A Quantitative Assessment of Methane-Derived Carbon Cycling at the Cold Seeps in the Northwestern South China Sea
Abstract
:1. Introduction
2. Geological Background
3. Materials and Methods
3.1. Seafloor Observations
3.2. Sampling and Analytical Methods
3.3. Reaction-Transport Model
4. Results
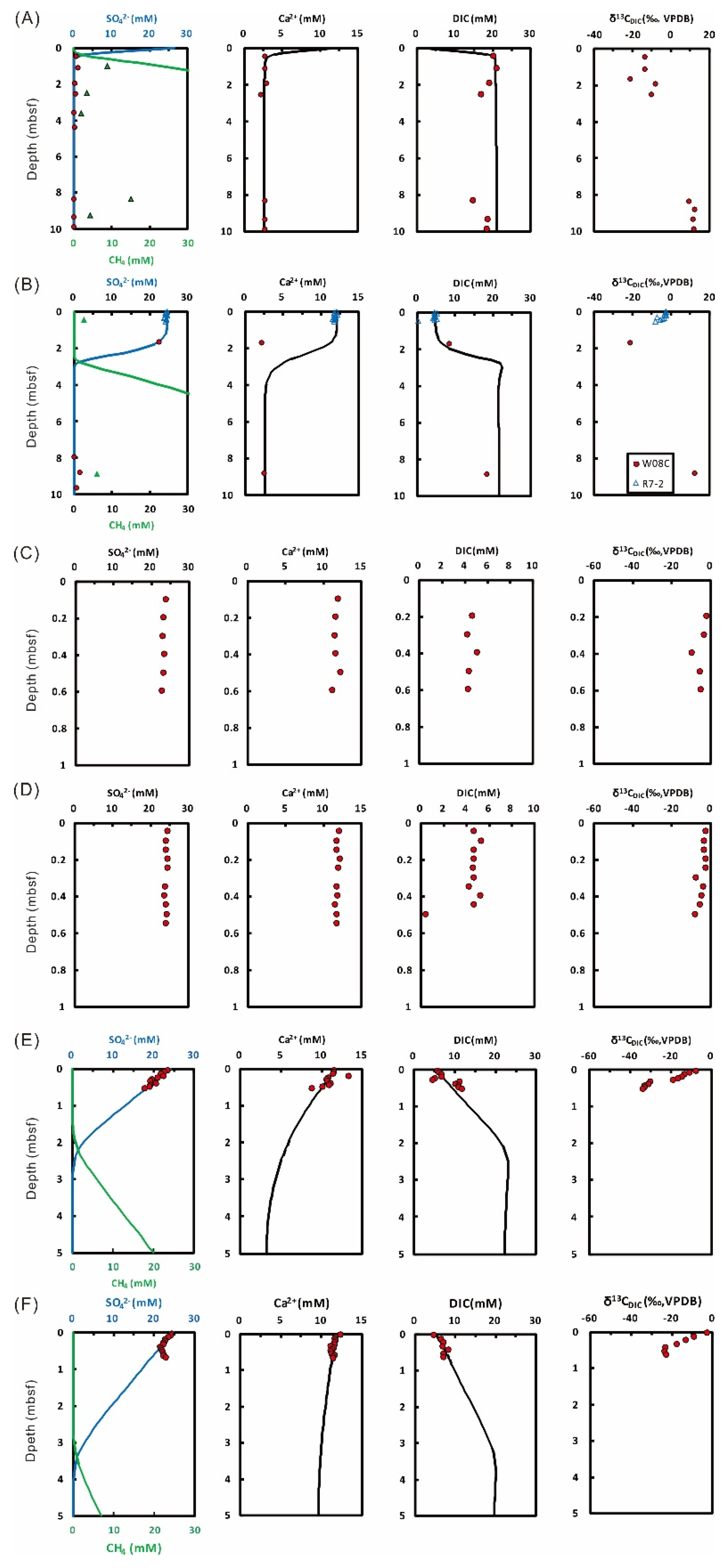
4.1. Site Characteristics
4.2. General Geochemical Trends
4.3. Reaction-Transport Modeling
5. Discussion
5.1. Methane-Related Carbon Cycling at Cold Seep Areas
5.2. Potential Contribution of Fossil Carbon from Cold Seeps to Bottom-Water Carbon Pool
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Rate |
|---|---|
| Particulate organic carbon (POC) | −RPOC |
| Sulfate (SO42−) | −RSR − RAOM |
| Methane (CH4) | −RAOM + RMG |
| Dissolved inorganic carbon (DIC) | RPOC/fPOC − RMG + RAOM − RCP |
| Calcium (Ca2+) | −RCP |
| Parameter | W08B | W08C | W09 | R7-1 | R7-3 | HM-1 | Unit |
|---|---|---|---|---|---|---|---|
| Temperature (T)a | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | 2.9 | °C |
| Salinity (S) | 34 | 34 | 34 | 34 | 34 | 34 | PSU |
| Pressure (P)b | 17.7 | 17.7 | 17.6 | 17.7 | 17.7 | 14.2 | MPa |
| Density of dry solids (ρs) c | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | g cm−3 |
| Density of porewater (ρpw) c | 1.033 | 1.033 | 1.033 | 1.033 | 1.033 | 1.033 | g cm−3 |
| Sedimentation rate (ω) d | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | 0.017 | cm yr−1 |
| Porosity (Φ) e | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | - |
| Initial age of POC (a0) f | 950 | 950 | 950 | 950 | 950 | 650 | kyr |
| Molecular diffusion coefficient of SO42− in free seawater g | 191 | 191 | 191 | 191 | 191 | 191 | cm2 yr−1 |
| Molecular diffusion coefficient of CH4 in free seawater g | 294 | 294 | 294 | 294 | 294 | 294 | cm2 yr−1 |
| Molecular diffusion coefficient of DIC in free seawater g | 203 | 203 | 203 | 203 | 203 | 203 | cm2 yr−1 |
| Molecular diffusion coefficient of Ca2+ in free seawater g | 142 | 142 | 142 | 142 | 142 | 142 | cm2 yr−1 |
| Michaelis–Menten constant for POC degradation ()h | 1 × 10−4 | 1 × 10−4 | 1 × 10−4 | 1 × 10−4 | 1 × 10−4 | 1 × 10−4 | mM |
| Rate constant for AOM (kAOM) | 400 | 50 | 50 | 50 | 50 | 400 | cm3 yr−1 mmol−1 |
| Rate constant for carbonate precipitation/dissolution (kCa) | 1.2 × 10−3 | 6.0 × 10−6 | 2.0 × 10−7 | 1.0 × 10−6 | 1.0 × 10−7 | 1.1 × 10−5 | mmol cm−3 yr−1 |
| Upper boundary condition for POC | 1.2 | 1.2 | 1.3 | 1.2 | 1.2 | 0.9 | wt.% |
| Upper boundary condition for SO42− | 25 | 25 | 27 | 24 | 25 | 29 | mM |
| Upper boundary condition for DIC | 4.6 | 4.6 | 4.8 | 5.3 | 4.8 | 3.5 | mM |
| Upper boundary condition for Ca2+ | 12.5 | 12.1 | 9.1 | 12.0 | 11.8 | 12.5 | μM |
| Upper boundary condition for CH4 | 0 | 0 | 0 | 0 | 0 | 0 | mM |
| Lower boundary condition for SO42− | - | ||||||
| Lower boundary condition for DIC | - | ||||||
| Lower boundary condition for Ca2+ | - | ||||||
| Lower boundary condition for CH4 | - | - | - | 13 | 4.5 | - | mM |
Appendix B
| Depth (cmbsf) | SO42− (mM) | Ca2+ (mM) | DIC (mM) | δ13CDIC (‰,VPDB) | Depth (cmbsf) | CH4 (mM) |
|---|---|---|---|---|---|---|
| W08B | ||||||
| 47 | 0.7 | 2.7 | 20.3 | −13.5 | - | - |
| 114 | 1.2 | 2.7 | 21.2 | −13.4 | 102 | 9 |
| 196 | 0.4 | 3.0 | 19.4 | −7.6 | - | - |
| 255 | 0.6 | 2.2 | 17.2 | −9.8 | 253 | 3.6 |
| 361 | 0.2 | - | - | - | - | - |
| 441 | 0.4 | - | - | - | - | - |
| 835 | 0.3 | 2.7 | 14.9 | 9.8 | 835 | 15.3 |
| 935 | 0.1 | 2.7 | 18.9 | 11.6 | 925 | 4.4 |
| 990 | 0.1 | 2.7 | 18.7 | 12.1 | - | - |
| W08C | ||||||
| 170 | 22.5 | 2.2 | -21.1 | 8.6 | 50 | 2.8 |
