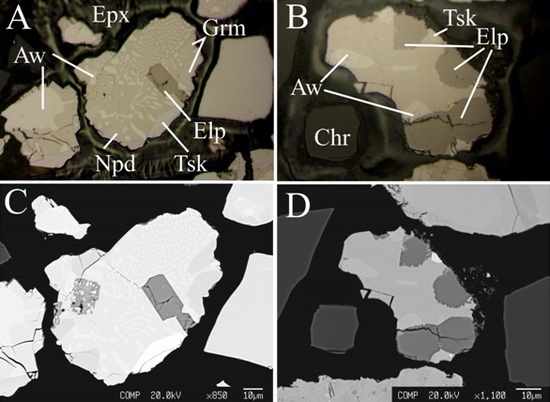
Eliopoulosite, V7S8, A New Sulfide from the Podiform Chromitite of the Othrys Ophiolite, Greece
Abstract
:1. Introduction
2. Geological Background and Occurrence of Eliopoulosite
3. Analytical Methods
4. Physical and Optical Properties
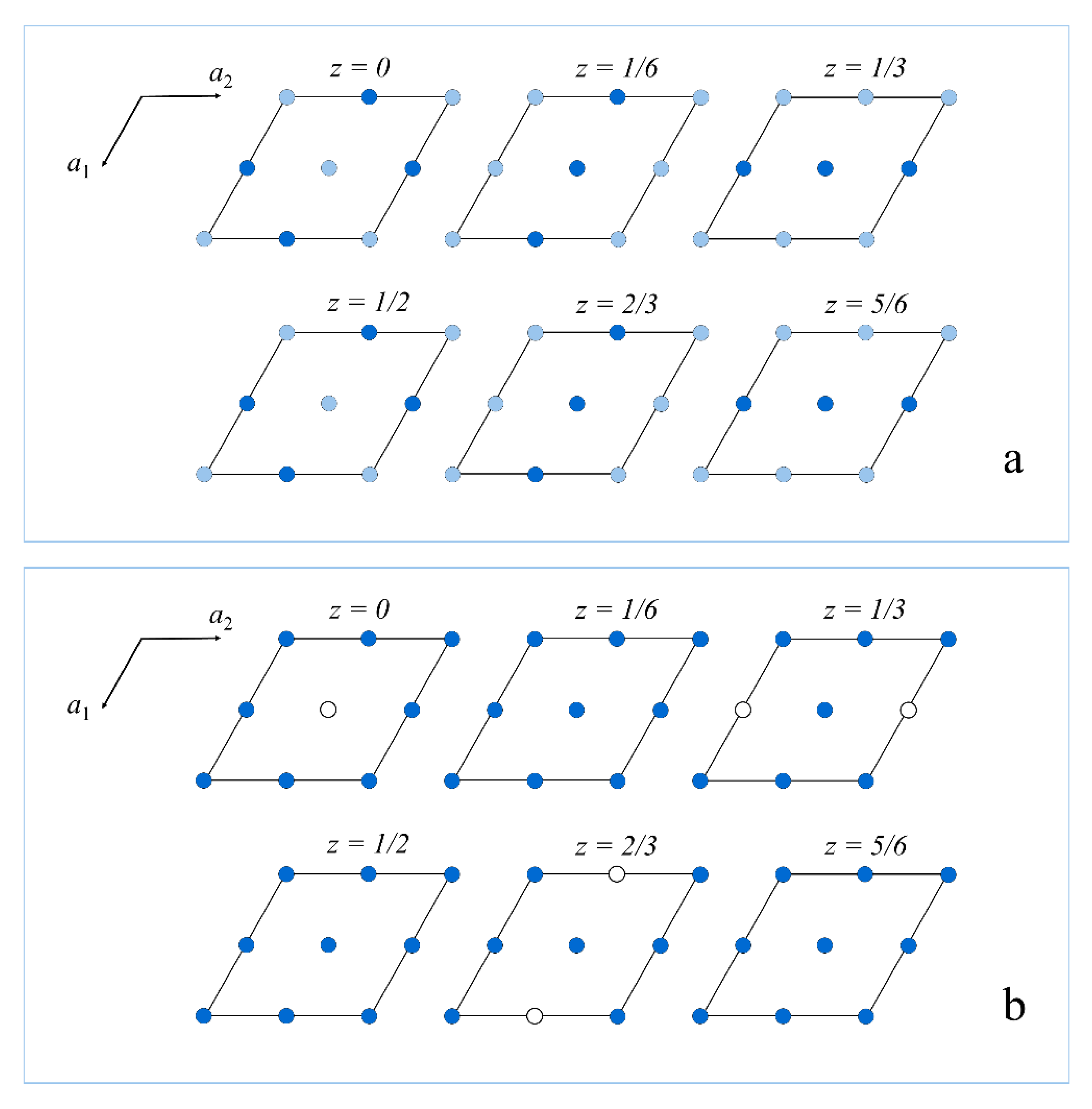
5. X-Ray Crystallography and Chemical Composition
6. Remarks on the Origin of Eliopoulosite
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostrooumov, M.; Taran, Y.; Arellano-Jimenez, M.; Ponse, A.; Reyes-Gasga, J. Colimaite, K3VS4—A new potassium-vanadium sulfide mineral from the Colima volcano, State of Colima (Mexico). Rev. Mex. Cienc. Geol. 2009, 26, 600–608. [Google Scholar]
- Zachariasen, W.H. X-Ray examination of colusite, (Cu,Fe,Mo,Sn)4(S,As,Te)3–4. Am. Mineral. 1933, 18, 534–537. [Google Scholar]
- Spiridonov, E.M.; Kachalovskaya, V.M.; Kovachev, V.V.; Krapiva, L.Y. Germanocolusite Cu26V2(Ge,As)6S32—a new mineral. Vest. Moskov. Univers., Ser. 4, Geologiya 1992, 1992, 50–54. (In Russian) [Google Scholar]
- Jaszczak, J.A.; Rumsey, M.S.; Bindi, L.; Hackney, S.A.; Wise, M.A.; Stanley, C.J.; Spratt, J. Merelaniite, Mo4Pb4VSbS15, a new molybdenum-essential member of the cylindrite group, from the Merelani Tanzanite Deposit, Lelatema Mountains, Manyara Region, Tanzania. Minerals 2016, 6, 115. [Google Scholar] [CrossRef] [Green Version]
- Kovalenker, V.A.; Evstigneeva, T.L.; Malov, V.S.; Trubkin, N.V.; Gorshkov, A.I.; Geinke, V.R. Nekrasovite Cu26V2Sn6S32—A new mineral of the colusite group. Mineral. Zh. 1984, 6, 88–97. [Google Scholar]
- Hillibrand, W.F. Vanadium sulphide, patronite, and its mineral associates from Minasragra, Peru. Am. J. Sci. 1907, 24, 141–151. [Google Scholar] [CrossRef]
- Spiridonov, E.M.; Badalov, A.S.; Kovachev, V.V. Stibiocolusite Cu26V2(Sb,Sn,As)6S32: A new mineral. Dokl. Akad. Nauk 1992, 324, 411–414. (In Russian) [Google Scholar]
- Trojer, F.J. Refinement of the structure of sulvanite. Am. Mineral. 1996, 51, 890–894. [Google Scholar]
- Zaccarini, F.; Bindi, L.; Ifandi, E.; Grammatikopoulos, T.; Stanley, C.; Garuti, G.; Mauro, D. Tsikourasite, Mo3Ni2P1 + x (x < 0.25), a new phosphide from the chromitite of the Othrys Ophiolite, Greece. Minerals 2019, 9, 248. [Google Scholar]
