Molecular Dynamics Simulation on Microstructure and Physicochemical Properties of FexO-SiO2-CaO-MgO-“NiO” Slag in Nickel Matte Smelting under Modulating CaO Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Method
2.2.1. Molecular Dynamics Simulation
2.2.2. Phase and Microstructure Characterization of Slag
2.2.3. Determination of Physicochemical Properties
3. Results and Discussions
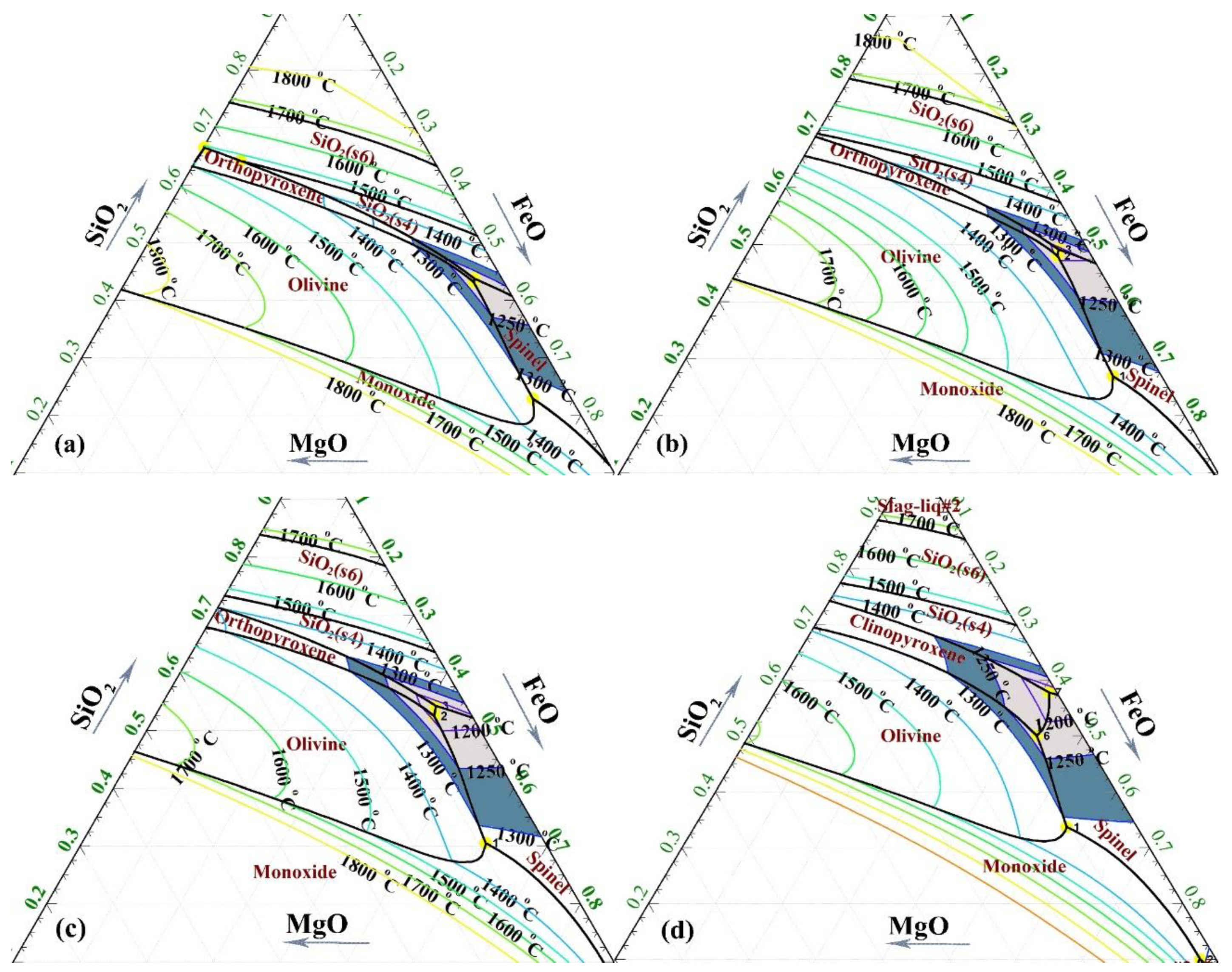
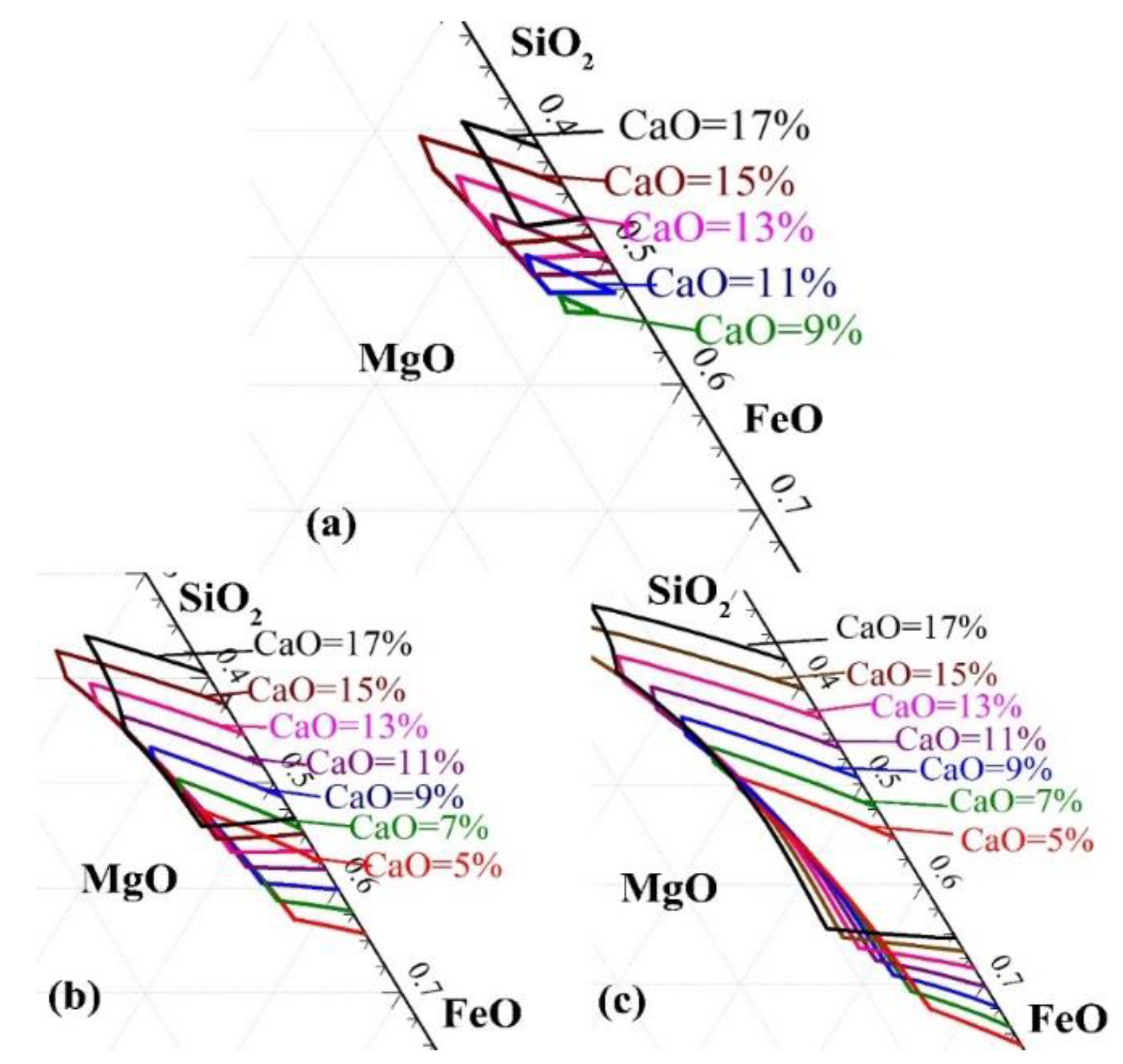
3.1. Effect of CaO on Liquidus Temperature of Slag
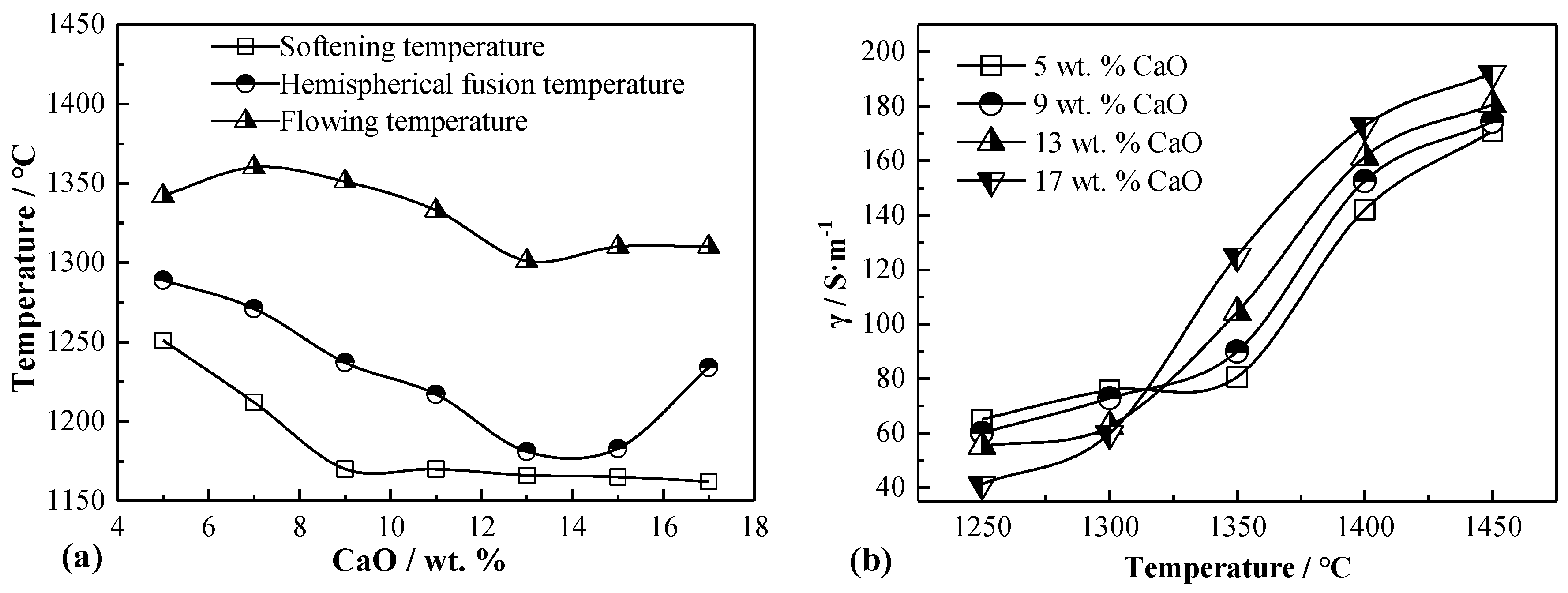
3.2. Influence of CaO on Melting Temperature and Conductivity
3.3. Effect of CaO on Phase Composition and Microstructure of Nickel Slag
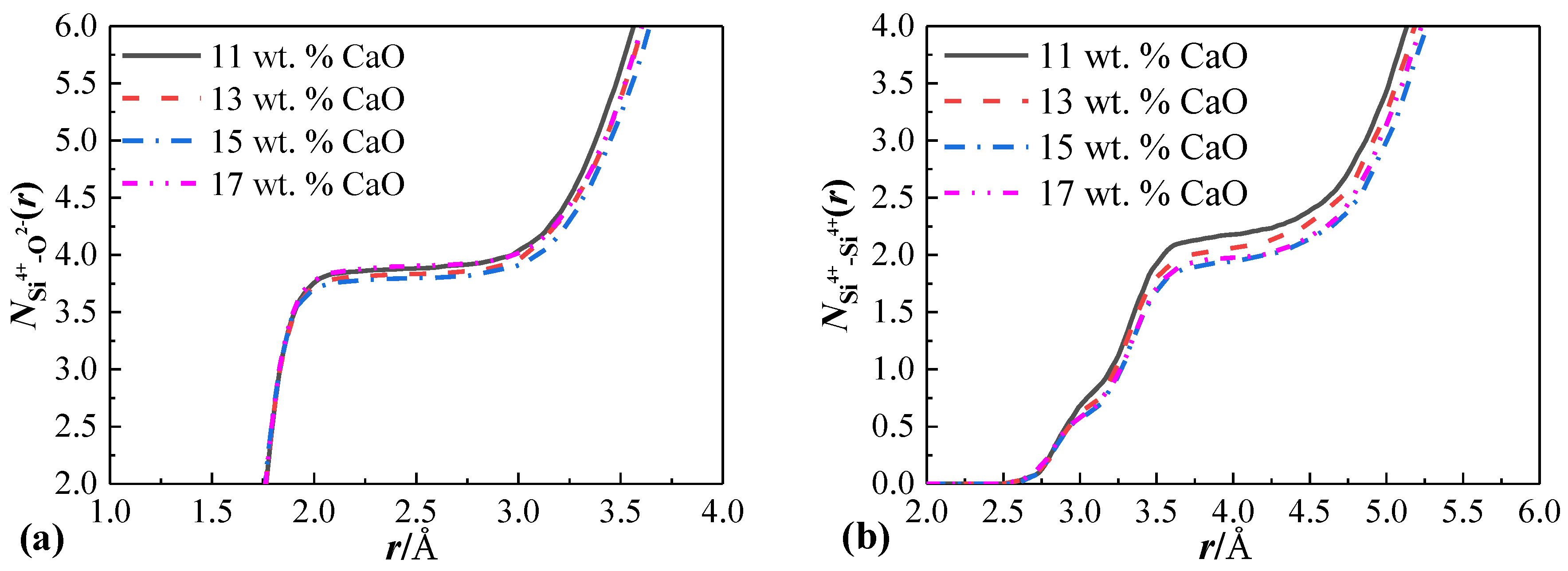
3.3.1. Molecular Dynamics Simulation on Phase Composition and Microstructure
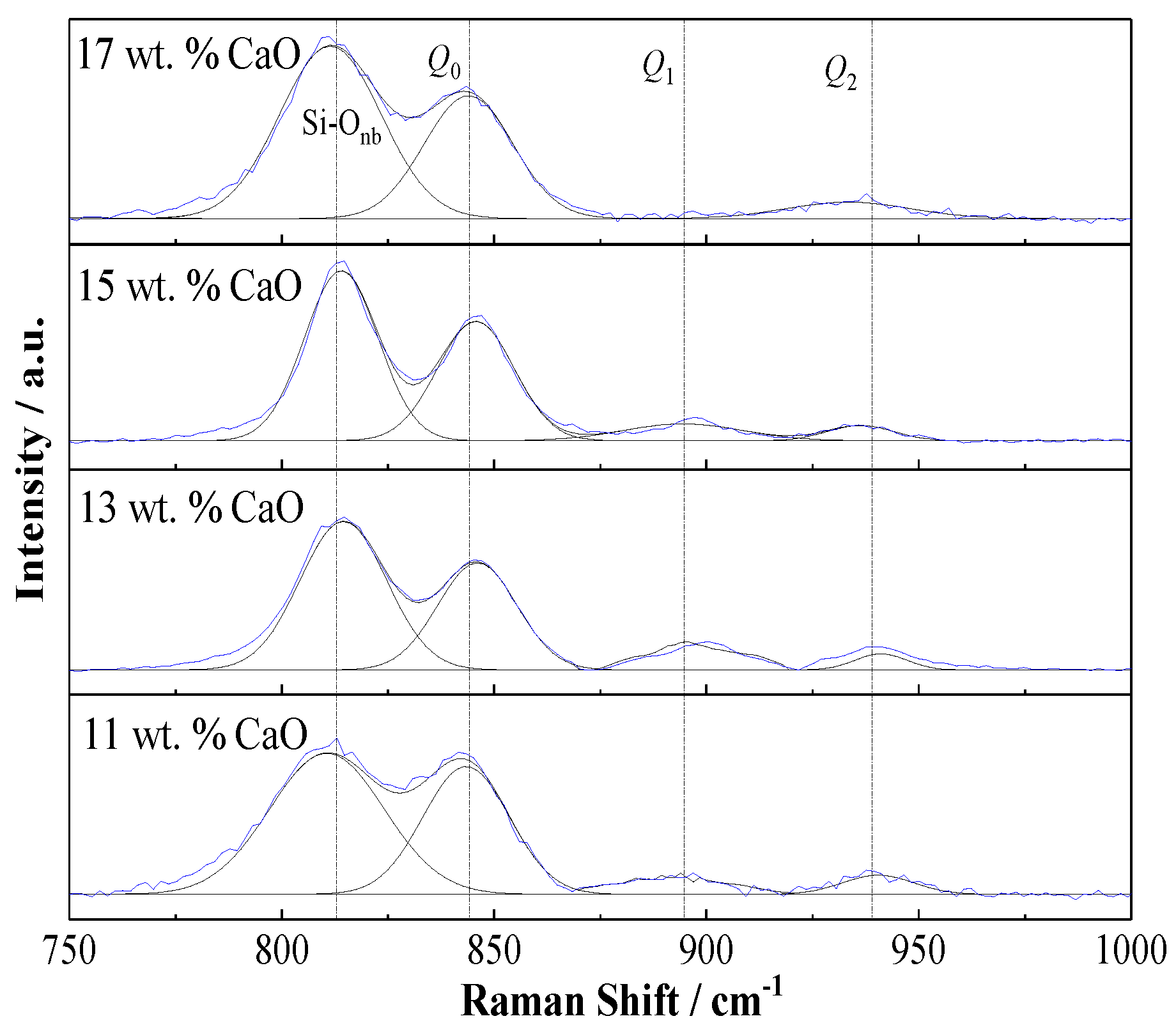
3.3.2. Experimental Characterization of Phase Composition and Microstructure
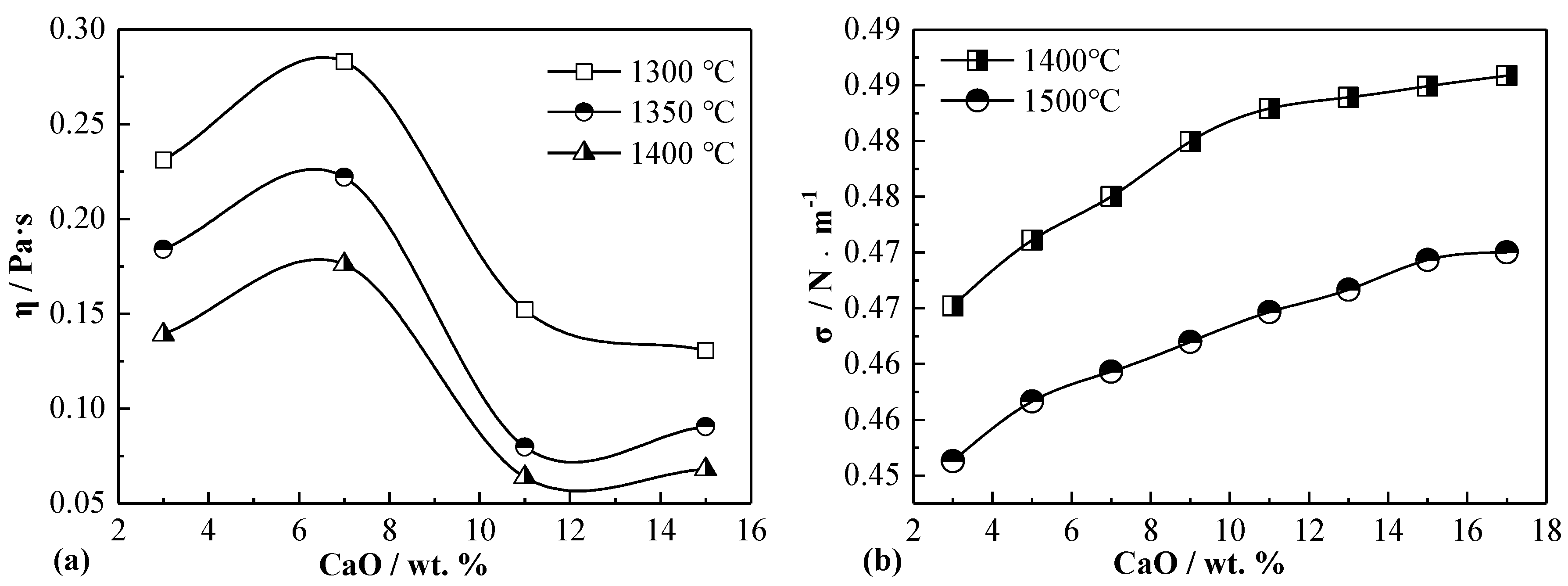
3.4. Fluidity and Interface Separation of Nickel Slag with Varied CaO Content
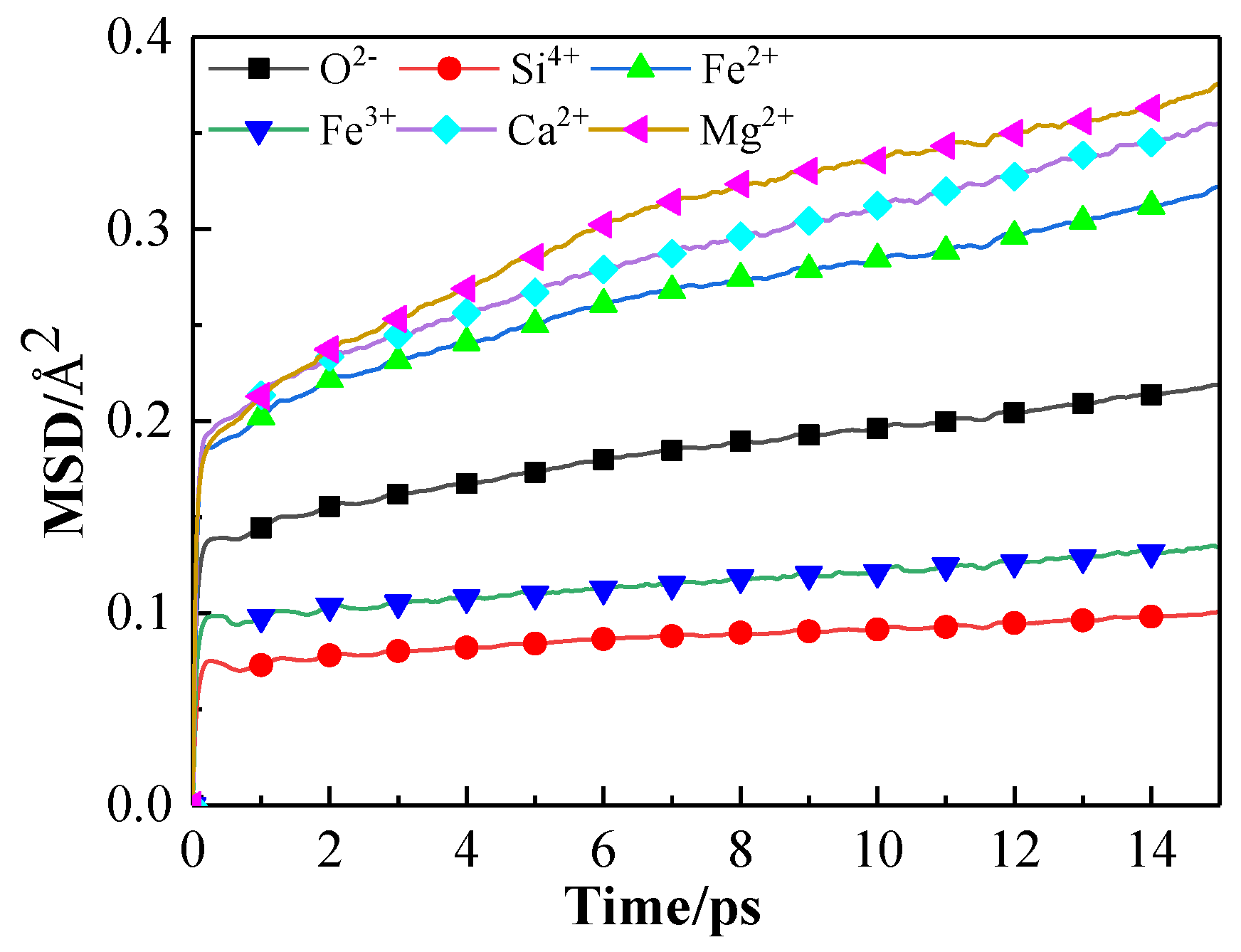
3.4.1. Kinetic Properties of Molecular Dynamics Simulation
3.4.2. Experimental Characterization of Separation Characteristics of Slag Matte