| 796 | 0.1 | - | - | - | - | - |
| 880 | 1.6 | 2.6 | 12.5 | 18.5 | 890 | 6.2 |
| 963 | 0.7 | - | - | - | - | - |
| W09 | ||||||
| 60 | 27.3 | 9.5 | 4.7 | −10.0 | - | - |
| 160 | 26.4 | 9.0 | 5.3 | −14.4 | 170 | 0 |
| 260 | 25.9 | 9.2 | 5.4 | −16.9 | 270 | 0 |
| 360 | 24.2 | 8.7 | 6.7 | −23.9 | 370 | 0 |
| 460 | 17.3 | 8.2 | 9.9 | −34.4 | 470 | 0 |
| 851 | 1.5 | 2.7 | 19.7 | −29.6 | 800 | 0.2 |
| 919 | 0.4 | 1.4 | 19.6 | −26.2 | 984 | 1.2 |
| 1036 | 0.4 | 1.7 | 17.6 | −20.4 | - | - |
| 1127 | 0.2 | 1.6 | 19.0 | −14.9 | - | - |
| 1189 | 0.2 | 2.5 | 20.3 | −8.0 | 1176 | 8.9 |
| 1610 | 3.9 | 1.2 | 18.3 | 19.3 | - | - |
| 1836 | 2.6 | 2.0 | - | - | - | - |
| Depth (cmbsf) | SO42− (mM) | Ca2+ (mM) | DIC (mM) | δ13CDIC (‰,VPDB) |
|---|---|---|---|---|
| R5-1 | ||||
| 10 | 24.4 | 12.1 | 4.7 | - |
| 20 | 24.3 | 12.0 | 4.7 | −3.1 |
| 30 | 24.6 | 11.7 | 4.7 | −3.6 |
| 40 | 24.3 | 11.6 | 4.8 | −3.0 |
| 50 | 24.5 | 12.1 | 4.9 | −3.3 |
| 60 | 24.4 | 11.6 | 4.9 | −3.6 |
| 70 | 25.1 | 11.7 | 5.0 | −3.2 |
| R7 | ||||
| 10 | 24.0 | 12.1 | - | - |
| 20 | 23.5 | 11.7 | 4.7 | −1.8 |
| 30 | 23.3 | 11.6 | 4.3 | −3.0 |
| 40 | 23.7 | 11.7 | 5.1 | −9.3 |
| 50 | 23.5 | 12.3 | 4.4 | −4.9 |
| 60 | 22.9 | 11.3 | 4.3 | −4.6 |
| R7-1 | ||||
| 5 | 23.5 | 11.6 | 6.0 | −7.5 |
| 10 | 22.7 | 11.6 | 6.4 | −10.4 |
| 15 | 22.1 | 11.5 | 6.8 | −13.1 |
| 20 | 22.6 | 13.4 | 6.9 | −14.0 |
| 25 | 21.2 | 10.9 | 5.3 | −16.2 |
| 30 | 19.8 | 10.7 | 4.6 | −18.5 |
| 35 | 19.4 | 10.7 | 11.2 | −29.8 |
| 40 | 20.8 | 11.2 | 10.3 | −30.5 |
| 45 | 19.4 | 11.0 | 11.1 | −32.7 |
| 50 | 19.1 | 10.1 | 11.1 | −32.5 |
| 55 | 18.0 | 8.9 | 12.0 | −33.3 |
| R7-2 | ||||
| 5 | 24.7 | 12.1 | 4.7 | −2.3 |
| 10 | 24.3 | 11.8 | 5.4 | −2.9 |
| 15 | 24.2 | 11.8 | 4.7 | −2.9 |
| 20 | 24.6 | 12.3 | 4.7 | −2.3 |
| 25 | 24.7 | 12.0 | 4.6 | −2.2 |
| 30 | - | - | 4.7 | −7.0 |
| 35 | 24.1 | 11.8 | 4.2 | −3.3 |
| 40 | 23.8 | 12.0 | 5.3 | −4.1 |
| 45 | 24.4 | 11.6 | 4.7 | −5.1 |
| 50 | 24.5 | 11.8 | 0.5 | −7.8 |
| 55 | 24.3 | 11.8 | - | - |
| R7-3 | ||||
| 5 | 24.5 | 12.5 | 4.8 | −2.3 |
| 10 | 24.2 | 11.8 | - | - |
| 15 | 23.8 | 11.7 | 6.6 | −8.8 |
| 20 | 23.0 | 11.8 | - | - |
| 25 | 22.9 | 11.7 | 7.2 | −12.8 |
| 30 | 22.4 | 11.7 | - | - |
| 35 | 22.4 | 11.3 | 7.0 | −17.2 |
| 40 | 21.7 | 11.6 | - | - |
| 45 | 21.8 | 11.4 | 8.4 | −22.7 |
| 50 | 22.0 | 11.2 | - | - |
| 55 | 22.2 | 11.3 | 7.3 | −23.3 |
| 60 | 22.5 | 11.7 | - | - |
| 65 | 22.5 | 11.6 | 7.3 | −22.5 |
| 70 | 23.0 | 11.5 | - | - |
| HM-1 | ||||
| 10 | 23.1 | 11.1 | 7.0 | −19.8 |
| 15 | 20.7 | 10.3 | 9.7 | −35.2 |
| 20 | 18.5 | 9.4 | 11.9 | −40.6 |
| 25 | 14.9 | 8.7 | 16.2 | −44.3 |
| 30 | 13.4 | 8.2 | 17.4 | −48.2 |
| 35 | 11.3 | 7.6 | 19.4 | −48.8 |
| 40 | 9.5 | 7.1 | 22.7 | −49.9 |
| Depth (cmbsf) | SO42− (mM) | Ca2+ (mM) | Alk (mM) | PO43− (μM) |
| CL48 | ||||
| 55 | 25.7 | 8.7 | 3.6 | 14.3 |
| 115 | 25.5 | 8.6 | 3.8 | 12.8 |
| 175 | 25.3 | 8.8 | 3.8 | 12.0 |
| 235 | 25.4 | 8.5 | 3.9 | 15.5 |
| 295 | 25.3 | 8.5 | 3.9 | 14.5 |
| 355 | 25.3 | 8.7 | 3.9 | 14.3 |
| 415 | 25.3 | 8.6 | 3.9 | 12.3 |
| 475 | 25.2 | 8.6 | 3.9 | 11.5 |
| 535 | 24.7 | 9.6 | 4.0 | 14.3 |
| 595 | 24.6 | 8.5 | 4.3 | 17.6 |
| 655 | 24.7 | 8.3 | 4.4 | 18.8 |
| 715 | 24.1 | 9.0 | 4.9 | 22.6 |
| Site | Samle ID | Sampling Method | pH | TA (mM) | δ13CDIC (‰,VPDB) | 14C age (yr BP) | Δ14C (‰,VPDB) | δ13CCS (‰,VPDB) | Fcs (%) | AlkMD (mM) |
|---|---|---|---|---|---|---|---|---|---|---|
| ROV05 | R-05-shell | T,P-tight | 7.7 | 3.1 | −3.7 | 800 | −95 | −17.2 | 21.6 | 0.7 |
| ROV05 | ROV05 | CTD | 7.7 | 3.2 | −1.6 | 1230 | −142 | −6.3 | 25.9 | 0.8 |
| ROV05 | ROV05-1 | Water on the top of the tubes | 7.7 | 3.2 | −1.1 | −4.6 | 25.0 | 0.8 | ||