- Bindi, L.; Zaccarini, F.; Ifandi, E.; Tsikouras, B.; Stanley, C.; Garuti, G.; Mauro, D. Grammatikopoulosite, NiVP, a new phosphide from the chromitite of the Othrys Ophiolite, Greece. Minerals 2020, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.G.; Rassios, A. The evolution of ideas for the origin and emplacement of the western Hellenic ophiolites. Geol. Soc. Am. Spec. Pap. 2003, 373, 337–350. [Google Scholar]
- Hynes, A.J.; Nisbet, E.G.; Smith, G.A.; Welland, M.J.P.; Rex, D.C. Spreading and emplacement ages of some ophiolites in the Othris region (Eastern Central Greece). Z. Deutsch Geol. Ges. 1972, 123, 455–468. [Google Scholar]
- Smith, A.G.; Hynes, A.J.; Menzies, M.; Nisbet, E.G.; Price, I.; Welland, M.J.; Ferrière, J. The stratigraphy of the Othris Mountains, Eastern Central Greece: A deformed Mesozoic continental margin sequence. Eclogue Geol. Helv. 1975, 68, 463–481. [Google Scholar]
- Rassios, A.; Smith, A.G. Constraints on the formation and emplacement age of western Greek ophiolites (Vourinos, Pindos, and Othris) inferred from deformation structures in peridotites. In Ophiolites and Oceanic Crust: New Insights from Field Studies and the Ocean Drilling Program; Dilek, Y., Moores, E., Eds.; Geological Society of America Special Paper: Boulder, CO, USA, 2001; pp. 473–484. [Google Scholar]
- Bortolotti, V.; Chiari, M.; Marcucci, M.; Photiades, A.; Principi, G.; Saccani, E. New geochemical and age data on the ophiolites from the Othrys area (Greece): Implication for the Triassic evolution of the Vardar ocean. Ofioliti 2008, 33, 135–151. [Google Scholar]
- Barth, M.G.; Mason, P.R.D.; Davies, G.R.; Drury, M.R. The Othris Ophiolite, Greece: A snapshot of subduction initiation at a mid-ocean ridge. Lithos 2008, 100, 234–254. [Google Scholar] [CrossRef]
- Barth, M.; Gluhak, T. Geochemistry and tectonic setting of mafic rocks from the Othris Ophiolite, Greece. Contr. Mineral. Petrol. 2009, 157, 23–40. [Google Scholar] [CrossRef]
- Dijkstra, A.H.; Barth, M.G.; Drury, M.R.; Mason, P.R.D.; Vissers, R.L.M. Diffuse porous melt flow and melt-rock reaction in the mantle lithosphere at a slow-spreading ridge: A structural petrology and LA-ICP-MS study of the Othris Peridotite Massif (Greece). Geochem. Geophys. Geosyst. 2003, 4, 278. [Google Scholar] [CrossRef]