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.X.; Zhao, Z.Y.; Cui, Y.R.; Shi, R.M.; Tang, W.D.; Li, X.M.; Shang, N. New Slag for Nickel Matte Smelting Process and Subsequent Fe Extraction. MMTB 2018, 49, 304–310. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Deng, J.; Lu, T.; Wang, S.; Li, J.; Chen, D.; Liu, Y.; Wang, S. Environmental-Friendly Process for Recovering Copper and Nickel from Jinchuan Tailings by Silica-Based Selective Adsorbents. Ind. Eng. Chem. Res. 2014, 53, 11137–11144. [Google Scholar] [CrossRef]
- Huang, F.; Liao, Y.; Zhou, J.; Wang, Y.; Li, H. Selective recovery of valuable metals from nickel converter slag at elevated temperature with sulfuric acid solution. Sep. Purif. Technol. 2015, 156, 572–581. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, D.; Pan, J.; Zhang, F. Mineralogical Characteristics and Preliminary Beneficiation of Nickel Slag from Reduction Roasting-Ammonia Leaching. Minerals 2017, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Zheng, G.L.; Zhu, D.Q.; Zhou, X.L. Utilization of nickel slag using selective reduction followed by magnetic separation. Trans. Nonferrous Met. Soc. China 2013, 23, 3421–3427. [Google Scholar] [CrossRef]
- Minovich, A.K. The studies on nickel concentrate production from metallurgical products slag. Med. Dosw. Mikrobiol. 2013, 15, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Ilyushechkin, A.; Hayes, P.C.; Jak, E. Effects of Al2O3, CaO and Cr2O3 on liquidus temperatures of Fe–Mg–Si–O slags. Can. Metall. Q. 2014, 54, 179–191. [Google Scholar] [CrossRef]