| R7 | R-07 | T,P-tight | 7.6 | 2.9 | −2.0 | 1170 | −136 | −12.5 | 16.1 | 0.5 |
| R7 | ROV07 | CTD | 7.7 | 3.2 | −1.1 | 1580 | −178 | −4.4 | 25.8 | 0.8 |
| R7 | ROV07+v | Water on the top of the tubes | 7.8 | 2.9 | −2.0 | −11.1 | 18.4 | 0.5 | ||
| ROV7-1 | R-07-1 | T,P-tight | 7.9 | 3.0 | −4.3 | 860 | −101 | −22.7 | 18.8 | 0.6 |
| ROV7-1 | R01-2018 | CTD | 7.9 | 3.1 | −1.8 | 590 | −71 | −8.0 | 22.0 | 0.7 |
| ROV7-1 | ROV07-1 | Water on the top of the tubes | 7.8 | 3.2 | −1.7 | −7.1 | 24.4 | 0.8 | ||
| HM-ROV | HM-2-vent | T,P-tight | 7.7 | 2.9 | −2.1 | 880 | −104 | −12.3 | 16.8 | 0.5 |
| HM-ROV | HM-3-vent | T,P-tight | 7.7 | 2.9 | −3.4 | 1250 | −144 | −21.2 | 16.1 | 0.5 |
| HM-ROV | HM-2 | CTD | 7.9 | 2.9 | −2.1 | −11.5 | 18.0 | 0.5 | ||
| HM-ROV | HM-3 | CTD | 7.7 | 3.2 | −1.3 | −5.2 | 24.8 | 0.8 | ||
| HM-ROV05-1 | HM-R003-1 | T,P-tight | 7.7 | 3.0 | −1.5 | 1300 | −149 | −7.5 | 19.9 | 0.6 |
| HM-ROV05-1 | HM-1 | CTD | 7.7 | 3.2 | −1.7 | −6.4 | 26.0 | 0.8 |
References
- Judd, A.; Hovland, M. Seabed Fluid Flow; Cambridge University Press: Cambridge, UK, 2007; p. 408. [Google Scholar]
- Suess, E. Marine cold seeps and their manifestations: Geological control, biogeochemical criteria and environmental conditions. Int. J. Earth Sci. 2014, 103, 1889–1916. [Google Scholar] [CrossRef]
- Barnes, R.O.; Goldberg, E.D. Methane production and consumption in anoxic marine sediments. Geology 1976, 4, 297–300. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, R.; Jørgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Luff, R.; Greinert, J.; Wallmann, K.; Klaucke, I.; Suess, E. Simulation of long-term feedbacks from authigenic carbonate crust formation at cold vent sites. Chem. Geol. 2005, 216, 157–174. [Google Scholar] [CrossRef]
- Boetius, A.; Wenzhöfer, F. Seafloor oxygen consumption fuelled by methane from cold seeps. Nat. Geosci. 2013, 6, 725–734. [Google Scholar] [CrossRef]
- Egger, M.; Riedinger, N.; Mogollón, J.M.; Jørgensen, B.B. Global diffusive fluxes of methane in marine sediments. Nat. Geosci. 2018, 11, 421–425. [Google Scholar] [CrossRef]
- Orphan, V.J.; Hinrichs, K.U.; Paull, C.K.; Taylor, L.T.; Sylva, S.P.; Hayes, J.M.; Delong, E.F. Comparative analysis of methane-oxidizing archaea and sulfate-reducing bacteria in anoxic marine sediments. Appl. Environ. Microbiol. 2001, 67, 1922–1934. [Google Scholar] [CrossRef] [Green Version]
- Sahling, H.; Rickert, D.; Lee, R.W.; Linke, P.; Suess, E. Macrofaunal community structure and sulfide flux at gas hydrate deposits from the Cascadia convergent margin, NE Pacifc. Mar. Ecol. Prog. Ser. 2002, 231, 121–138. [Google Scholar] [CrossRef]
- Aharon, P.; Graber, E.R.; Roberts, H.H. Dissolved carbon and 33-133-133-1 anomalies in the water column caused by hydrocarbon seeps on the northwestern Gulf of Mexico slope. Geo-Mar. Lett. 1992, 12, 33–40. [Google Scholar] [CrossRef]
- Wang, X.C.; Chen, R.F.; Whelan, J.; Eglinton, L. Contribution of “Old” carbon from natural marine hydrocarbon seeps to sedimentary and dissolved organic carbon pools in the Gulf of Mexico. Geophys. Res. Lett. 2001, 28, 3313–3316. [Google Scholar] [CrossRef]
- Snyder, G.T.; Hiruta, A.; Matsumoto, R.; Dickens, G.R.; Tomaru, H.; Takeuchi, R.; Komatsubarad, J.; Ishida, Y.; Yu, H. Pore water profiles and authigenic mineralization in shallow marine sediments above the methane-charged system on Umitaka Spur, Japan Sea. Deep Sea Res. Part II 2007, 54, 1216–1239. [Google Scholar] [CrossRef]
- Pohlman, J.W.; Bauer, J.E.; Waite, W.F.; Osburn, C.L.; Chapman, N.R. Methane hydrate-bearing seeps as a source of aged dissolved organic carbon to the oceans. Nat. Geosci. 2011, 4, 37–41. [Google Scholar] [CrossRef]