- Magganas, A.; Koutsovitis, P. Composition, melting and evolution of the upper mantle beneath the Jurassic Pindos ocean inferred by ophiolitic ultramafic rocks in East Othris, Greece. Int. J. Earth Sci. 2015, 104, 1185–1207. [Google Scholar] [CrossRef]
- Garuti, G.; Zaccarini, F.; Economou-Eliopoulos, M. Paragenesis and composition of laurite from chromitites of Othrys (Greece): Implications for Os-Ru fractionation in ophiolite upper mantle of the Balkan Peninsula. Mineral. Dep. 1999, 34, 312–319. [Google Scholar] [CrossRef]
- Tsikouras, B.; Ifandi, E.; Karipi, S.; Grammatikopoulos, T.A.; Hatzipanagiotou, K. Investigation of platinum-group minerals (PGM) from Othrys chromitites (Greece) using superpanning concentrates. Minerals 2016, 6, 94. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.H.F. Overview of the genesis and emplacement of Mesozoic ophiolites in the Eastern Mediterranean Tethyan region. Lithos 2002, 65, 1–67. [Google Scholar] [CrossRef]
- Robertson, A.H.F.; Clift, P.D.; Degnan, P.; Jones, G. Palaeogeographic and palaeotectonic evolution of the Eastern Mediterranean Neotethys. Palaeogeogr. Palaeoclim. Palaeoecol. 1991, 87, 289–343. [Google Scholar] [CrossRef]
- Economou, M.; Dimou, E.; Economou, G.; Migiros, G.; Vacondios, I.; Grivas, E.; Rassios, A.; Dabitzias, S. Chromite deposits of Greece. In Chromites, UNESCO’s IGCP197 Project Metallogeny of Ophiolites; Petrascheck, W., Karamata, S., Eds.; Theophrastus Publ. S.A.: Athens, Greece, 1986; pp. 129–159. [Google Scholar]
- Ifandi, E.; Zaccarini, F.; Tsikouras, B.; Grammatikopoulos, T.; Garuti, G.; Karipi, S.; Hatzipanagiotou, K. First occurrences of Ni-V-Co phosphides in chromitite of Agios Stefanos mine, Othrys ophiolite, Greece. Ofioliti 2018, 43, 131–145. [Google Scholar]
- Zaccarini, F.; Ifandi, E.; Tsikouras, B.; Grammatikopoulos, T.; Garuti, G.; Mauro, D.; Bindi, L.; Stanley, C. Occurrences of new phosphides and sulfide of Ni, Co, V, and Mo from chromitite of the Othrys ophiolite complex (Central Greece). Per. Mineral. 2019, 88. [Google Scholar] [CrossRef]
- Bruker. APEX3; Bruker AXS Inc.: Madison, WI, USA, 2016. Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/single-crystal-x-ray-diffraction/sc-xrd-software/apex3.html (accessed on 6 March 2020).