- Gao, Y.m.; Wang, S.b.; Hong, C.; Ma, X.j.; Yang, F. Effects of basicity and MgO content on the viscosity of the SiO2-CaO-MgO-9 wt. % Al2O3 slag system. Int. J. Miner. Metall. Mater. 2014, 21, 353–362. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Xu, J.W.; Chou, J.L. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO. Powder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Dai, X.; Bai, J.; Li, D.T.; Yuan, P.; Yan, T.G.; Kong, L.X.; Li, W. Experimental and theoretical investigation on relationship between structures of coal ash and its fusibility for Al2O3-SiO2-CaO-FeO system. J. Fuel Chem. Technol. 2019, 47, 641–648. [Google Scholar] [CrossRef]
- Deng, L.B.; Zhang, X.F.; Zhang, M.X.; Jia, X.L. Effect of CaF2 on viscosity, structure and properties of CaO-Al2O3-MgO-SiO2 slag glass ceramics. J. Non Cryst. Solids 2018, 500, 310–316. [Google Scholar] [CrossRef]
- Siakati, C.; Macchieraldo, R.; Kirchner, B.; Tielens, F.; Peys, A.; Seveno, D.; Pontikes, Y. Unraveling the nano-structure of a glassy CaO-FeO-SiO2 slag by molecular dynamics simulations. J. Non Cryst. Solids 2020, 528, 119771. [Google Scholar] [CrossRef]
- Liang, D.; Yan, Z.M.; Lv, X.W.; Zhang, J.; Bai, C.G. Transition of blast furnace slag from silicate-based to aluminate-based: Structure evolution by molecular dynamics simulation and raman spectroscopy. MMTB 2017, 48, 573–581. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, S.; Jahanshahi, S. Molecular dynamics simulations of silicate slags and slag–solid interfaces. J. Non Cryst. Solids 2001, 282, 24–29. [Google Scholar] [CrossRef]
- Yoshioka, T.; Asaeda, M.; Tsuru, T. A molecular dynamics simulation of pressure-driven gas permeation in a micropore potential field on silica membranes. J. Membr. Sci. 2007, 293, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, M.; Guo, Y.F.; Jiang, T.; Zhao, B.J. Comparison of phase equilibria between FactSage predictions and experimental results in titanium oxide-containing system. Calphad 2018, 63, 77–81. [Google Scholar] [CrossRef]
- Nikolic, S.; Hayes, P.C.; Jak, E. Phase equilibria in ferrous calcium silicate slags: Part I. Intermediate oxygen partial pressures in the temperature range 1200 °C to 1350 °C. MMTB 2008, 39, 179–188. [Google Scholar] [CrossRef]
- Nikolic, S.; Hayes, P.C.; Jak, E. Phase equilibria in ferrous calcium silicate slags: Part III. Copper-saturated slag at 1250 °C and 1300 °C at an oxygen partial pressure of 10−6 atm. MMTB 2008, 39, 200–209. [Google Scholar] [CrossRef]
- Nikolic, S.; Hayes, P.C.; Jak, E. Phase equilibria in ferrous calcium silicate slags: Part IV. Liquidus temperatures and solubility of copper in “Cu2O”-FeO-Fe2O3-CaO-SiO2 slags at 1250 °C and 1300 °C at an oxygen partial pressure of 10−6 atm. MMTB 2008, 39, 210–217. [Google Scholar] [CrossRef]