- Xu, C.; Wu, N.; Sun, Z.; Zhang, X.; Geng, W.; Cao, H.; Wang, L.; Zhang, X.; Xu, G. Methane seepage inferred from pore water geochemistry in shallow sediments in the western slope of the Mid-Okinawa Trough. Mar. Petrol. Geol. 2018, 98, 306–315. [Google Scholar] [CrossRef]
- Zachos, J.C.; Röhl, U.; Schellenberg, S.A.; Sluijs, A.; Hodell, D.A.; Kelly, D.C.; Thomas, E.; Nicolo, M.; Raffi, I.; Lourens, L.J.; et al. Rapid acidifcation of the ocean during the Paleocene-Eocene thermal maximum. Science 2005, 308, 1611–1615. [Google Scholar] [CrossRef] [Green Version]
- Archer, D.; Buffett, B.; Brovkin, V. Ocean methane hydrates as a slow tipping point in the global carbon cycle. Proc. Natl. Acad. Sci. USA 2009, 106, 20596–20601. [Google Scholar] [CrossRef] [Green Version]
- Biastoch, A.; Treude, T.; Rüpke, L.H.; Riebesell, U.; Roth, C.; Burwicz, E.B.; Park, W.; Latif, M.; Böning, C.W.; Madec, G.; et al. Rising Arctic Ocean temperatures cause gas hydrate destabilization and ocean acidification. Geophys. Res. Lett. 2011, 38, L08602. [Google Scholar] [CrossRef] [Green Version]
- Suess, E. Marine cold seeps: background and recent advances. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; Wilkes, H., Ed.; Springer: Berlin, Germany, 2018; pp. 1–21. [Google Scholar]
- Hong, W.L.; Sauer, S.; Panieri, G.; Ambrose Jr, W.G.; James, R.H.; Plaza-Faverola, A.; Schneider, A. Removal of methane through hydrological, microbial, and geochemical processes in the shallow sediments of pockmarks along eastern Vestnesa Ridge (Svalbard). Limnol. Oceanogr. 2016, 61, S324–S343. [Google Scholar] [CrossRef]
- Luo, M.; Dale, A.W.; Haffert, L.; Haeckel, M.; Koch, S.; Crutchley, G.; De Stigter, H.; Chen, D.; Greinert, J. A quantitative assessment of methane cycling in Hikurangi Margin sediments (New Zealand) using geophysical imaging and biogeochemical modeling. Geochem. Geophys. Geosyst. 2016, 17, 4817–4835. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Qiu, J.W.; Hu, Y.; Peckmann, J.; Guan, H.; Tong, H.; Cheng, C.; Chen, J.; Gong, S.; Li, N.; et al. Cold seep systems in the South China Sea: An overview. J. Asian Earth Sci. 2018, 168, 3–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, M.; Hu, Y.; Wang, H.; Chen, D. An areal assessment of subseafloor carbon cycling in cold seeps and hydrate-bearing areas in the northern South China Sea. Geofluids 2019, 2019, 1–14. [Google Scholar]
- Liang, Q.; Hu, Y.; Feng, D.; Peckmann, J.; Chen, L.; Yang, S.; Liang, J.; Tao, J.; Chen, D. Authigenic carbonates from newly discovered active cold seeps on the northwestern slope of the South China Sea: Constraints on fluid sources, formation environments, and seepage dynamics. Deep-Sea Res. Part I 2017, 124, 31–41. [Google Scholar] [CrossRef]
- Bai, Y.; Song, H.; Guan, Y.; Chen, J.; Liu, B. Structural characteristics and genesis of pockmarks in the northwest of the South China Sea derived from reflective seismic and multibeam data. Chin. J. Geophys. 2014, 57, 2208–2222, (In Chinese with English Abstract). [Google Scholar]
- Liu, B.; Liu, S. Gas bubble plumes observed at north slope of South China Sea from multi-beam water column data. Acta Oceanol. Sin. 2017, 39, 83–89, (in Chinese with English abstract). [Google Scholar]
- Yang, L.; Liu, B.; Xu, M.; Liu, S.; Guan, Y.; Gu, Y. Characteristics of active cold seepages in Qiongdongnan Sea Area of the northern South China Sea. Chin. J. Geophys. 2018, 61, 2905–2914, (In Chinese with English Abstract). [Google Scholar]