- Bruker. SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2016. Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/single-crystal-x-ray-diffraction/sc-xrd-software/apex3.html (accessed on 6 March 2020).
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.J.C. International Tables for Crystallography: Mathematical, Physical, and Chemical Tables; International Union of Crystallography: Chester, UK, 1992; Volume 3. [Google Scholar]
- Grønvold, F.; Haraldsen, H.; Pedersen, B.; Tufte, T. X-ray and magnetic study of vanadium sulfides in the range V5S4 to V5S8. Rev. Chim. Minéral. 1969, 6, 215. [Google Scholar]
- Nakano, A.; Tokonami, M.; Morimoto, N. Refinement of 3C pyrrhotite, Fe7S8. Acta Crystallogr. 1979, B35, 722–724. [Google Scholar] [CrossRef]
- Morimoto, N. Crystal structure of a monoclinic pyrrhotite. Rec. Progr. Nat. Sci. Japan 1978, 3, 183–206. [Google Scholar]
- Smith, D.G.W.; Nickel, E.H. A system for codification for unnamed minerals: report of the Subcommittee for Unnamed Minerals of the IMA Commission on New Minerals, Nomenclature and Classification. Can. Mineral. 2007, 45, 983–1055. [Google Scholar] [CrossRef]
- Cámara, F.; Bindi, L.; Pagano, A.; Pagano, R.; Gain, S.E.M.; Griffin, W.L. Dellagiustaite: A novel natural spinel containing V2+. Minerals 2019, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, M.A.; Ma, C.; Lorenz, C.A.; Franchi, I.A.; Kononkova, N.N. A new unusual bencubbinite (cba), Sierra Gorda 013 with unique V-rich sulfides. Met. Plan. Sci. 2019, 54, 6149. [Google Scholar]
- Selezneva, N.V.; Ibrahim, P.N.G.; Toporova, N.M.; Sherokalova, E.M.; Baranov, N.V. Crystal structure and magnetic properties of pyrrhotite-type compounds Fe7-yVyS8. Acta Phys. Polon. 2018, A133, 450–452. [Google Scholar] [CrossRef]
- Malvoisin, B.; Chopin, C.; Brunet, F.; Matthieu, E.; Galvez, M.E. Low-temperature wollastonite formed by carbonate reduction: a marker of serpentinite redox conditions. J. Petrol. 2012, 53, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Etiope, G.; Tsikouras, B.; Kordella, S.; Ifandi, E.; Christodoulou, D.; Papatheodorou, G. Methane flux and origin in the Othrys ophiolite hyperalkaline springs, Greece. Chem. Geol. 2013, 347, 161–174. [Google Scholar] [CrossRef]
- Etiope, G.; Ifandi, E.; Nazzari, M.; Procesi, M.; Tsikouras, B.; Ventura, G.; Steele, A.; Tardini, R.; Szatmari, P. Widespread abiotic methane in chromitites. Sci. Rep. 2018, 8, 8728. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Q.; Griffin, W.L.; Huang, J.X.; Gain, S.E.M.; Toledo, V.; Pearson, N.J.; O’Reilly, S.Y. Super-reduced mineral assemblages in “ophiolitic” chromitites and peridotites: The view from Mount Carmel. Eur. J. Mineral. 2017, 29, 557–570. [Google Scholar] [CrossRef]