- Nikolic, S.; Henao, H.; Hayes, P.C.; Jak, E. Phase equilibria in ferrous calcium silicate slags: Part II. Evaluation of experimental data and computer thermodynamic models. MMTB 2008, 39, 189–199. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, Z.; Yang, F.; Sridhar, S. Molecular dynamics study of the structural properties of calcium aluminosilicate slags with varying Al2O3/SiO2 ratios. ISIJ Int. 2012, 52, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H. Structure–property correlations of CaO–SiO2–MnO slag derived from Raman spectroscopy. ISIJ Int. 2012, 52, 1627–1636. [Google Scholar] [CrossRef] [Green Version]
- Portillo, H.; Zuluaga, M.C.; Ortega, L.A.; Alonso-Olazabal, A.; Murelaga, X.; Martinez-Salcedo, A. XRD, SEM/EDX and micro-Raman spectroscopy for mineralogical and chemical characterization of iron slags from the Roman archaeological site of Forua (Biscay, North Spain). Microchem. J. 2018, 138, 246–254. [Google Scholar] [CrossRef]
- Xing, X.; Pang, Z.; Mo, C.; Wang, S.; Ju, J. Effect of MgO and BaO on viscosity and structure of blast furnace slag. J. Non Cryst. Solids 2020, 530, 119801. [Google Scholar] [CrossRef]
- Jiang, C.H.; Zhang, H.X.; Xiong, Z.; Chen, S.; Li, K.; Zhang, J.L.; Liang, W.; Sun, M.M.; Wang, Z.M.; Wang, L. Molecular dynamics investigations on the effect of Na2O on the structure and properties of blast furnace slag under different basicity conditions. J. Mol. Liq. 2019, 112195. [Google Scholar] [CrossRef]