- Zhao, B.; Liu, S.; Li, L.; Guo, J. Distribution pattern of cold seeps in South China Sea and its geological significance. Mar. Geol. Front. 2018, 34, 32–43, (In Chinese with English Abstract). [Google Scholar]
- Wei, J.; Liang, J.; Lu, J.; Zhang, W.; He, Y. Characteristics and dynamics of gas hydrate systems in the northwestern South China Sea-Results of the fifth gas hydrate drilling expedition. Mar. Petrol. Geol. 2019, 110, 287–298. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Feng, D.; Hu, Y.; Bayon, G.; Liang, Q.; Tong, H.; Gong, S.; Tao, J.; Chen, D. Using chemical compositions of sediments to constrain methane seepage dynamics: a case study from Haima cold seeps of the South China Sea. J. Asian Earth Sci. 2018, 168, 137–144. [Google Scholar] [CrossRef]
- Feng, J.; Yang, S.; Wang, H.; Liang, J.; Fang, Y.; Luo, M. Methane source and turnover in the shallow sediments to the west of Haima cold seeps on the northwestern slope of the South China Sea. Geofluids 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Luo, M.; Liang, Q.; Chen, L.; Feng, D.; Yang, S.; Liang, J.; Chen, D. Pore fluid compositions and inferred fluid flow patterns at the Haima cold seeps of the South China Sea. Mar. Petrol. Geol. 2019, 103, 29–40. [Google Scholar] [CrossRef]
- Taylor, B.; Hayes, D.E. The tectonic evolution of the South China Basin. In Tectonic and Geologic Evolution of Southeast Asian Seas and Islands; Hayes, D.E., Ed.; Geophysical Monograph of AGU: Washington DC, USA, 1980; pp. 89–104. [Google Scholar]
- Xie, X.; Müller, R.D.; Li, S.; Gong, Z.; Steinberger, B. Origin of anomalous subsidence along the northern South China Sea margin and its relationship to dynamic topography. Mar. Petrol. Geol. 2006, 23, 745–765. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, H.; Yang, J.; Yang, X.; Xu, H. Models of the rapid post-rift subsidence in the eastern Qiongdongnan Basin, South China Sea: implications for the development of the deep thermal anomaly. Basin Res. 2017, 29, 340–362. [Google Scholar] [CrossRef]
- Sun, Q.; Cartwright, J.; Lüdmann, T.; Wu, S.; Zhong, G.; Yao, G. Three-dimensional seismic characterization of a complex sediment drift in the South China Sea: evidence for unsteady flow regime. Sedimentology 2017, 64, 832–853. [Google Scholar] [CrossRef]
- Hui, G.; Li, S.; Guo, L.; Zhang, G.; Gong, Y.; Somerville, I.D.; Zhang, Y.; Zheng, Q.; Zang, Y. Source and accumulation of gas hydrate in the northern margin of the South China Sea. Mar. Petrol. Geol. 2016, 69, 127–145. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.; Kong, X.; Ma, B.; Li, W.; Wang, D.; Gao, J.; Chen, W. Subsurface fluid flow at an active cold seep area in the Qiongdongnan Basin, northern South China Sea. J. Asian Earth Sci. 2018, 168, 17–26. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, J.; Yang, X.; Su, P.; Wan, Z. The formation mechanism of mud diapirs and gas chimneys and their relationship with natural gas hydrates: insights from the deep-water area of Qiongdongnan Basin, northern South China Sea. Int. Geol. Rev. 2018, 1–22. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, W.; Lu, J.A.; Wei, J.; Kuang, Z.; He, Y. Geological occurrence and accumulation mechanism of natural gas hydrates in the eastern Qiongdongnan Basin of the South China Sea: Insights from site GMGS5-W9-2018. Mar. Geol. 2019, 418, 106042. [Google Scholar] [CrossRef]
- Ye, J.; Wei, J.; Liang, J.; Lu, J.; Lu, H.; Zhang, W. Complex gas hydrate system in a gas chimney, South China Sea. Mar. Petrol. Geol. 2019, 104, 29–39. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis, 3rd Completely Revised and Extended ed.; Wiley-VCH: Weinheim, Germany, 1999; p. 160. [Google Scholar]