- Pasek, M.A.; Hammeijer, J.P.; Buick, R.; Gull, M.; Atlas, Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc. Nat. Acad. Sci. U.S.A. 2013, 110, 100089–100094. [Google Scholar] [CrossRef] [Green Version]
- Ballhaus, C.; Wirth, R.; Fonseca, R.O.C.; Blanchard, H.; Pröll, W.; Bragagni, A.; Nagel, T.; Schreiber, A.; Dittrich, S.; Thome, V.; et al. Ultra-high pressure and ultra-reduced minerals in ophiolites may form by lightning strikes. Geochem. Perspec. Lett. 2017, 5, 42–46. [Google Scholar] [CrossRef]




| Analysis | S | V | Ni | Fe | Co | Mo | Total |
|---|---|---|---|---|---|---|---|
| 1 | 41.34 | 53.08 | 1.24 | 0.87 | 0.48 | 0.55 | 99.01 |
| 2 | 41.35 | 53.45 | 1.33 | 0.92 | 0.52 | 0.57 | 99.29 |
| 3 | 41.36 | 53.55 | 1.39 | 0.97 | 0.55 | 0.57 | 99.37 |
| 4 | 41.39 | 53.71 | 1.41 | 1.00 | 0.55 | 0.57 | 99.43 |
| 5 | 41.42 | 53.90 | 1.51 | 1.01 | 0.61 | 0.58 | 99.72 |
| 6 | 41.44 | 53.91 | 1.54 | 1.09 | 0.63 | 0.59 | 99.75 |
| 7 | 41.57 | 53.97 | 1.55 | 1.09 | 0.63 | 0.59 | 99.77 |
| 8 | 41.65 | 54.03 | 1.58 | 1.09 | 0.63 | 0.60 | 99.82 |
| 9 | 41.67 | 54.03 | 1.65 | 1.10 | 0.64 | 0.63 | 99.86 |
| 10 | 41.73 | 54.06 | 1.67 | 1.11 | 0.64 | 0.64 | 99.92 |
| 11 | 41.76 | 54.09 | 1.69 | 1.11 | 0.65 | 0.65 | 100.18 |
| 12 | 41.82 | 54.16 | 1.70 | 1.12 | 0.68 | 0.67 | 100.20 |
| 13 | 41.90 | 54.21 | 1.72 | 1.12 | 0.69 | 0.68 | 100.26 |
| 14 | 41.94 | 54.25 | 1.72 | 1.13 | 0.72 | 0.68 | 100.27 |
| 15 | 41.99 | 54.40 | 1.94 | 1.16 | 0.72 | 0.69 | 100.28 |
| 16 | 42.03 | 54.40 | 1.99 | 1.16 | 0.73 | 0.69 | 100.31 |
| 17 | 42.12 | 54.56 | 2.02 | 1.18 | 0.76 | 0.70 | 100.78 |
| 18 | 42.28 | 54.64 | 2.13 | 1.19 | 0.78 | 0.75 | 100.78 |
| 19 | 42.33 | 54.80 | 2.22 | 1.21 | 0.79 | 0.81 | 100.84 |
| 20 | 42.62 | 55.02 | 2.27 | 1.47 | 0.91 | 0.90 | 100.84 |
| average | 41.78 | 54.11 | 1.71 | 1.1 | 0.67 | 0.66 | 100.03 |
| λ (nm) | Ro (%) | Re’ (%) |
|---|---|---|
| 400 | 32.0 | 33.1 |
| 420 | 32.7 | 33.7 |
| 440 | 33.5 | 34.5 |
| 460 | 34.3 | 35.3 |
| 470 | 34.8 | 35.7 |
| 480 | 35.2 | 36.1 |
| 500 | 36.1 | 37.0 |
| 520 | 36.9 | 37.8 |
| 540 | 37.7 | 38.7 |
| 546 | 38.0 | 39.0 |
| 560 | 38.6 | 39.7 |
| 580 | 39.5 | 40.8 |
| 589 | 40.0 | 41.3 |
| 600 | 40.5 | 41.9 |
| 620 | 41.2 | 42.9 |
| 640 | 42.1 | 43.8 |
| 650 | 42.5 | 44.2 |
| 660 | 42.8 | 44.6 |
| 680 | 43.5 | 45.5 |
| 700 | 44.3 | 46.2 |
| Atom | Site Occupancy | x/a | y/b | z/c | Uiso |
