The Record of Environmental and Microbial Signatures in Ancient Microbialites: The Terminal Carbonate Complex from the Neogene Basins of Southeastern Spain
Abstract
1. Introduction
2. Geological Setting
3. Terminology
- Cements (i) and peloids (ii), as well as various microstructures and microfossils (Table 2) were considered exclusively as in situ components;
- Micrite (iii) and organic matter particles (iv) were either formed in situ and/or were trapped;
- Siliciclastic grains (v), ooids (vi), fecal pellets (vii), and bioclasts (viii), except borers and encrusters, were considered exclusively as trapped grains.


4. Methods
5. Microbialite Associations and Fabrics
5.1. Previous Interpretations
5.2. Microbialite Association 1: Low Energy, Hypersaline
5.2.1. Description
5.2.2. Paleoenvironmental Interpretation
5.3. Microbialite Associations 2a and 2b: High Energy, Normal Marine to Hypersaline
5.3.1. Description
5.3.2. Paleoenvironmental Interpretation
6. Stratigraphy of TCC Microbialites
6.1. Sorbas Basin
6.1.1. Cariatiz
6.1.2. Góchar
6.1.3. Moras
6.1.4. Sorbas
6.1.5. Hueli
6.2. Agua Amarga Depression
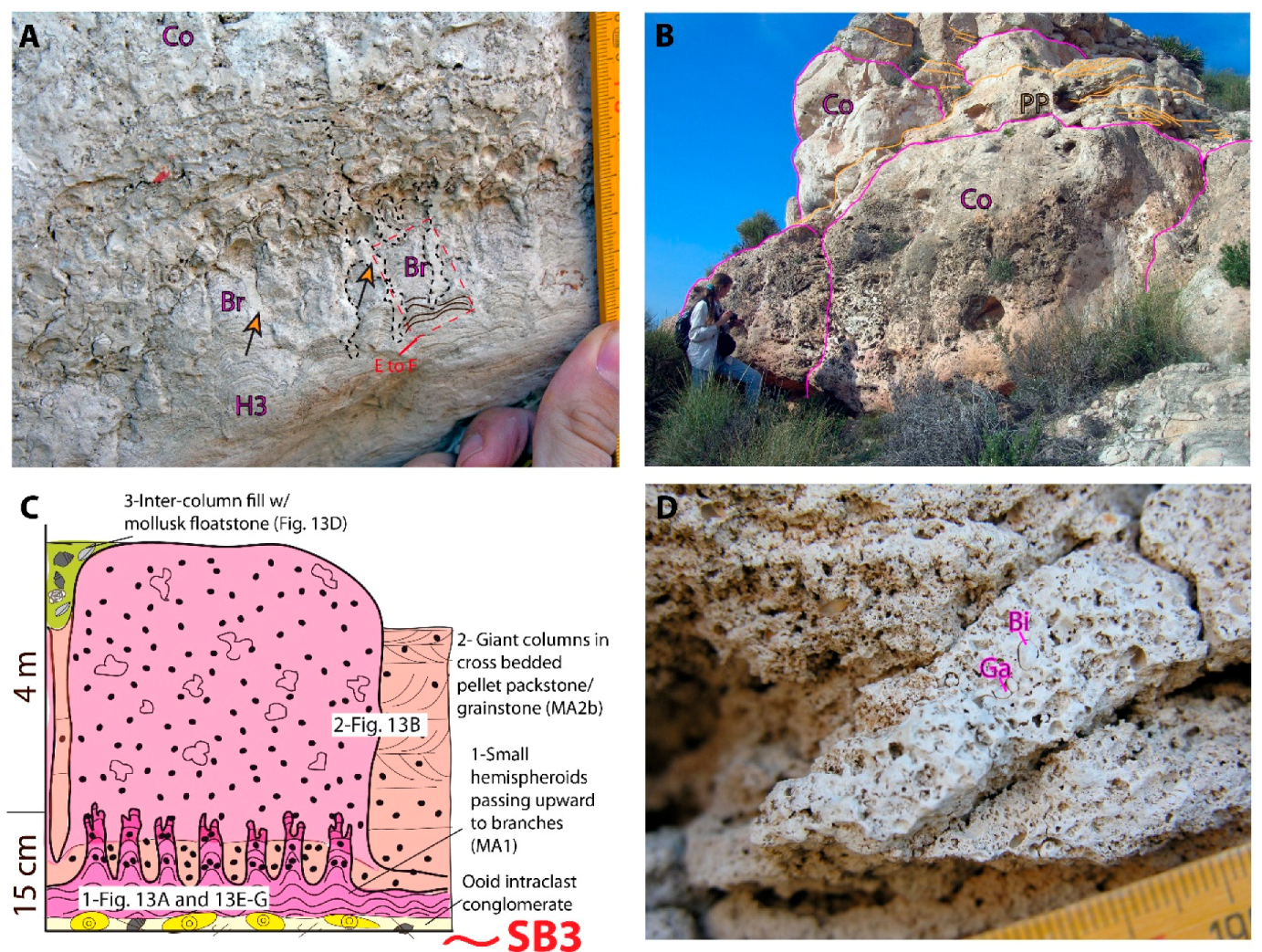
Las Negras
6.3. Bajo Segura Basin
6.3.1. Benejuzar
6.3.2. Santa Pola
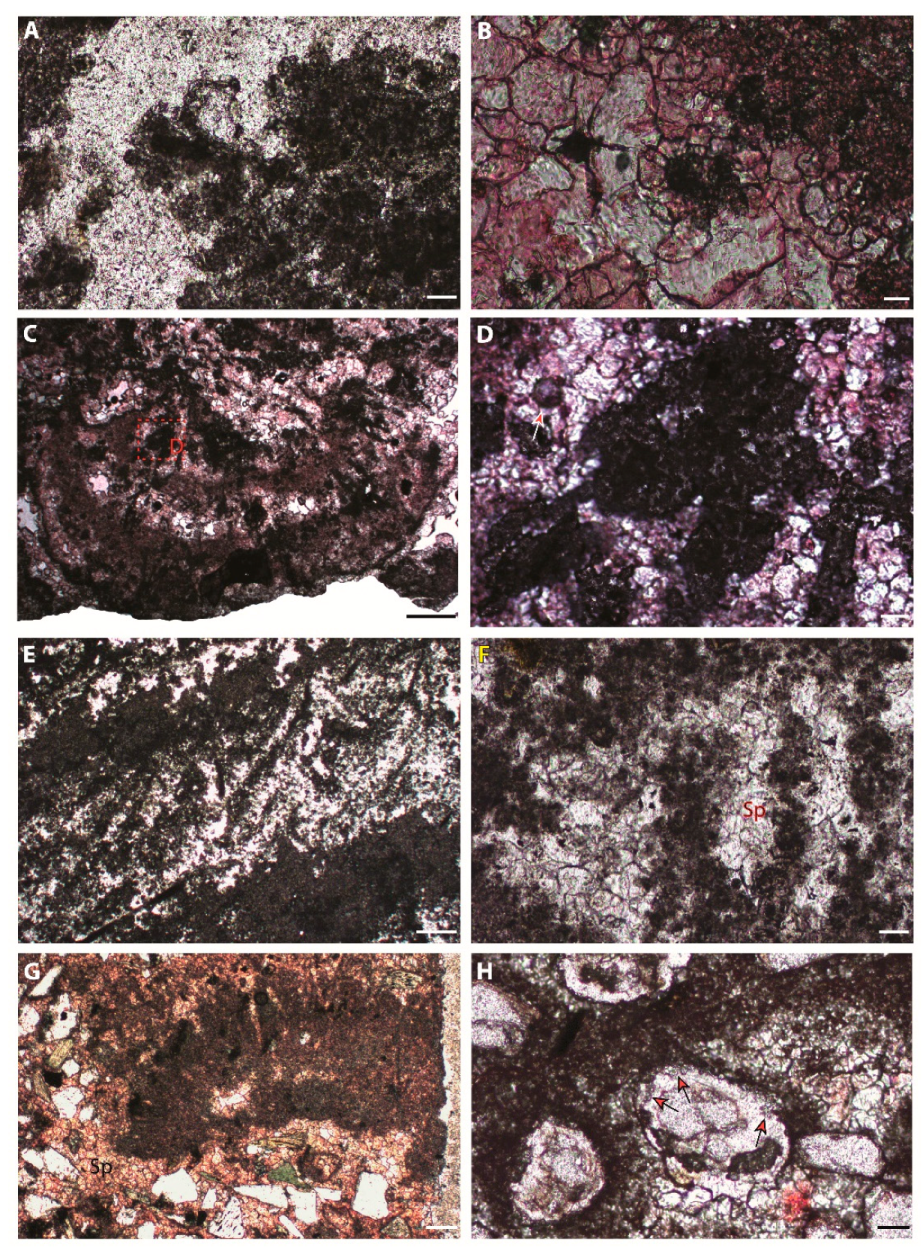
7. Microbialite Mineralogy, Microstructures, and Microfossils
7.1. Peloids
7.1.1. Description
7.1.2. Interpretation
7.2. Bushy Peloid Aggregates
7.2.1. Description
7.2.2. Interpretation
7.3. Filaments, Tepee-Like, and Flabellate Structures
7.3.1. Description
7.3.2. Interpretation
7.4. Microborings
7.4.1. Description
7.4.2. Interpretation
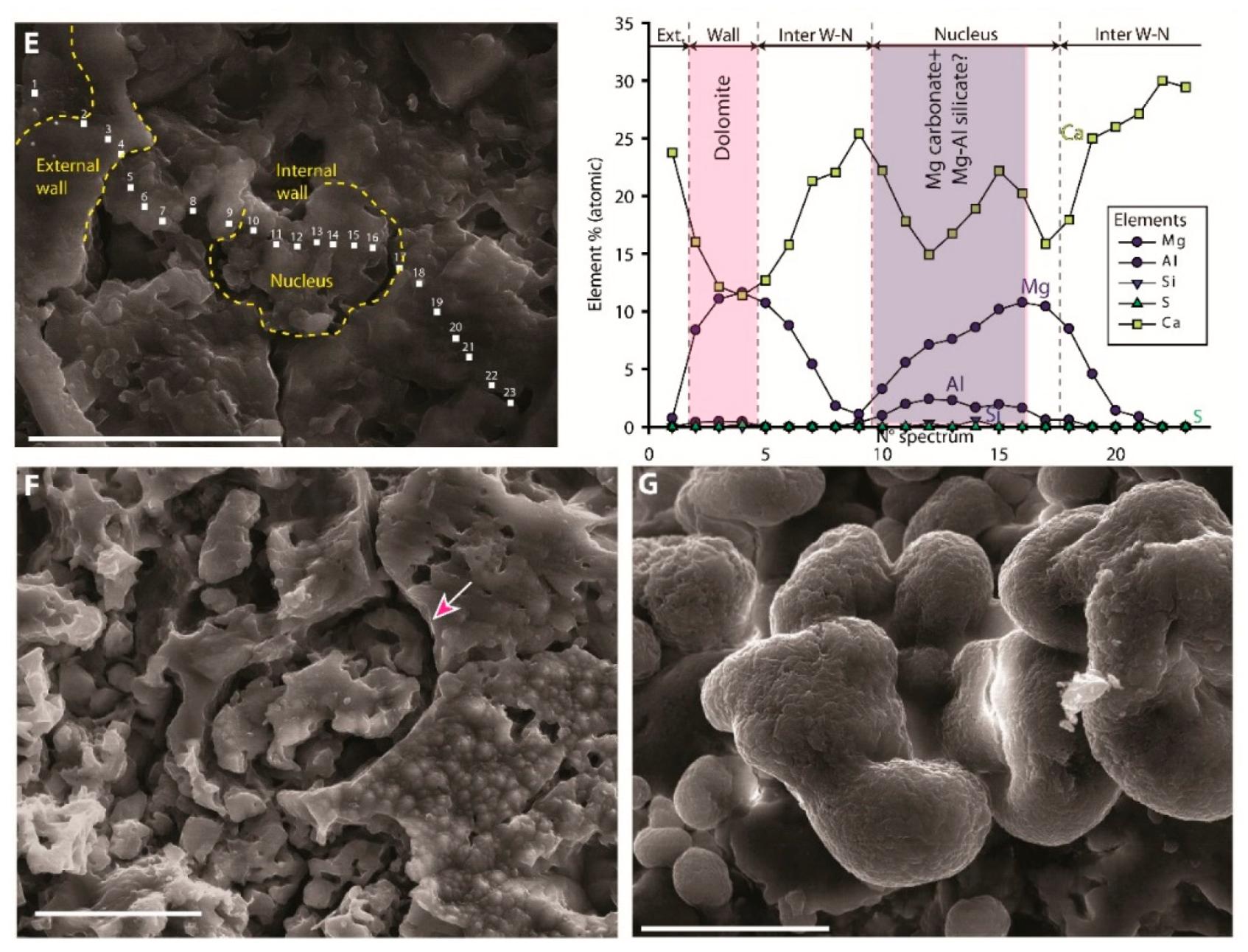
7.5. Microspheres
7.5.1. Description
7.5.2. Interpretation
7.6. Bean-Shaped Structures
7.6.1. Description
7.6.2. Interpretation
7.7. Microballs and Dumbbells
7.7.1. Description
7.7.2. Interpretation
8. Discussion
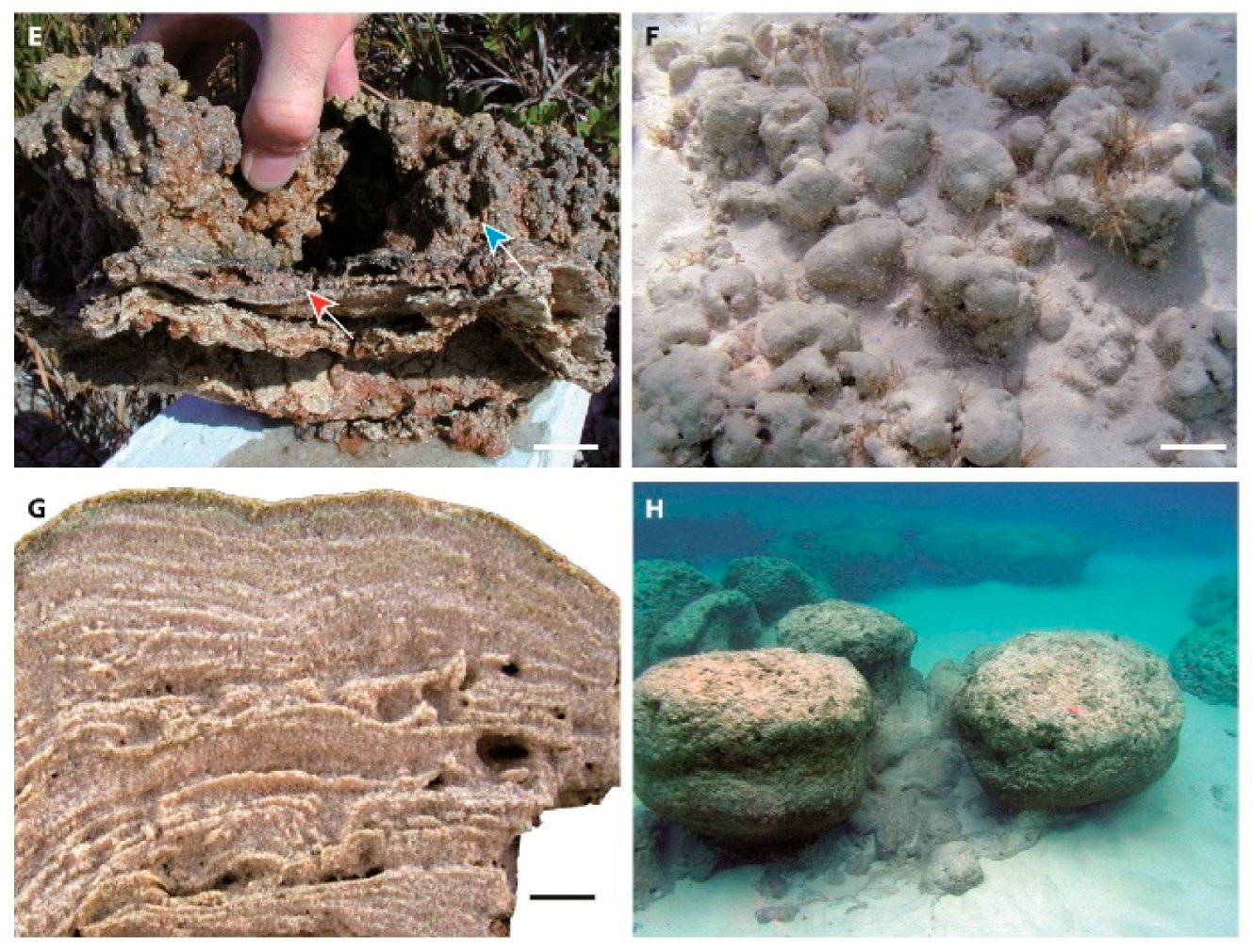
8.1. Salinity and Sedimentary Dynamics Controls on Microbialite Associations: Comparison with Modern Counterparts
8.2. Long-Term Controls on Microbialites Distribution: Water Level, Substratum, and Evaporite Deformation/Dissolution
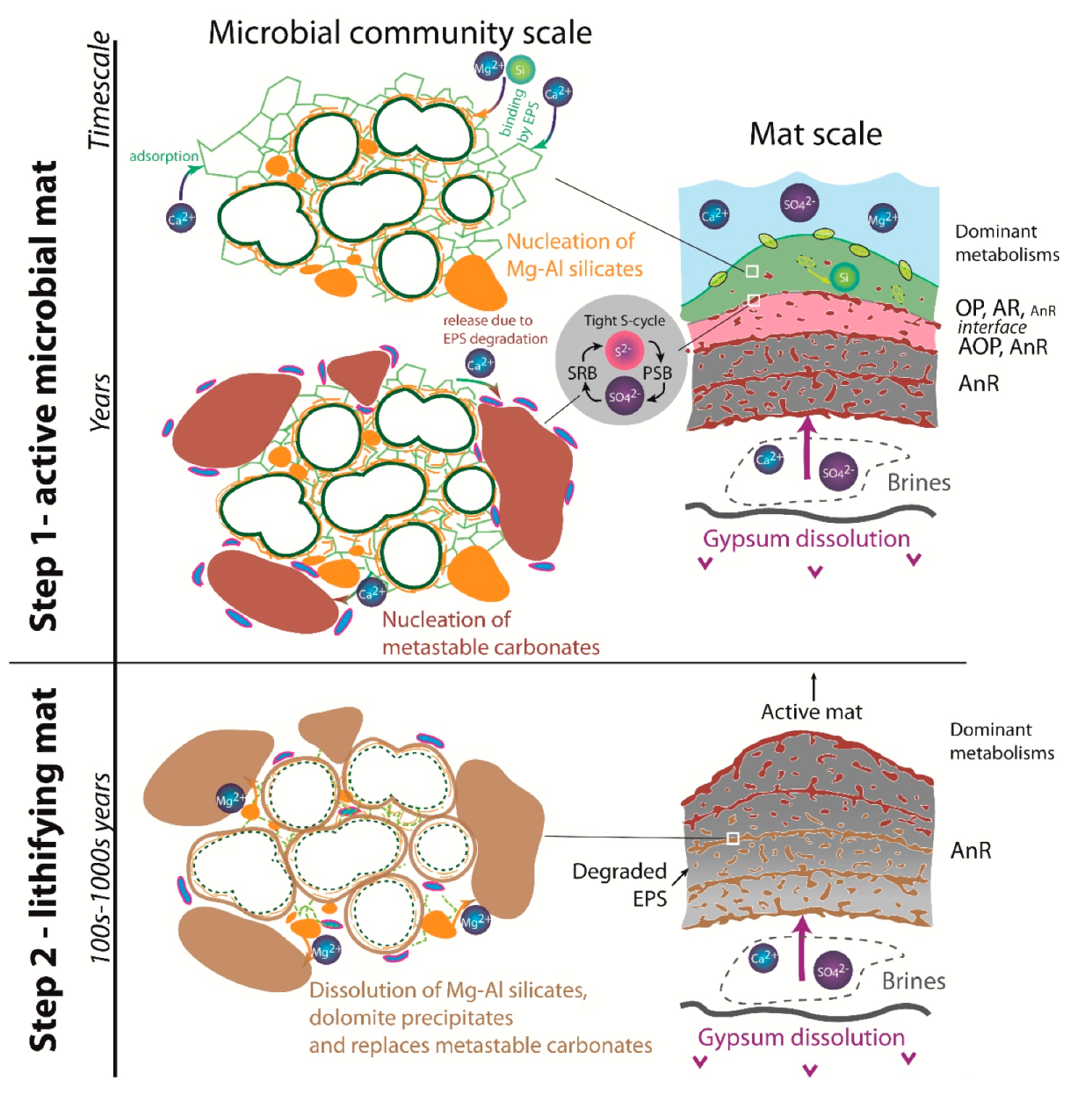
8.3. Microbial and Diagenetic Influence on Microfabrics and Mineral Precipitation
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allwood, A.C.; Walter, M.R.; Kamber, B.S.; Marshall, C.P.; Burch, I.W. Stromatolite reef from the early archaean era of Australia. Nature 2006, 441, 714–718. [Google Scholar] [CrossRef]
- Nutman, A.P.; Bennett, V.C.; Friend, C.R.L.; Van Kranendonk, M.J.; Chivas, A.R. Rapid emergence of life shown by discovery of 3700-million-year-old microbial structures. Nature 2016, 537, 535–538. [Google Scholar] [CrossRef]
- Nutman, A.P.; Bennett, V.C.; Friend, C.R.L.; Van Kranendonk, M.J.; Rothacker, L.; Chivas, A.R. Cross-examining Earth’s oldest stromatolites: Seeing through the effects of heterogeneous deformation, metamorphism and metasomatism affecting Isua (Greenland) ∼3700 Ma sedimentary rocks. Precambrian Res. 2019, 331, 105347. [Google Scholar] [CrossRef]
- Des Marais, D.J. Evolution—When did photosynthesis emerge on earth? Science 2000, 289, 1703–1705. [Google Scholar]
- Arp, G.; Reimer, A.; Reitner, J. Photosynthesis-induced biofilm calcification and calcium concentrations in phanerozoic oceans. Science 2001, 292, 1701–1704. [Google Scholar] [CrossRef]
- Aloisi, G. The calcium carbonate saturation state in cyanobacterial mats throughout Earth’s history. Geochim. Cosmochim. Acta 2008, 72, 6037–6060. [Google Scholar] [CrossRef]
- Dupraz, C.; Visscher, P.T. Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol. 2005, 13, 429–438. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonate abundance compared with fluctuations in metazoan diversity over geological time. Sediment. Geol. 2006, 185, 229–238. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon mineralization: From natural analogues to engineered systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Dravis, J.J. Hardened subtidal stromatolites, Bahamas. Science 1983, 219, 385–386. [Google Scholar] [CrossRef]
- Reid, R.P.; Visscher, P.T.; Decho, A.W.; Stolz, J.F.; Bebout, B.M.; Dupraz, C.; Macintyre, I.G.; Paerl, H.W.; Pinckney, J.L.; Prufert-Bebout, L.; et al. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 2000, 406, 989–992. [Google Scholar] [CrossRef]