- Donahue, D.J.; Linick, T.W.; Jull, A.J.T. Isotope-ratio and background corrections for accelerator mass-spectrometry radiocarbon measurements. Radiocarbon 1990, 32, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Shen, C.; Ding, P.; Yi, W.; Zhu, S.; Liu, K.; Xu, X. Recent progress in reporting and calculation of 14C for dating and tracing. Geochimica 2018, 47, 442–451, (In Chinese with English Abstract). [Google Scholar]
- Wallmann, K.; Aloisi, G.; Haeckel, M.; Obzhirov, A.; Pavlova, G.; Tishchenko, P. Kinetics of organic matter degradation, microbial methane generation, and gas hydrate formation in anoxic marine sediments. Geochim. Cosmochim. Acta 2006, 70, 3905–3927. [Google Scholar] [CrossRef]
- Chuang, P.; Dale, A.W.; Wallmann, K.; Haeckel, M.; Yang, T.; Chen, N.; Chen, H.; Chen, H.; Lin, S.; Sun, C. Relating sulfate and methane dynamics to geology: Accretionary prism offshore SW Taiwan. Geochem. Geophy. Geosy. 2013, 14, 2523–2545. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Dale, A.W.; Wallmann, K.; Hensen, C.; Gieskes, J.; Yan, W.; Chen, D. Estimating the time of pockmark formation in the SW Xisha Uplift (South China Sea) using reaction-transport modeling. Mar. Geol. 2015, 364, 21–31. [Google Scholar] [CrossRef]
- Middelburg, J.J. A simple rate model for organic matter decomposition in marine sediments. Geochim. Cosmochim. Acta 1989, 53, 1577–1581. [Google Scholar] [CrossRef]
- Whiticar, M.J.; Faber, E.; Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation–Isotope evidence. Geochim. Cosmochim. Acta 1986, 50, 693–709. [Google Scholar] [CrossRef]
- Solomon, E.A.; Spivack, A.J.; Kastner, M.; Torres, M.E.; Robertson, G. Gas hydrate distribution and carbon sequestration through coupled microbial methanogenesis and silicate weathering in the Krishna-Godavari Basin, offshore India. Mar. Petrol. Geol. 2014, 58, 233–253. [Google Scholar] [CrossRef] [Green Version]
- Millero, F.J. Thermodynamics of the carbon dioxide system in the oceans. Geochim. Cosmochim. Acta 1995, 59, 661–677. [Google Scholar] [CrossRef]
- Zeebe, R.E.; Wolf-Gladrow, D.A. CO2 in Seawater: Equilibrium, Kinetics and Isotopes; Elsevier: London, UK, 2001; p. 347. [Google Scholar]
- Kastner, M.; Claypool, G.; Robertson, G. Geochemical constraints on the origin of the pore fluids and gas hydrate distribution at Atwater Valley and Keathley Canyon, northern Gulf of Mexico. Mar. Petrol. Geol. 2008, 25, 860–872. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dickens, G.R.; Bhatnagar, G.; Chapman, W.G.; Dugan, B.; Snyder, G.T.; Hirasaki, G.J. Pore water sulfate, alkalinity, and carbon isotope profiles in shallow sediment above marine gas hydrate systems: A numerical modeling perspective. J. Geophys. Res. 2011, 116, B09103. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Jiang, S.; Ge, L.; Yang, J.; Wu, N.; Zhang, G.; Liu, J.; Chen, D. Geochemistry of pore waters from HQ-1PC of the Qiongdongnan Basin, northern South China Sea, and its implications for gas hydrate exploration. Sci. China Earth Sci. 2013, 56, 521–529. [Google Scholar] [CrossRef]
- Wu, L.; Yang, S.; Liang, J.; Su, X.; Fu, S.; Sha, Z.; Yang, T. Variations of pore water sulfate gradients in sediments as indicator for underlying gas hydrate in Shenhu Area, the South China Sea. Sci. China Earth Sci. 2013, 56, 530–540. [Google Scholar] [CrossRef]