|---|---|---|---|---|---|
| V1 | V0.749(6) | 0.0001(6) | 0 | 2/3 | 0.0088(6) |
| V2 | V0.762(6) | 0.5005(6) | 0 | 2/3 | 0.0095(6) |
| V3 | V0.755(6) | 0.0006(7) | 0 | 1/6 | 0.0113(6) |
| V4 | V0.749(7) | 0.5010(7) | 0 | 1/6 | 0.0187(8) |
| V5 | V1.00 | 0.4996(4) | 0.4997(5) | 0.16664(5) | 0.0060(3) |
| V6 | V1.00 | 0.4997(5) | 0.4996(5) | 0.33327(5) | 0.0120(4) |
| S1 | S1.00 | 0.1663(7) | 0.3343(6) | 0.08309(11) | 0.0227(4) |
| S2 | S1.00 | 0.1659(7) | 0.3328(7) | 0.41639(11) | 0.0226(4) |
| S3 | S1.00 | 0.1661(7) | 0.3331(6) | 0.74976(11) | 0.0226(4) |
| S4 | S1.00 | 0.3333(6) | 0.6673(7) | 0.25009(11) | 0.0228(4) |
| V1-S2 | 2.409(5) (×2) | V5-S1 | 2.413(4) |
| V1-S3 | 2.411(3) (×2) | V5-S4 | 2.416(3) |
| V1-S1 | 2.418(4) (×2) | V5-S1 | 2.417(4) |
| mean | 2.413 | V5-S2 | 2.418(4) |
| V5-S4 | 2.419(3) | ||
| V2-S1 | 2.412(4) (×2) | V5-S3 | 2.419(4) |
| V2-S2 | 2.413(5) (×2) | mean | 2.417 |
| V2-S4 | 2.414(3) (×2) | ||
| mean | 2.413 | V6-S1 | 2.407(4) |
| V6-S4 | 2.411(4) | ||
| V3-S3 | 2.414(4) (×2) | V6-S3 | 2.413(4) |
| V3-S2 | 2.418(3) (×2) | V6-S3 | 2.413(4) |
| V3-S1 | 2.422(3) (×2) | V6-S2 | 2.415(4) |
| mean | 2.418 | V6-S4 | 2.417(4) |
| mean | 2.413 | ||
| V4-S4 | 2.413(3) (×2) | ||
| V4-S2 | 2.417(4) (×2) | ||
| V4-S3 | 2.421(5) (×2) | ||
| mean | 2.417 | ||
| V1-V3 | 2.9006(10) (×2) | ||
| V2-V5 | 2.9001(13) (×2) | ||
| V4-V6 | 2.9018(14) (×2) | ||
| V5-V6 | 2.8998(12) (×2) |
| hkl | dcalc | Icalc |
|---|---|---|
| 200 | 2.8964 | 29 |
| 023 | 2.5914 | 45 |
| 026 | 2.0495 | 100 |
| 220 | 1.6723 | 40 |
| 029 | 1.6082 | 10 |
| 012 | 1.4503 | 8 |
| 400 | 1.4482 | 3 |
| 046 | 1.2957 | 20 |
| 2212 | 1.0956 | 15 |
| 246 | 1.0242 | 12 |
| 600 | 0.9655 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindi, L.; Zaccarini, F.; Bonazzi, P.; Grammatikopoulos, T.; Tsikouras, B.; Stanley, C.; Garuti, G. Eliopoulosite, V7S8, A New Sulfide from the Podiform Chromitite of the Othrys Ophiolite, Greece. Minerals 2020, 10, 245. https://doi.org/10.3390/min10030245
Bindi L, Zaccarini F, Bonazzi P, Grammatikopoulos T, Tsikouras B, Stanley C, Garuti G. Eliopoulosite, V7S8, A New Sulfide from the Podiform Chromitite of the Othrys Ophiolite, Greece. Minerals. 2020; 10(3):245. https://doi.org/10.3390/min10030245
Chicago/Turabian StyleBindi, Luca, Federica Zaccarini, Paola Bonazzi, Tassos Grammatikopoulos, Basilios Tsikouras, Chris Stanley, and Giorgio Garuti. 2020. "Eliopoulosite, V7S8, A New Sulfide from the Podiform Chromitite of the Othrys Ophiolite, Greece" Minerals 10, no. 3: 245. https://doi.org/10.3390/min10030245