- Visscher, P.T.; Reid, R.P.; Bebout, B.M.; Hoeft, S.E.; Macintyre, I.G.; Thompson, J.A. Formation of lithified micritic laminae in modern marine stromatolites (Bahamas): The role of sulfur cycling. Am. Miner. 1998, 83, 1482–1493. [Google Scholar] [CrossRef]
- Dupraz, C.; Visscher, P.T.; Baumgartner, L.K.; Reid, R.P. Microbe-mineral interactions: Early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas). Sedimentology 2004, 51, 745–765. [Google Scholar] [CrossRef]
- Glunk, C.; Dupraz, C.; Braissant, O.; Gallagher, K.L.; Verrecchia, E.P.; Visscher, P.T. Microbially mediated carbonate precipitation in a hypersaline lake, Big Pond (Eleuthera, Bahamas). Sedimentology 2011, 58, 720–736. [Google Scholar] [CrossRef]
- Bouton, A.; Vennin, E.; Pace, A.; Bourillot, R.; Dupraz, C.; Thomazo, C.; Brayard, A.; Désaubliaux, G.; Visscher, P.T. External controls on the distribution, fabrics and mineralization of modern microbial mats in a coastal hypersaline lagoon, Cayo Coco (Cuba). Sedimentology 2016, 63, 972–1016. [Google Scholar] [CrossRef]
- Pace, A.; Bourillot, R.; Bouton, A.; Vennin, E.; Braissant, O.; Dupraz, C.; Duteil, T.; Bundeleva, I.; Patrier, P.; Galaup, S.; et al. Formation of stromatolite lamina at the interface of oxygenic–anoxygenic photosynthesis. Geobiology 2018, 16, 378–398. [Google Scholar] [CrossRef]
- Bouton, A.; Vennin, E.; Boulle, J.; Pace, A.; Bourillot, R.; Thomazo, C.; Brayard, A.; Désaubliaux, G.; Goslar, T.; Yokoyama, Y.; et al. Linking the distribution of microbial deposits from the Great Salt Lake (Utah, USA) to tectonic and climatic processes. Biogeosciences 2016, 13, 5511–5526. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Knoll, A.H. Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks? Annu. Rev. Earth Planet. Sci. 1999, 27, 313–358. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Pace, A.; Bourillot, R.; Bouton, A.; Vennin, E.; Galaup, S.; Bundeleva, I.; Patrier, P.; Dupraz, C.; Thomazo, C.; Sansjofre, P.; et al. Microbial and diagenetic steps leading to the mineralisation of Great Salt Lake microbialites. Sci. Rep. 2016, 6, 31495. [Google Scholar] [CrossRef]
- Sforna, M.C.; Daye, M.; Philippot, P.; Somogyi, A.; van Zuilen, M.A.; Medjoubi, K.; Gérard, E.; Jamme, F.; Dupraz, C.; Braissant, O.; et al. Patterns of metal distribution in hypersaline microbialites during early diagenesis: Implications for the fossil record. Geobiology 2017, 15, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Montero, M.E.; Rodriguez-Aranda, J.P.; Garcia Del Cura, M.A. Dolomite–silica stromatolites in Miocene lacustrine deposits from the Duero Basin, Spain: The role of organotemplates in the precipitation of dolomite. Sedimentology 2008, 55, 729–750. [Google Scholar] [CrossRef]
- Esteban, M. Significance of the upper miocene coral reefs of the western mediterranean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1979, 29, 169–188. [Google Scholar] [CrossRef]
- Rouchy, J.-M.; Saint Martin, J.-P. Late Miocene events in the mediterranean as recorded by carbonate-evaporite relations. Geology 1992, 20, 629–632. [Google Scholar] [CrossRef]
- Braga, J.C.; Martin, J.M.; Riding, R. Controls on microbial dome fabric along a carbonate-siliciclastic shelf-basin transect, Miocene, SE Spain. Palaios 1995, 10, 347–361. [Google Scholar] [CrossRef]
- Franseen, E.K.; Goldstein, R.H.; Farr, M.R. Quantitative controls on location and architecture of carbonate depositional sequences. J. Sediment. Res. 1998, 68, 283–298. [Google Scholar] [CrossRef]
- Bourillot, R.; Vennin, E.; Rouchy, J.-M.; Blanc-Valleron, M.-M.; Caruso, A.; Durlet, C. The end of the Messinian Salinity Crisis in the western Mediterranean: Insights from the carbonate platforms of south-eastern Spain. Sediment. Geol. 2010, 229, 224–253. [Google Scholar] [CrossRef]
- Mas, G.; Fornós, J. La Crisis de Salinidad del Messiniense en la cuenca sedimentaria de Palma (Mallorca, Islas Baleares). Geogaceta 2012, 52, 57–60. [Google Scholar]
- Esteban, M.; Braga, J.C.; Martin, J.; de Santisteban, C. Western mediterranean reef complexes. In Models for Carbonate Stratigraphy from Miocene Reef Complexes of Mediterranean Regions; Franseen, E.K., Esteban, M., Ward, W.C., Rouchy, J.M., Eds.; Concepts in Sedimentology and Paleontology; SEPM: Tulsa, OK, USA, 1996; Volume 5, pp. 56–72. [Google Scholar]
- Bourillot, R.; Vennin, E.; Rouchy, J.M.; Durlet, C.; Rommevaux, V.; Kolodka, C.; Knap, F. Structure and evolution of a Messinian mixed carbonate-siliciclastic platform: The role of evaporites (Sorbas Basin, South-east Spain). Sedimentology 2010, 57, 477–512. [Google Scholar] [CrossRef]
