzgerald, N.M.; Jiang, W.T.; Lv, G. Palygorskite for the uptake and removal of pharmaceuticals for wastewater treatment. Process Saf. Environ. Prot. 2016, 101, 80–87. [Google Scholar] [CrossRef]
- Middea, A.; Spinelli, L.S.; Souza, F.G.; Neumann, R.; Gomes, O.D.F.M.; Fernandes, T.L.A.P.; De Lima, L.C.; Barthem, V.M.T.S.; De Carvalho, F.V. Synthesis and characterization of magnetic palygorskite nanoparticles and their application on methylene blue remotion from water. Appl. Surf. Sci. 2015, 346, 232–239. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Z.; Wang, B.; An, H.; Chen, Z.; Cui, H. Adsorption and photocatalytic degradation of tetracycline hydrochloride using a palygorskite-supported Cu2O-TiO2 composite. Appl. Clay Sci. 2016, 119, 311–320. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Q.; Zhu, X.; Wang, J.; Chen, J.; Shi, Y.; Yang, Z.; Cui, H.; Hu, Y.; Zhang, L.; et al. Palygorskite supported BiVO4 photocatalyst for tetracycline hydrochloride removal. Appl. Clay Sci. 2017, 137, 249–258. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Kwok, C.; Aita, C.R. Near-band gap optical behavior of sputter deposited α- and α + β − ZrO2 films. J. Appl. Phys. 1989, 66, 2756–2758. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.; He, H. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interface Sci. 2010, 341, 153–161. [Google Scholar] [CrossRef]
- Vdovic, N.; Jurina, I.; Škapin, S.D.; Sondi, I. The surface properties of clay minerals modified by intensive dry milling—Revisited. Appl. Clay Sci. 2010, 48, 575–580. [Google Scholar] [CrossRef]
- Sarabadan, M.; Bashiri, H.; Mousavi, S.M. Adsorption of crystal violet dye by zeolite-montmorillonite: Modeling, kinetic and equilibrium studies. Clay Miner. 2019, 1–45. [Google Scholar] [CrossRef]
- Trigueiro, P.; Pedetti, S.; Rigaud, B.; Balme, S.; Janot, J.-M.; dos Santos, I.M.G.; Gougeon, R.; Fonseca, M.G.; Georgelin, T.; Jaber, M. Going through the wine fining: Intimate dialogue between organics and clays. Colloids Surf. B Biointerfaces 2018, 166, 79–88. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J.J. Zr-doped TiO2 supported on delaminated clay materials for solar photocatalytic treatment of emerging pollutants. J. Hazard. Mater. 2017, 322, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Belver, C.; Bedia, J.; Álvarez-Montero, M.A.; Rodriguez, J.J. Solar photocatalytic purification of water with Ce-doped TiO2/clay heterostructures. Catal. Today 2016, 266, 36–45. [Google Scholar] [CrossRef]
- Chen, H.; Wang, A. Kinetic and isothermal studies of lead ion adsorption onto palygorskite clay. J. Colloid Interface Sci. 2007, 307, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.A.S.; Figueiras, A.; Veiga, F.; de Freitas, R.M.; Nunes, L.C.C.; da Silva Filho, E.C.; da Silva Leite, C.M. The systems containing clays and clay minerals from modified drug release: A review. Colloids Surf. B Biointerfaces 2013, 103, 642–651. [Google Scholar] [CrossRef]
- Miranda, M.O.; de Araújo, F.P.; Osajima, J.A.; Silva Filho, E.C. Incorporation of zirconium oxide on the surface of palygorskite clay for photodegradation of industrial dye. Mater. Sci. Forum 2016, 869, 768–772. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Tang, C.; Lv, J.; Zhong, H.; Zhao, Y.; Wang, X. Palygorskite and SnO2–TiO2 for the photodegradation of phenol. Appl. Clay Sci. 2011, 51, 68–73. [Google Scholar] [CrossRef]
- Yuan, P.; Yin, X.; He, H.; Yang, D.; Wang, L.; Zhu, J. Investigation on the delaminated-pillared structure of TiO2-PILC synthesized by TiCl4 hydrolysis method. Microporous Mesoporous Mater. 2006, 93, 240–247. [Google Scholar] [CrossRef]
- Idakiev, V.; Tabakova, T.; Naydenov, A.; Yuan, Z.Y.; Su, B.L. Gold catalysts supported on mesoporous zirconia for low-temperature water-gas shift reaction. Appl. Catal. B Environ. 2006, 63, 178–186. [Google Scholar] [CrossRef]