- Jiang, C.H.; Li, K.; Zhang, J.L.; Qin, Q.H.; Liu, Z.J.; Sun, M.M.; Wang, Z.M.; Liang, W. Effect of MgO/Al2O3 ratio on the structure and properties of blast furnace slags: A molecular dynamics simulation. J. Non Cryst. Solids 2018, 502, 76–82. [Google Scholar] [CrossRef]



| CaO/wt.% | Number of Ions | Density/(g/cm3) | Fe2+/Fe3+ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ni2+ | Fe2+ | Fe3+ | Si4+ | Ca2+ | Mg2+ | O2− | Sum | |||
| 11 | 3 | 625 | 48 | 516 | 196 | 223 | 2154 | 3766 | 3.50 | 12.93 |
| 13 | 3 | 607 | 50 | 504 | 232 | 223 | 2147 | 3766 | 3.50 | 12.09 |
| 15 | 3 | 578 | 62 | 491 | 267 | 223 | 2146 | 3771 | 3.49 | 9.30 |
| 17 | 3 | 554 | 69 | 478 | 303 | 223 | 2142 | 3773 | 3.49 | 8.02 |
| Ioni | Ionj | Aij, eV | βij, 1/Å | Cij, eV Å6 | R2 |
|---|---|---|---|---|---|
| O2− | O2− | 117,584.04 | 7.31 | 0.00 | 0.996 |
| O2− | Ni2+ | 367,217.18 | 6.64 | 0.00 | 0.997 |
| O2− | Fe2+ | 274,205.07 | 6.08 | 0.00 | 0.992 |
| O2− | Ca2+ | 152,301.61 | 4.93 | 0.00 | 0.997 |
| O2− | Si4+ | 198,320.86 | 5.77 | 0.00 | 0.988 |
| O2− | Mg2+ | 105,782.04 | 4.95 | 0.00 | 0.992 |
| Ni2+ | Ni2+ | 761,382.64 | 6.49 | 0.00 | 0.999 |
| Ni2+ | Fe2+ | 671,045.87 | 6.31 | 0.00 | 0.999 |
| Ni2+ | Ca2+ | 487,920.85 | 5.88 | 0.00 | 0.999 |