- Lipinski, C.J.; Franseen, E.K.; Goldstein, R.H. Reservoir analog model for oolite-microbialite sequences, Miocene terminal carbonate complex, Spain. AAPG Bull. 2013, 97, 2035–2057. [Google Scholar] [CrossRef]
- Feldmann, M.; McKenzie, J.A. Messinian stromatolite-thrombolite associations, Santa Pola, SE Spain: An analogue for the palaeozoic? Sedimentology 1997, 44, 893–914. [Google Scholar] [CrossRef]
- Riding, R.; Braga, J.C.; Martin, J.M. Oolite stromatolites and thrombolites, Miocene, Spain: Analogue of Recent giant Bahamian examples. Sediment. Geol. 1991, 71, 121–127. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A. Microbial mediation of modern dolomite precipitation and diagenesis under anoxic conditions (Lagoa Vermelha, Rio de Janeiro, Brazil). J. Sediment. Res. 1997, 67, 378–390. [Google Scholar]
- Van Lith, Y.; Warthmann, R.; Vasconcelos, C.; McKenzie, J.A. Sulphate-reducing bacteria induce low-temperature Ca-dolomite and high Mg-calcite formation. Geobiology 2003, 1, 71–79. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; Vasconcelos, C.; Warthmann, R.J.; Lundberg, R.; McKenzie, J.A. Dolomite-mediating bacterium isolated from the sabkha of Abu Dhabi (UAE). Terra Nova 2012, 24, 248–254. [Google Scholar] [CrossRef]
- Gebelein, C.D.; Hoffman, P. Algal origin of dolomite laminations in stromatolitic limestones. J. Sediment. Petrol. 1973, 43, 603–613. [Google Scholar]
- Krijgsman, W.; Fortuin, A.R.; Hilgen, F.J.; Sierro, F.J. Astrochronology for the Messinian Sorbas basin (SE Spain) and orbital (precessional) forcing for evaporite cyclicity. Sediment. Geol. 2001, 140, 43–60. [Google Scholar] [CrossRef]
- Rouchy, J.M.; Caruso, A. The Messinian salinity crisis in the Mediterranean basin: A reassessment of the data and an integrated scenario. Sediment. Geol. 2006, 188–189, 35–67. [Google Scholar] [CrossRef]
- Esteban, M. Guia de campo del arrecife messiniense de Santa Pola. 1977; 1–50. [Google Scholar]
- Bourillot, R. Evolution des Plates-Formes Carbonatées Pendant la crise de Salinité Messinienne: De la Déformation des Évaporites aux Communautés Microbialithiques (Sud-Est de l’Espagne). Unpublished. Ph.D. Thesis, Université de Bourgogne, Dijon, France, 2009. [Google Scholar]
- Ott d’Estevou, P.; Montenat, C. Le bassin de Sorbas-Tabernas. In Les bassins Neogènes du domaine Bétique oriental (Espagne). Tectonique et Sédimentation Dans un Couloir de Décrochement. Première partie: Étude Régionale; Montenat, C., Ed.; Documents et travaux de L’IGAL; IGAL: Paris, France, 1990; Volume 12–13, pp. 101–128. [Google Scholar]
- Burne, R.V.; Moore, L.S. Microbialites; organosedimentary deposits of benthic microbial communities. Palaios 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Kalkowsky, E. Oolith und stromatolith in norddeutschen buntsandstein. Dtsch. Geol. Ges. 1908, 60, 68–125. [Google Scholar]
- Aitken, J.D. Classification and environmental significance of cryptalgal limestones and dolomites with illustrations from the Cambrian and Ordovician of southwestern Alberta. J. Sediment. Petrol. 1967, 37, 1163–1178. [Google Scholar] [CrossRef]
- Monty, C. Le Miocène supérieur de la région de Benejuzar (Province d’Alicante) et stromatolithes associés. Ann. Société Géologique Belg. 1981, 104, 109–114. [Google Scholar]
- Rouchy, J.M. La Genèse des Évaporites Messiniennes de Méditerannée; Mémoires du Muséum National d’Histoire Naturelle: Paris, France, 1982; Volume L. [Google Scholar]
- Dickson, J.A.D. Carbonate identification and genesis as revealed by staining. J. Sediment. Res. 1966, 36, 491–505. [Google Scholar]
- Al-Hashimi, W.S.; Hemingway, J.E. Recent dedolomitization and the origin of the rusty crusts of Northumberland. J. Sediment. Res. 1973, 43, 82–91. [Google Scholar] [CrossRef]
- Lumsden, D.N. Discrepancy between thin-section and X-ray estimates of dolomite in limestone. J. Sediment. Res. 1979, 49, 429–435. [Google Scholar]
- Roduit, N. Jmicrovision: Un Logiciel D’analyse D’images Pétrographiques Polyvalent; Université de Genève: Genève, Geneva, 2007. [Google Scholar]
- Braga, J.C.; Martín, J.M. Subaqueous siliciclatic stromatolites: A case history from Late Miocene beach deposits in the Sorbas Basin of SE Spain. In Microbial Sediments; Riding, R., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Goldstein, R.H.; Franseen, E.K.; Lipinski, C.J. Topographic and sea level controls on oolite-microbialite-coralgal reef sequences: The terminal carbonate complex of southeast Spain. AAPG Bull. 2013, 97, 1997–2034. [Google Scholar] [CrossRef]