- Chen, D.; Du, Y.; Zhu, H.; Deng, Y. Synthesis and characterization of a microfibrous TiO2-CdS/palygorskite nanostructured material with enhanced visible-light photocatalytic activity. Appl. Clay Sci. 2014, 87, 285–291. [Google Scholar] [CrossRef]
- Grudzien, R.M.; Grabicka, B.E.; Jaroniec, M. Effect of organosilane/polymer ratio on adsorption properties of periodic mesoporous ethane-silica. Colloids Surf. A Physicochem. Eng. Asp. 2007, 300, 235–244. [Google Scholar] [CrossRef]
- Silva, G.T.M.; Silva, C.P.; Gehlen, M.H.; Oake, J.; Bohne, C.; Quina, F.H. Organic/inorganic hybrid pigments from flavylium cations and palygorskite. Appl. Clay Sci. 2018, 162, 478–486. [Google Scholar] [CrossRef]
- Stathatos, E.; Papoulis, D.; Aggelopoulos, C.A.; Panagiotaras, D.; Nikolopoulou, A. TiO2/palygorskite composite nanocrystalline films prepared by surfactant templating route: Synergistic effect to the photocatalytic degradation of an azo-dye in water. J. Hazard. Mater. 2012, 211–212, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Dali Youcef, L.; Belaroui, L.S.; López-Galindo, A. Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl. Clay Sci. 2019, 179, 105145. [Google Scholar] [CrossRef]
- Pan, J.; Zou, X.; Wang, X.; Guan, W.; Yan, Y.; Han, J. Selective recognition of 2,4-dichlorophenol from aqueous solution by uniformly sized molecularly imprinted microspheres with β-cyclodextrin/attapulgite composites as support. Chem. Eng. J. 2010, 162, 910–918. [Google Scholar] [CrossRef]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benítez-Guerrero, M.; Pozo, E.; Martín Rubí, J.A. Microwave assisted acid treatment of sepiolite: The role of composition and “crystallinity”. Appl. Clay Sci. 2014, 102, 15–27. [Google Scholar] [CrossRef]
- Chahi, A.; Petit, S.; Decarreau, A. Infrared evidence of dioctahedral-trioctahedral site occupancy in palygorskite. Clays Clay Miner. 2002, 50, 306–313. [Google Scholar] [CrossRef]
- Qi, Z.; Ye, H.; Xu, J.; Peng, J.; Chen, J.; Guo, B. Synthesis and characterizations of attapulgite reinforced branched poly (butylene succinate) nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 26–33. [Google Scholar] [CrossRef]
- Ma, J.; Zou, J.; Li, L.; Yao, C.; Kong, Y.; Cui, B.; Zhu, R.; Li, D. Nanocomposite of attapulgite–Ag3PO4 for Orange II photodegradation. Appl. Catal. B Environ. 2014, 144, 36–40. [Google Scholar] [CrossRef]
- Kim, C.S.; Jeong, H.D. Band gap tuning in nanoporous TiO2-ZrO2 hybrid thin films. Bull. Korean Chem. Soc. 2007, 28, 2333–2337. [Google Scholar]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Rezaei, M.; Habibi-Yangjeh, A. Simple and large scale refluxing method for preparation of Ce-doped ZnO nanostructures as highly efficient photocatalyst. Appl. Surf. Sci. 2013, 265, 591–596. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Nikolopoulou, A.; Tsolis-Katagas, P.; Panagiotaras, D.; Kacandes, H.G.; Zhang, P.; Yin, S.; Sato, T.; Katsuki, H. Palygorskite- and Halloysite-TiO2 nanocomposites: Synthesis and photocatalytic activity. Appl. Clay Sci. 2010, 50, 118–124. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar light induced and TiO2 assisted degradation of textile dye reactive blue 4. Chemosphere 2002, 46, 1173–1181. [Google Scholar] [CrossRef]
- Egerton, T.A.; Purnama, H. Does hydrogen peroxide really accelerate TiO2 UV-C photocatalyzed decolouration of azo-dyes such as Reactive Orange 16? Dye. Pigment. 2014, 101, 280–285. [Google Scholar] [CrossRef]
- Santos, W.G.; Tominaga, T.T.; Nascimento, O.R.; Schmitt, C.C.; Neumann, M.G. Phototransients of 2-ethylaminodiphenylborinate generated by direct photolysis and photosensitization. J. Photochem. Photobiol. A Chem. 2012, 236, 14–20. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Zheng, W.; Zou, H.-F.; Lv, S.-W.; Lin, Y.-H.; Wang, M.; Yan, F.; Sheng, Y.; Song, Y.-H.; Chen, J.; Zheng, K.-Y. The effect of nano-TiO2 photocatalysis on the antioxidant activities of Cu, Zn-SOD at physiological pH. J. Photochem. Photobiol. B Biol. 2017, 174, 251–260. [Google Scholar] [CrossRef]