| Ni2+ | Si4+ | 622,188.81 | 6.73 | 60.51 | 0.997 |
| Ni2+ | Mg2+ | 282,774.68 | 5.71 | 0.00 | 0.999 |
| Fe2+ | Fe2+ | 848,529.41 | 6.89 | 0.00 | 0.999 |
| Fe2+ | Ca2+ | 387,583.35 | 5.53 | 0.00 | 0.999 |
| Fe2+ | Si4+ | 579,168.00 | 6.64 | 65.58 | 0.996 |
| Fe2+ | Mg2+ | 251,819.69 | 5.59 | 0.00 | 0.999 |
| Ca2+ | Ca2+ | 255,057.57 | 4.68 | 0.00 | 0.999 |
| Ca2+ | Si4+ | 243,110.79 | 5.52 | 7.65 | 0.998 |
| Ca2+ | Mg2+ | 181,673.62 | 5.08 | 0.00 | 0.999 |
| Si4+ | Si4+ | 832,193.36 | 8.77 | 27.51 | 0.999 |
| Si4+ | Mg2+ | 585,831.01 | 7.64 | 46.54 | 0.999 |
| Mg2+ | Mg2+ | 416,041.84 | 6.75 | 69.12 | 0.999 |
| O2− | Fe3+ | 315,438.74 | 6.27 | 0.00 | 0.999 |
| Ni2+ | Fe3+ | 740,398.65 | 6.56 | 0.00 | 0.999 |
| Fe2+ | Fe3+ | 1,040,710.00 | 7.23 | 26.53 | 0.999 |
| Fe3+ | Ca2+ | 419,396.09 | 5.68 | 0.00 | 0.999 |
| Fe3+ | Si4+ | 575,165.97 | 6.75 | 64.99 | 0.999 |
| Fe3+ | Mg2+ | 336,931.32 | 6.20 | 0.00 | 0.999 |
| Fe3+ | Fe3+ | 1,143,420.00 | 7.59 | 0.00 | 0.999 |
| Raman Shift of Si-O | Composition of Nickel Slag | Vibration Mode | |||
|---|---|---|---|---|---|
| Mass Fraction of CaO | 11% | 13% | 15% | 17% | |
| Raman shift/cm−1 | 811 | 814 | 814 | 811 | Si-Onb bending vibration |
| 843 | 846 | 846 | 844 | Si-Ob of Q0 symmetrical stretching vibration | |
| 890 | 894 | 894 | Si-Ob of Q1 symmetrical stretching vibration | ||
| 940 | 940 | 936 | 933 | Si-Ob of Q2 symmetrical stretching vibration | |