- Calvet, F.; Zamarreno, I.; Vallès, D. Late Miocene reefs of the Alicante-Elche basin, southeast Spain. In Models for Carbonate Stratigraphy from Miocene of Mediterranean Regions; Franseen, E.K., Esteban, M., Ward, W.C., Rouchy, J.M., Eds.; SEPM: Tulsa, OK, USA, 1996; pp. 177–190. [Google Scholar]
- Reid, R.P.; James, N.P.; Macintyre, I.G.; Dupraz, C.P.; Burne, R.V. Shark Bay stromatolites: Microfabrics and reinterpretation of origins. Facies 2003, 49, 299–324. [Google Scholar] [CrossRef]
- Roche, A.; Vennin, E.; Bouton, A.; Olivier, N.; Wattinne, A.; Bundeleva, I.; Deconinck, J.-F.; Virgone, A.; Gaucher, E.C.; Visscher, P.T. Oligo-Miocene lacustrine microbial and metazoan buildups from the Limagne Basin (French Massif Central). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 504, 34–59. [Google Scholar] [CrossRef]
- Andres, M.S.; Reid, P.R. Growth morphologies of modern marine stromatolites: A case study from Highborne Cay, Bahamas. Sediment. Geol. 2006, 185, 319–328. [Google Scholar] [CrossRef]
- Vennin, E.; Bouton, A.; Bourillot, R.; Pace, A.; Roche, A.; Brayard, A.; Thomazo, C.; Virgone, A.; Gaucher, E.C.; Desaubliaux, G.; et al. The lacustrine microbial carbonate factory of the successive Lake Bonneville and Great Salt Lake, Utah, USA. Sedimentology 2019, 66, 165–204. [Google Scholar] [CrossRef]
- Planavsky, N.; Ginsburg, R.N. Taphonomy of modern marine bahamian microbialites. Palaios 2009, 24, 5–17. [Google Scholar] [CrossRef]
- Bernhard, J.M.; Edgcomb, V.P.; Visscher, P.T.; McIntyre-Wressnig, A.; Summons, R.E.; Bouxsein, M.L.; Louis, L.; Jeglinski, M. Insights into foraminiferal influences on microfabrics of microbialites at Highborne Cay, Bahamas. Proc. Natl. Acad. Sci. USA 2013, 110, 9830–9834. [Google Scholar] [CrossRef] [PubMed]
- Planavsky, N.; Reid, R.P.; Lyons, T.W.; Myshrall, K.L.; Visscher, P.T. Formation and diagenesis of modern marine calcified cyanobacteria. Geobiology 2009, 7, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, C.; Strasser, A. Microbialites and micro-encrusters in shallow coral bioherms (Middle to Late Oxfordian, Swiss Jura Mountains). Facies 1999, 40, 101–129. [Google Scholar] [CrossRef]
- Dupraz, C.; Strasser, A. Nutritional modes in coral-microbialite reefs (Jurassic, Oxfordian, Switzerland): Evolution of trophic structure as a response to environmental change. Facies 2002, 17, 449–471. [Google Scholar] [CrossRef]
- Camoin, G.; Cabioch, G.; Eisenhauer, A.; Braga, J.C.; Hamelin, B.; Lericolais, G. Environmental significance of microbialites in reef environments during the last deglaciation. Sediment. Geol. 2006, 185, 277–295. [Google Scholar] [CrossRef]
- Bourillot, R.; Vennin, E.; Kolodka, C.; Rouchy, J.M.; Caruso, A.; Durlet, C.; Chaix, C.; Rommevaux, V. The role of topography and erosion in the development and architecture of shallow-water coral bioherms (Tortonian-Messinian, Cabo de Gata, SE Spain). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 281, 92–114. [Google Scholar] [CrossRef]
- Roep, T.B.; Dabrio, C.J.; Fortuin, A.R.; Polo, M.D. Late highstand patterns of shifting and stepping coastal barriers and washover-fans (late Messinian, Sorbas basin, SE Spain). Sediment. Geol. 1998, 116, 27–56. [Google Scholar] [CrossRef]
- Meyers, W.J.; Lu, F.H.; Zachariah, J.K. Dolomitization by mixed evaporative brines and freshwater, Upper Miocene carbonates, Nijar, Spain. J. Sediment. Res. 1997, 67, 898–912. [Google Scholar]
- Monty, C. The origin and development of cryptalgal fabrics. In Stromatolites; Walter, M.R., Ed.; Sptinger: Berlin, Germany, 1976; pp. 193–249. [Google Scholar]
- Gerdes, G.; Krumbein, W.E. Peritidal potential stromatolites-A synopsis. In Phanerozoic Stromatolites II; Bertrand-Sarfati, J., Monty, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1994; pp. 101–129. [Google Scholar]
- Arp, G.; Reimer, A.; Reitner, J. Microbialite formation in seawater of increased alkalinity, satonda crater Lake, indonesia. J. Sediment. Res. 2003, 73, 105–127. [Google Scholar] [CrossRef]
- Flügel, E. Microfacies of Carbonate Rocks; Springer: Berlin, Germany, 2004. [Google Scholar]
- Trichet, J.; Défarge, C.; Tribble, J.; Tribble, G.; Sansone, F. Christmas island lagoonal lakes, models for the deposition of carbonate–evaporite–organic laminated sediments. Sediment. Geol. 2001, 140, 177–189. [Google Scholar] [CrossRef]