- Chen, N.C.; Yang, T.F.; Hong, W.L.; Chen, H.W.; Chen, H.C.; Hu, C.Y.; Huang, Y.C.; Lin, S.; Lin, L.H.; Su, C.C.; et al. Production, consumption, and migration of methane in accretionary prism of southwestern Taiwan. Geochem. Geophy. Geosyst. 2017, 18, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Yang, S.; Liang, J.; Fang, Y.; He, Y.; Luo, M.; Chen, D. Methane seepage inferred from the porewater geochemistry of shallow sediments in the Beikang Basin of the southern South China Sea. J. Asian Earth Sci. 2018, 168, 77–86. [Google Scholar] [CrossRef]
- Masuzawa, T.; Handa, N.; Kitagawa, H.; Kusakabe, M. Sulfate reduction using methane in sediments beneath a bathyal “cold seep” giant clam community off Hatsushima Island, Sagami Bay, Japan. Earth Planet. Sci. Lett. 1992, 110, 39–50. [Google Scholar] [CrossRef]
- Masuzawa, T.; Kitagawa, H.; Nakatsuka, T.; Handa, N.; Nakamura, T. AMS 14C measurements of dissolved inorganic carbon in pore waters from a deep-sea “cold seep” giant clam community off Hatsushima Island, Sagami Bay, Japan. Radiocarbon 1995, 37, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Suess, E.; Torres, M.E.; Bohrmann, G.; Collier, R.W.; Greinert, J.; Linke, P.; Rehder, G.; Trehu, A.; Wallmann, K.; Winckler, G.; et al. Gas hydrate destabilization: enhanced dewatering, benthic material turnover and large methane plumes at the Cascadia convergent margin. Earth Planet. Sci. Lett. 1999, 170, 1–15. [Google Scholar] [CrossRef]
- Garcia-Tigreros, F.; Kessler, J.D. Limited acute influence of aerobic methane oxidation on ocean carbon dioxide and pH in Hudson Canyon, northern U.S. Atlantic margin. J. Geophys. Res.-Biogeosci. 2018, 123, 2135–2144. [Google Scholar] [CrossRef]
- Sauvage, J.; Spivack, A.J.; Murray, R.W.; D’Hondt, S. Determination of in situ dissolved inorganic carbon concentration and alkalinity for marine sedimentary porewater. Chem. Geol. 2014, 387, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.L.; Wang, L.W.; Wang, C.H.; Gong, G.C. Vertical distribution of δ13C of dissolved inorganic carbon in the northeastern South China Sea. Deep-Sea Res. Part I 1999, 46, 757–775. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, L.; Liu, K.; Xu, X. Radiocarbon in the maritime air and sea surface water of the South China Sea. Radiocarbon 2019, 61, 461–472. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Fan, D.; Xu, C.; Wang, L.; Zhang, X.; Geng, W.; Luan, X. Compositional characteristics and sources of DIC and DOC in seawater of the Okinawa Trough, East China Sea. Cont. Shelf Res. 2019, 174, 108–117. [Google Scholar] [CrossRef]
- Hu, X.; Cai, W.J.; Wang, Y.; Luo, S.; Guo, X. Pore-water geochemistry of two contrasting brine-charged seep sites in the northern Gulf of Mexico continental slope. Mar. Chem. 2010, 118, 99–107. [Google Scholar] [CrossRef]
- Komada, T.; Burdige, D.J.; Crispo, S.M.; Druffel, E.R.; Griffin, S.; Johnson, L.; Le, D. Dissolved organic carbon dynamics in anaerobic sediments of the Santa Monica Basin. Geochim. Cosmochim. Acta 2013, 110, 253–273. [Google Scholar] [CrossRef] [Green Version]
- Pohlman, J.W.; Riedel, M.; Bauer, J.E.; Canuel, E.A.; Paull, C.K.; Lapham, L.; Grabowski, K.S.; Coffin, R.B.; Spence, G.D. Anaerobic methane oxidation in low-organic content methane seep sediments. Geochim. Cosmochim. Acta 2013, 108, 184–201. [Google Scholar] [CrossRef] [Green Version]
- Paull, C.K.; Iii, W.U.; Peltzer, E.T.; Brewer, P.G.; Keaten, R.; Mitts, P.J. Authigenic carbon entombed in methane-soaked sediments from the northeastern transform margin of the Guaymas Basin, Gulf of California. Deep-Sea Res. II 2007, 54, 1240–1267. [Google Scholar] [CrossRef]
- China Geological Survey. Available online: http://www.cgs.gov.cn/xwl/ddyw/201607/t20160702_337003.html (accessed on 26 June 2016).