| Area Ratio of Qi | Composition of Nickel Slag | Vibration Mode | |||
|---|---|---|---|---|---|
| Mass Fraction of CaO | 11% | 13% | 15% | 17% | |
| Area ratio/% | 79.68 | 82.13 | 81.83 | 79.68 | Si-Ob of Q0 symmetrical stretching vibration |
| 10.59 | 10.08 | 8.61 | 10.59 | Si-Ob of Q1 symmetrical stretching vibration | |
| 9.74 | 7.79 | 9.56 | 9.74 | Si-Ob of Q2 symmetrical stretching vibration | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Cui, Y.; Li, X.; Yang, S.; Zhao, J.; Tang, H.; Li, X. Molecular Dynamics Simulation on Microstructure and Physicochemical Properties of FexO-SiO2-CaO-MgO-“NiO” Slag in Nickel Matte Smelting under Modulating CaO Content. Minerals 2020, 10, 149. https://doi.org/10.3390/min10020149
Wang G, Cui Y, Li X, Yang S, Zhao J, Tang H, Li X. Molecular Dynamics Simulation on Microstructure and Physicochemical Properties of FexO-SiO2-CaO-MgO-“NiO” Slag in Nickel Matte Smelting under Modulating CaO Content. Minerals. 2020; 10(2):149. https://doi.org/10.3390/min10020149
Chicago/Turabian StyleWang, Guohua, Yaru Cui, Xiaoming Li, Shufeng Yang, Junxue Zhao, Hongliang Tang, and Xuteng Li. 2020. "Molecular Dynamics Simulation on Microstructure and Physicochemical Properties of FexO-SiO2-CaO-MgO-“NiO” Slag in Nickel Matte Smelting under Modulating CaO Content" Minerals 10, no. 2: 149. https://doi.org/10.3390/min10020149