- Spadafora, A.; Perri, E.; McKenzie, J.A.; Vasconcelos, C. Microbial biomineralization processes forming modern Ca:Mg carbonate stromatolites. Sedimentology 2010, 57, 27–40. [Google Scholar] [CrossRef]
- Riding, R. Calcified cyanobacteria. In Calcareous Algae and Stromatolites; Riding, R., Ed.; Springer: Berlin, Germany, 1991; pp. 55–87. [Google Scholar]
- Davaud, E.; Strasser, A.; Jedoui, Y. Stromatolite and serpulid bioherms in a holocene restricted lagoon. In Stromatolites II; Bertrand Sarfati, J., Monty, C., Eds.; Springer: Dordrecht, The Nerterlands, 1994; pp. 131–151. [Google Scholar]
- Dupraz, C.; Fowler, A.; Tobias, C.; Visscher, P.T. Stromatolitic knobs in Storr’s Lake (San Salvador, Bahamas): A model system for formation and alteration of laminae. Geobiology 2013, 11, 527–548. [Google Scholar] [CrossRef]
- Bertrand-Sarfati, J.; Freytet, P.; Plaziat, J. Microstructures in Tertiary nonmarine stromatolites (France). Comparison with Proterozoic. In Stromatolites II; Bertrand Sarfati, J., Monty, C., Eds.; Springer: Dordrecht, The Nerterlands, 1994. [Google Scholar]
- Rasmussen, K.A.; Macintyre, I.G.; Prufert, L. Modern stromatolite reefs fringing a brackish coastline, Chetumal Bay, Belize. Geology 1993, 21, 199–202. [Google Scholar] [CrossRef]
- Freytet, P.; Verrecchia, E.P. Freshwater organisms that build stromatolites: A synopsis of biocrystallization by prokaryotic and eukaryotic algae. Sedimentology 1998, 45, 535–563. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Kading, T.J.; Braissant, O.; Dupraz, C.; Visscher, P.T. Inside the alkalinity engine: The role of electron donors in the organomineralization potential of sulfate-reducing bacteria. Geobiology 2012, 10, 518–530. [Google Scholar] [CrossRef]
- Jones, B.; Peng, X. Multiphase calcification associated with the atmophytic cyanobacterium Scytonema julianum. Sediment. Geol. 2014, 313, 91–104. [Google Scholar] [CrossRef]
- Bouton, A.; Vennin, E.; Amiotte-Suchet, P.; Thomazo, C.; Sizun, J.-P.; Virgone, A.; Gaucher, E.C.; Visscher, P.T. Prediction of the calcium carbonate budget in a sedimentary basin: A “source-to-sink” approach applied to Great Salt Lake, Utah, USA. Basin Res. 2019. [Google Scholar] [CrossRef]
- Gebelein, C.D. Distribution, morphology, and accretion rate of recent subtidal algal stromatolites, Bermuda. J. Sediment. Res. 1969, 39, 49–69. [Google Scholar]
- Golubic, S.; Focke, J.W. Phormidium hendersonii Howe; identity and significance of a modern stromatolite building microorganism. J. Sediment. Res. 1978, 48, 751–764. [Google Scholar]
- Macintyre, I.G.; Prufert-Bebout, L.; Reid, R.P. The role of endolithic cyanobacteria in the formation of lithified laminae in Bahamian stromatolites. Sedimentology 2000, 47, 915–921. [Google Scholar] [CrossRef]
- Awramik, S.M. Stromatolites with Coccoid and Filamentous Blue-Green Algae of Messinian Age From Site 374—Ionian Abyssal Plain; In Initial Reports; DSDP: Washington, WA, USA, 1978; Volume 1, pp. 665–668. [Google Scholar]
- Défarge, C.; Trichet, J.; Couté, A. On the appearance of cyanobacterial calcification in modern stromatolites. Sed. Geol. 1994, 94, 11–19. [Google Scholar] [CrossRef]
- Zeyen, N.; Benzerara, K.; Li, J.; Groleau, A.; Balan, E.; Robert, J.-L.; Estève, I.; Tavera, R.; Moreira, D.; López-García, P. Formation of low-T hydrated silicates in modern microbialites from Mexico and implications for microbial fossilization. Front. Earth Sci. 2015, 3, 64. [Google Scholar] [CrossRef]
- Burne, R.V.; Moore, L.S.; Christy, A.G.; Troitzsch, U.; King, P.L.; Carnerup, A.M.; Hamilton, P.J. Stevensite in the modern thrombolites of Lake Clifton, Western Australia: A missing link in microbialite mineralization? Geology 2014, 42, 575–578. [Google Scholar] [CrossRef]
- Visscher, P.T.; Dupraz, C.; Braissant, O.; Gallagher, K.L.; Glunk, C.; Casillas, L.; Reed, R.E.S. Biogeochemistry of carbon cycling in hypersaline mats: Linking the present to the past through biosignatures. In Microbial Mats: Modern and Ancient Microorganisms in Stratified Systems; Seckbach, J., Oren, A., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 443–468. ISBN 978-90-481-3799-2. [Google Scholar]
- Mann, C.J.; Nelson, W.M. Microbialitic structures in Storr’s Lake, San Salvador island, Bahamas Islands. Palaios 1989, 4, 287–293. [Google Scholar] [CrossRef]