- Saquib, M.; Muneer, M. Semiconductor mediated photocatalysed degradation of an anthraquinone dye, Remazol Brilliant Blue R under sunlight and artificial light source. Dye. Pigment. 2002, 53, 237–249. [Google Scholar] [CrossRef]
- Chang, J.P.; Lin, Y.S.; Chu, K. Rapid thermal chemical vapor deposition of zirconium oxide for metal-oxide-semiconductor field effect transistor application. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2001, 19, 1782–1787. [Google Scholar] [CrossRef]
- Gayathri, P.V.; Yesodharan, S.; Yesodharan, E.P. Microwave/Persulphate assisted ZnO mediated photocatalysis (MW/PS/UV/ZnO) as an efficient advanced oxidation process for the removal of RhB dye pollutant from water. J. Environ. Chem. Eng. 2019, 7, 103122. [Google Scholar] [CrossRef]
- Lim, J.; Kwak, D.-Y.; Sieland, F.; Kim, C.; Bahnemann, D.W.; Choi, W. Visible light-induced catalytic activation of peroxymonosulfate using heterogeneous surface complexes of amino acids on TiO2. Appl. Catal. B Environ. 2018, 225, 406–414. [Google Scholar] [CrossRef]
- Nasirian, M.; Mehrvar, M. Photocatalytic degradation of aqueous Methyl Orange using nitrogen-doped TiO2 photocatalyst prepared by novel method of ultraviolet-assisted thermal synthesis. J. Environ. Sci. (China) 2018, 66, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Turolla, A.; Bestetti, M.; Antonelli, M. Optimization of heterogeneous photoelectrocatalysis on nanotubular TiO2 electrodes: Reactor configuration and kinetic modelling. Chem. Eng. Sci. 2018, 182, 171–179. [Google Scholar] [CrossRef]
- Hu, C.; Yu, J.C.; Hao, Z.; Wong, P.K. Effects of acidity and inorganic ions on the photocatalytic degradation of different azo dyes. Appl. Catal. B Environ. 2003, 46, 35–47. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, J.; Wang, J.; Ji, Z. Effect of sulfate ions on the crystallization and photocatalytic activity of TiO2/diatomite composite photocatalyst. Chem. Phys. Lett. 2016, 643, 53–60. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.A.; Mahmoud, S.A.; Zaki, A.H. Visible light assisted photocatalytic degradation of crystal violet, bromophenol blue and eosin Y dyes using AgBr–ZnO nanocomposite. Environ. Nanotechnol. Monit. Manag. 2018, 9, 164–173. [Google Scholar] [CrossRef]
- Wang, C.; Xue, Y.; Wang, P.; Ao, Y. Effects of water environmental factors on the photocatalytic degradation of sulfamethoxazole by AgI/UiO-66 composite under visible light irradiation. J. Alloys Compd. 2018, 748, 314–322. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, M.O.; Viana, B.C.; Honório, L.M.; Trigueiro, P.; Fonseca, M.G.; Franco, F.; Osajima, J.A.; Silva-Filho, E.C. Oxide-Clay Mineral as Photoactive Material for Dye Discoloration. Minerals 2020, 10, 132. https://doi.org/10.3390/min10020132
Miranda MO, Viana BC, Honório LM, Trigueiro P, Fonseca MG, Franco F, Osajima JA, Silva-Filho EC. Oxide-Clay Mineral as Photoactive Material for Dye Discoloration. Minerals. 2020; 10(2):132. https://doi.org/10.3390/min10020132
Chicago/Turabian StyleMiranda, Maicon O., Bartolomeu Cruz Viana, Luzia Maria Honório, Pollyana Trigueiro, Maria Gardênnia Fonseca, Francisco Franco, Josy A. Osajima, and Edson C. Silva-Filho. 2020. "Oxide-Clay Mineral as Photoactive Material for Dye Discoloration" Minerals 10, no. 2: 132. https://doi.org/10.3390/min10020132
APA StyleMiranda, M. O., Viana, B. C., Honório, L. M., Trigueiro, P., Fonseca, M. G., Franco, F., Osajima, J. A., & Silva-Filho, E. C. (2020). Oxide-Clay Mineral as Photoactive Material for Dye Discoloration. Minerals, 10(2), 132. https://doi.org/10.3390/min10020132