- Andersson, A.J.; Mackenzie, F.T.; Ver, L.M. Solution of shallow-water carbonates: an insignificant buffer against rising atmospheric CO2. Geology 2003, 31, 513–516. [Google Scholar] [CrossRef]
- Berner, R. Early Diagenesis: A Theoretical Approach; Princeton Series in Geochemistry; Princeton University Press: Princeton, NJ, USA, 1980. [Google Scholar]
- Boudreau, B.P. Diagenetic Models and Their Implementation: Modelling Transport and Reactions in Aquatic Sediments; Springer: Berlin, Germany, 1997; p. 414. [Google Scholar]
- Wang, P.; Prell, W.L.; Blum, P. Initial Reports. Proc. Ocean Drill. Prog. 2000, 184, 1–48. [Google Scholar]
- Huang, C.Y.; Wu, S.F.; Zhao, M.; Chen, M.T.; Wang, C.H.; Tu, X.; Yuan, P.B. Surface ocean and monsoon climate variability in the South China Sea since the last glaciation. Mar. Micropaleontol. 1997, 32, 71–94. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Helgeson, H.C. Calculation of activity coefficients and degrees of formation of neutral ion pairs in supercritical electrolyte solutions. Geochim. Cosmochim. Acta 1991, 55, 1235–1251. [Google Scholar] [CrossRef]
- Schulz, H.D. Quantification of early diagenesis: dissolved constituents in pore water and signals in the solid phase. In Marine Geochemistry; Schulz, H.D., Zabel, M., Eds.; Springer: Berlin, Germany, 2006. [Google Scholar]





| Site | Water Depth (m) | Seafloor Temperature (°C) | Core Length (cm) |
|---|---|---|---|
| QH-ROV05 | 1722 | 2.2 | R5-1(78 cm), R5-2 (50 cm), QH-CL48 (763 cm) |
| QH-ROV07 | 1737 | 2.2 | R7 (60 cm), R7-1 (60 cm), R7-2 (50 cm), R7-3 (78 cm) |
| HM-ROV | 1405 | 2.9 | HM-1(70 cm) |
| Rate | Kinetic Rate Law* |
|---|---|
| Total POC degradation (wt.% C yr−1) | |
| POM degradation via sulfate reduction (mmol cm−3 yr−1 of SO42−) | |
| Methanogenesis (mmol cm−3 yr−1 of CH4) | |
| Anaerobic oxidation of methane (mmol cm−3 yr−1 of CH4) | |
| Authigenic carbonate precipitation (mmol cm−3 yr−1 of Ca2+) |
| Depth-Integrated Flux | W08B | W08C | W09 | R7-1 | R7-3 | HM-1 | Unit |
|---|---|---|---|---|---|---|---|
| FPOC: Total POC mineralization rate | 2.0 | 2.6 | 5.9 | 1.1 | 1.1 | 1.5 | mmol m−2 yr− 1 of C |
| FOSR: Sulfate reduction via POC degradation | 0.1 | 0.8 | 1.8 | 0.3 | 0.4 | 0.3 | mmol m− 2 yr− 1 of SO42− |
| FME: Methanogenesis via POC degradation | 1.9 | 1.8 | 4 | 0.5 | 0.3 | 1.3 | mmol m− 2 yr− 1 of CH4 |
| FAOM: Anaerobic oxidation of methane | 591 | 383 | 241 | 80.3 | 59.0 | 378 | mmol m− 2 yr− 1 of CH4 |
| FCP: Authigenic CaCO3 precipitation | 165 | 97.9 | 18.7 | 20.2 | 5.2 | 80.1 | mmol m− 2 yr− 1 of C |
| Dissolved CH4 flux above GHOZ | 619 | 418 | 263 | 80.1 | 59.4 | 451 | mmol m− 2 yr− 1 of CH4 |
| CH4 efflux | 13.6 | 11.7 | 5.7 | 4.4×10−4 | 4.4×10−5 | 4.3 | mmol m− 2 yr− 1 of CH4 |
| DIC efflux | 427 | 291 | 224 | 58.3 | 51.5 | 296 | mmol m− 2 yr− 1 of CH4 |
| Site ID | FSO4 | FCH4 | RAOM | DIC Efflux | ZSMTZ (mbsf) | Profile above SMTZ | Reference |
|---|---|---|---|---|---|---|---|
| W08B | 592 | 619 | 591 | 427 | <0.5 | kink-type | This study |
| W08C | 393 | 418 | 383 | 291 | ~3 | kink-type | |
| W09 | 246 | 263 | 241 | 224 | ~7 | kink-type | |
| R7-1 | 80.6 | 80.1 | 80.3 | 58.3 | ~2.1 | linear | |
| R7-3 | 59.4 | 58.9 | 59.0 | 51.5 | ~3.1 | linear | |
| HM-1 | 378 | 389 | 410 | 327 | ~0.6 | linear | |
| R1 | 1226 | 4110 | 1225.7 | 1139 | 1.5 | kink-type | [31] |
| QDN-14A | 450 | 540 | 449.3 | 404 | 3 | kink-type | |
| QDN-14B | 193 | 507 | 193.1 | 131 | 5 | kink-type | |
| 2015XS-44 | 19.7 | 12.6 | 12.4 | 15 | 18.6 | kink-type | [22] |
| 2015XS-50 | 31.9 | 25.8 | 24.6 | 25 | 18 | kink-type | |
| 2015XS-R2 | 172 | 570.9 | 170.6 | 155 | 1.3 | kink-type | |
| CL30 | 39.3 | 31.4 | 35.3 | 21.7 | 4.7 | kink-type | [30] |
| CL44 | 98.3 | 73.3 | 74.3 | 87 | 7 | kink-type | |
| CL47 | 110 | 84.8 | 85 | 115 | 6.8 | kink-type | |
| 1PC | 59.5 | 59.5 | 7 | linear | [54] | ||
| C14 | 56 | 15.7 | 11 | 55 | 14.3 | linear | [46] |
| Shenhu | 2.0–40.0 | 2.0–37.0 | 7.8–30.5 | 10.1–31.7 | 7.7–87.9 | linear | [22,55] |
| Dongsha | 5.7–102 | 1.0–101.5 | 1.0–101.5 | 13.1–26.1 | 0.05–21.8 | linear and kink-type | [22,56] |
| Beikang | 34.5–62.7 | 24.5–62.7 | 27.5–43.1 | 32.3–50.1 | 5.3–8.8 | linear and kink-type | [57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Li, N.; Luo, M.; Liang, J.; Yang, S.; Wang, H.; Chen, D. A Quantitative Assessment of Methane-Derived Carbon Cycling at the Cold Seeps in the Northwestern South China Sea. Minerals 2020, 10, 256. https://doi.org/10.3390/min10030256
Feng J, Li N, Luo M, Liang J, Yang S, Wang H, Chen D. A Quantitative Assessment of Methane-Derived Carbon Cycling at the Cold Seeps in the Northwestern South China Sea. Minerals. 2020; 10(3):256. https://doi.org/10.3390/min10030256
Chicago/Turabian StyleFeng, Junxi, Niu Li, Min Luo, Jinqiang Liang, Shengxiong Yang, Hongbin Wang, and Duofu Chen. 2020. "A Quantitative Assessment of Methane-Derived Carbon Cycling at the Cold Seeps in the Northwestern South China Sea" Minerals 10, no. 3: 256. https://doi.org/10.3390/min10030256