- Myshrall, K.L.; Mobberley, J.M.; Green, S.J.; Visscher, P.T.; Havemann, S.A.; Reid, R.P.; Foster, J.S. Biogeochemical cycling and microbial diversity in the thrombolitic microbialites of Highborne Cay, Bahamas. Geobiology 2010, 8, 337–354. [Google Scholar] [CrossRef]
- Dupraz, C.; Pattisina, R.; Verrecchia, E.P. Translation of energy into morphology: Simulation of stromatolite morphospace using a stochastic model. Microb. Microb. Commun. 2006, 185, 185–203. [Google Scholar] [CrossRef]
- Dill, R.F.; Shinn, E.A.; Jones, A.T.; Kelly, K.; Steinen, R.P. Giant subtidal stromatolites forming in normal salinity waters. Nature 1986, 324, 55–58. [Google Scholar] [CrossRef]
- Bowlin, E.M.; Klaus, J.S.; Foster, J.S.; Andres, M.S.; Custals, L.; Reid, R.P. Environmental controls on microbial community cycling in modern marine stromatolites. Sediment. Geol. 2012, 263, 45–55. [Google Scholar] [CrossRef]
- Reid, P.R.; MacIntyre, I.G. Steneck A microbialite/algal ridge fringing reef complex, Highborne Cay, Bahamas. Atoll Res. Bull. 1999, 465, 1–18. [Google Scholar] [CrossRef][Green Version]
- Tarhan, L.G.; Planavsky, N.J.; Laumer, C.E.; Stolz, J.F.; Reid, R.P. Microbial mat controls on infaunal abundance and diversity in modern marine microbialites. Geobiology 2013, 11, 485–497. [Google Scholar] [CrossRef]
- Kolodka, C.; Vennin, E.; Bourillot, R.; Granjeon, D.; Desaubliaux, G. Stratigraphic modelling of platform architecture and carbonate production: A Messinian case study (Sorbas Basin, SE Spain). Basin Res. 2016, 28, 658–684. [Google Scholar] [CrossRef]
- Atwood, G.; Wambeam, T.J.; Anderson, N.J. Chapter 1—The present as a key to the past: Paleoshoreline correlation insights from great salt lake. In Developments in Earth Surface Processes; Oviatt, C.G., Shroder, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 20, pp. 1–27. ISBN 0928-2025. [Google Scholar]
- Baskin, R.; Wright, V.P.; Driscoll, N.; Kent, G.; Hepner, G. Microbialite bioherms in Great Salt Lake, Utah: Influence of active tectonics and anthropogenic effects. In Proceedings of the American Association of Petroleum Geologists Heidelberg Conference, Heidelberg, Germany, 4–8 June 2012. [Google Scholar]
- Bouton, A.; Vennin, E.; Mulder, T.; Pace, A.; Bourillot, R.; Thomazo, C.; Brayard, A.; Goslar, T.; Buoncristiani, J.-F.; Désaubliaux, G.; et al. Enhanced development of lacustrine microbialites on gravity flow deposits, great salt lake, utah, USA. Sediment. Geol. 2016, 341, 1–12. [Google Scholar] [CrossRef]
- Vanden Berg, M. Domes, rings, ridges, and polygons: Characteristics of microbialites from utah’s great salt lake. Sediment. Rec. 2019, 17, 4–10. [Google Scholar] [CrossRef]
- Carozzi, A.V. Observations on algal biostromes in the great salt lake, utah. J. Geol. 1962, 70, 246–252. [Google Scholar] [CrossRef]
- Ginsburg, R.N.; Planavsky, N.J. Diversity of bahamian microbialite substrates. In Links Between Geological Processes, Microbial Activities&Evolution of Life: Microbes and Geology; Dilek, Y., Furnes, H., Muehlenbachs, K., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2008; pp. 177–195. ISBN 978-1-4020-8306-8. [Google Scholar]
- Quataert, E.; Storlazzi, C.; van Rooijen, A.; Cheriton, O.; van Dongeren, A. The influence of coral reefs and climate change on wave-driven flooding of tropical coastlines. Geophys. Res. Lett. 2015, 42, 6407–6415. [Google Scholar] [CrossRef]
- Maloof, A.C.; Grotzinger, J.P. The Holocene shallowing-upward parasequence of north-west andros island, bahamas. Sedimentology 2012, 59, 1375–1407. [Google Scholar] [CrossRef]
- Warren, J. Evaporites: Sediments, Resources and Hydrocarbons; Springer: Heidelberg, Germany, 2006. [Google Scholar]
- Vennin, E.; Olivier, N.; Brayard, A.; Bour, I.; Thomazo, C.; Escarguel, G.; Fara, E.; Bylund, K.G.; Jenks, J.F.; Stephen, D.A.; et al. Microbial deposits in the aftermath of the end-Permian mass extinction: A diverging case from the Mineral Mountains (Utah, USA). Sedimentology 2015, 62, 753–792. [Google Scholar] [CrossRef]
- Neuweiler, F. Development of albian microbialites and microbialite reefs at marginal platform areas of the Vasco-Cantabrian Basin (Soba reef area, Cantabria, N. Spain). Facies 1993, 29, 231–249. [Google Scholar] [CrossRef]
- Visscher, P.T.; Stolz, J.F. Microbial Mats as Bioreactors: Populations, Processes, and Products; Elsevier: Amsterdam, The Netherlands, 2005; pp. 87–100. [Google Scholar]
- Baumgartner, L.K.; Reid, R.P.; Dupraz, C.; Decho, A.W.; Buckley, D.H.; Spear, J.R.; Przekop, K.M.; Visscher, P.T. Sulfate reducing bacteria in microbial mats: Changing paradigms, new discoveries. Sediment. Geol. 2006, 185, 131–145. [Google Scholar] [CrossRef]
- Visscher, P.T.; Reid, R.P.; Bebout, B.M. Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 2000, 28, 919–922. [Google Scholar] [CrossRef]
- Land, L.S. Failure to precipitate dolomite at 25 °C Fromdilute solution despite 1000-fold oversaturation after32 years. Aquat. Geochem. 1998, 4, 361–368. [Google Scholar] [CrossRef]
- Warren, J. Dolomite: Occurrence, evolution and economically important associations. Earth Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Xu, J.; Yan, C.; Zhang, F.; Konishi, H.; Xu, H.; Teng, H.H. Testing the cation-hydration effect on the crystallization of Ca–Mg–CO3 systems. Proc. Natl. Acad. Sci. USA 2013, 110, 17750–17755. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Warthmann, R.; McKenzie, J.A.; Visscher, P.T.; Bittermann, A.G.; van Lith, Y. Lithifying microbial mats in lagoa vermelha, brazil: Modern precambrian relics? Sediment. Geol. 2006, 185, 175–183. [Google Scholar] [CrossRef]
- Van Lith, Y.; Warthmann, R.; Vasconcelos, C.; McKenzie, J.A. Microbial fossilization in carbonate sediments: A result of the bacterial surface involvement in dolomite precipitation. Sedimentology 2003, 50, 237–245. [Google Scholar] [CrossRef]
- Kenward, P.A.; Fowle, D.A.; Goldstein, R.H.; Ueshima, M.; González, L.A.; Roberts, J.A. Ordered low-temperature dolomite mediated by carboxyl-group density of microbial cell walls. AAPG Bull. 2013, 97, 2113–2125. [Google Scholar] [CrossRef]
- Kenward, P.A.; Goldstein, R.H.; Gonzalez, L.A.; Roberts, J.A. Precipitation of low-temperature dolomite from an anaerobic microbial consortium: The role of methanogenic archaea. Geobiology 2009, 7, 556–565. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kenward, P.A.; Fowle, D.A.; Goldstein, R.H.; González, L.A.; Moore, D.S. Surface chemistry allows for abiotic precipitation of dolomite at low temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 14540–14545. [Google Scholar] [CrossRef]
- Konhauser, K. Introduction to Geomicrobiology; Blackwell Publishing: Malden, MA, USA, 2007. [Google Scholar]
- Ferris, F.G.; Fyfe, W.S.; Beveridge, T.J. Metallic ion binding by Bacillus subtilis: Implications for the fossilization of microorganisms. Geology 1988, 16, 149–152. [Google Scholar] [CrossRef]
- Grotzinger, J.; Al-Rawahi, Z. Depositional facies and platform architecture of microbialite-dominated carbonate reservoirs, ediacaran–Cambrian ara group, sultanate of oman. AAPG Bull. 2014, 98, 1453–1494. [Google Scholar] [CrossRef]
- Mettraux, M.; Homewood, P.; Al Balushi, S.; Erthal, M.M.; Matsuda, N.S. Neoproterozoic microbialites in outcrops of the qarn Alam salt dome, central oman. GeoArabia 2014, 19, 17–79. [Google Scholar]
- Perri, E.; Tucker, M.E.; Mawson, M. Biotic and abiotic processes in the formation and diagenesis of permian dolomitic stromatolites (Zechstein Group, NE England). J. Sediment. Res. 2013, 83, 896–914. [Google Scholar] [CrossRef]


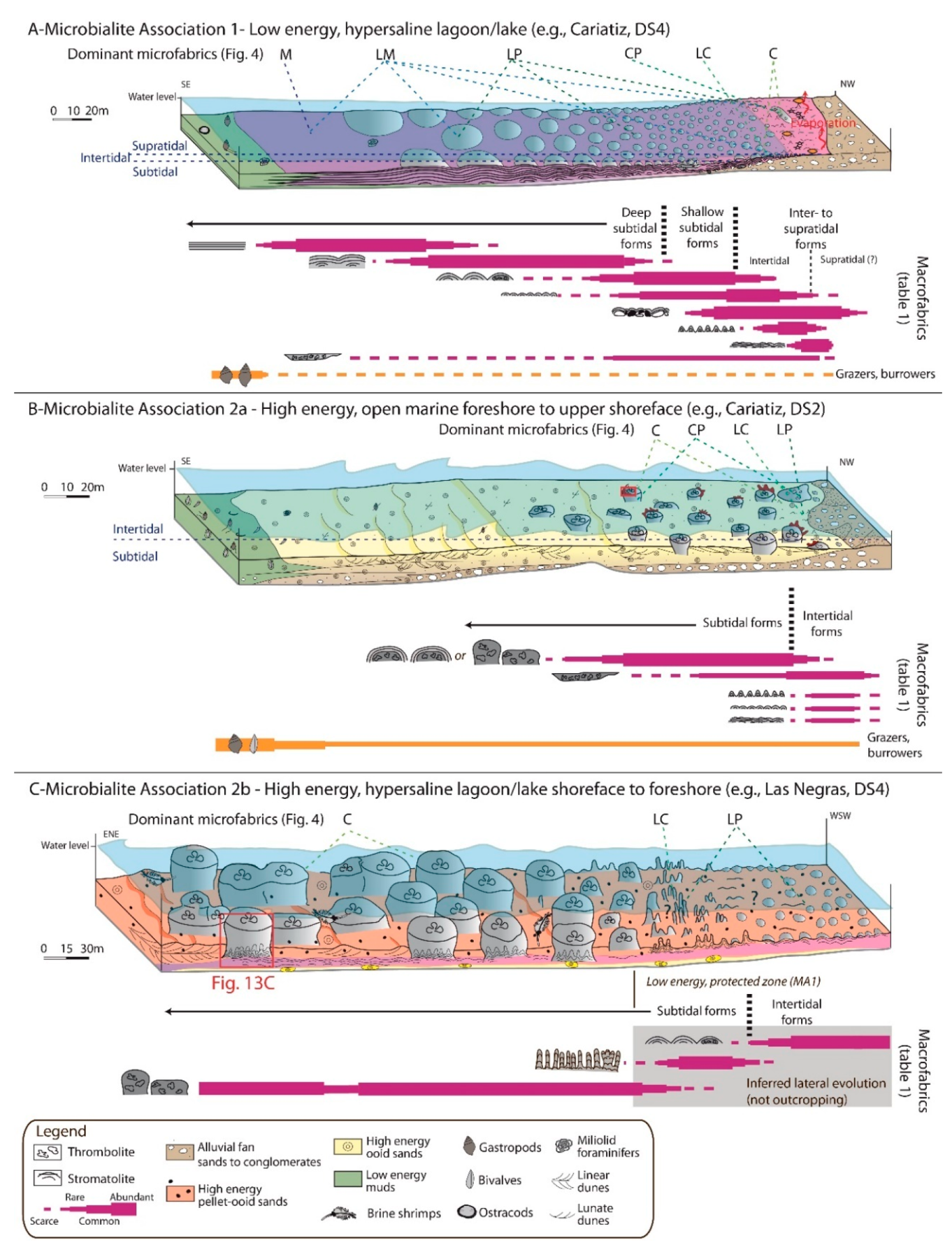















| Macrofabric (1st order) | 2nd order macrofabric | Mesofabric | Microfabric | Stratal pattern | Sedimentary features, associated sediments | Bioerosion, micro-encrusters, bioturbation | Paleoenvironment | Microbialite associations | ||||||||||
 M |  LM |  LP |  CP |  LC |  C | Lithophaga | Red algae | Serpulids | Vermetids | Disruption of lamination/clots | ||||||||
Pl-Planar | S | X | X | X | x | x | – | Ind. size: undefined Stratal geometry: Tabular Stratal dimensions: Thick: 0.1–2.8 m; Wide: 10 s–100 s m | 0-++ | Subtidal; Protected lagoon/salt lake | MA1 | |||||||
| LLH-Laterally Linked Hemispheroids | H1-Large | B/H2/H3 | S/T | x | X | X | x | x | x | Ind. size: Min. 0.2 × 0.5 m; Max. 1 × 2.5 m Stratal geometry: Tabular Stratal dimensions: Thick: 0.3–2 m; Wide: 10 s–100 s m | 0-++ | Subtidal; Protected lagoon/salt lake | MA1 | |||||
H2-Medium | H3 | – | X | X | x | X | x | Ind. size: Min. 0.05 × 0.1 m; Max. 0.2 × 0.5 m Stratal geometry: Tabular, lense Stratal dimensions: Thick: 0.1–9 m; Wide: 10 s–100 s m | Desiccation cracks; wave ripples | 0-++ | Subtidal to intertidal; Protected lagoon/salt lake | MA1 | ||||||
H3-Small | x | X | X | x | X | x | Ind. size: Min. 0.01 × 0.05 m; Max. 0.05 × 0.1 m Stratal geometry: Tabular, lense Stratal dimensions: Thick: 0.1–2.4 m; Wide: ms–10s m | Desiccation cracks; mangrove (?) root traces; gypsum pseudomorphs | 0-++ | Shallow subtidal to supratidal (?); Protected lagoon/salt lake, sabkha (?) | MA1 | |||||||
| SH-Stacked Hemispheroids  | T-S | – | x | X | – | X | x | Ind. size: Min. 1.25 × 3 m; Max. 3 × 5 m Stratal geometry: Shoal Stratal dimensions: Thick: 1.75–5 m; Wide: 10 s m–100 s m | Desiccation cracks; may develop within cross-bedded ooid pack- to grainstone | +-++ | Subtidal to intertidal; High energy shoreface to foreshore | MA2 | ||||||
W-Wavy | S | x | x | X | – | x | Ind. size: undefined Stratal geometry: Tabular, lense Stratal dimensions: Thick: 0.1–1.5 m; Wide: ms–10 s m | Desiccation cracks; boxwork structures; root traces (?) halite pseudomorphs, may pass laterally to cross-bedded ooid pack- to grainstone and gastropod floatstone | 0-+ | Intertidal to supratidal (?); High energy foreshore or protected lagoon/salt lake | MA1-2 | |||||||
Ci- Crinkles | H3 | S | – | X | X | – | x | x | Ind. size: Min. 0.035 × 0.015 m; Max. 0.5 × 0.3 m Stratal geometry: Tabular, lense Stratal dimensions: Thick: 0.035–0.8 m; Wide: ms–10 s m | Desiccation cracks, gypsum pseudomorphs | 0-++ | Shallow subtidal to supratidal (?); Protected lagoon/salt lake | MA1-2 | |||||
B- Branches | S | – | x | X | – | x | – | Ind. Size: Min. 0.04 × 0.01 m; Max. 0.08 × 0.015 m Stratal geometry: Tabular Stratal dimensions: Thick: 0.08–0.4 m; Wide: ms–10 s m | Desiccation cracks; may grow within pellet pack- to floatstone | 0 | Subtidal to intertidal; Protected lagoon/salt lake | MA1-2 | ||||||
| Cr-Crusts | Cr1- | S | – | x | X | x | X | x | Ind. size: Min. 0.03 × 0.05 m; Max. 0.75 × 1 m Stratal geometry: Tabular Stratal dimensions: Thick: 0.1–6.3 m; Wide: 10 s–100 s m | Desiccation cracks; wave ripples | 0-+++ | Subtidal to intertidal; Protected lagoon/salt lake | MA1-2 | |||||
Cr2- | T | x | x | x | X | – | X | Ind. size: Height: 0.1–1 m Stratal geometry: Tabular, lense, shoal Stratal dimensions: Thick: 0.1–1 m; Wide: ms–10 s m | May develop within cross-bedded ooid pack- to grainstone | x | x | x | x | +++ | Subtidal to intertidal; High energy shoreface to foreshore or Protected lagoon/salt lake | MA1-2 | ||
Co- Columns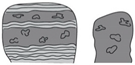 | H2/H3 | a-T b-S/T | – | x | x | X | x | X | Ind. size: Min. 1.1 × 0.8 m; 0.4 × 1 m Max. 4 × 3.5 m Stratal geometry: Tabular, shoal Stratal dimensions: Thick: 1–4 m; Wide: 10 sm–100 s m | Develop within cross-bedded ooid and/or pellet pack- to grainstone | x | x | x | x | ++-+++ | Subtidal; High energy shoreface to foreshore | MA2 | |
| Type, Dimensions | Mineralogy | Occurrence | Interpretation | |
|---|---|---|---|---|
| Peloids |  | Dolomite |
| Precipitation on EPS due to degradation by heterotrophic bacteria |
| Bushy peloid aggregates |  | Dolomite |
| Precipitation on EPS between filaments within cyanobacteria dominated mats |
| Filaments (a), flabellate (b) and tepees (c) structures | 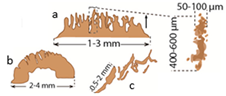 | Dolomite |
| Precipitation around or within filamentous cyanobacterial sheaths |
| Microspheres | 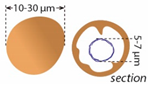 | External wall: Dolomite Internal wall: Mg-Al silicate? |
| Impregnation of coccoid cyanobacteria wall by (Ca)-Mg-Al silicates in elevated pH conditions. Subsequent reaction between silicates, metastable (?) carbonates and degraded EPS to form dolomite (see Figure 20) |
| Bean-shaped structures | 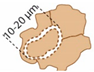 | Dolomite (rarely preserved) |
| Molds or mineralized cyanobacterial sheaths. Local enrichments in Si imply possible early silicate nucleation and dissolution |
| Microballs and dumbbells | 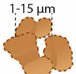 | Dolomite |
| EPS precipitates |
| Microborings | 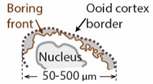 | None (porosity) |
| Boring of trapped grains by endolithic bacteria |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourillot, R.; Vennin, E.; Dupraz, C.; Pace, A.; Foubert, A.; Rouchy, J.-M.; Patrier, P.; Blanc, P.; Bernard, D.; Lesseur, J.; et al. The Record of Environmental and Microbial Signatures in Ancient Microbialites: The Terminal Carbonate Complex from the Neogene Basins of Southeastern Spain. Minerals 2020, 10, 276. https://doi.org/10.3390/min10030276
Bourillot R, Vennin E, Dupraz C, Pace A, Foubert A, Rouchy J-M, Patrier P, Blanc P, Bernard D, Lesseur J, et al. The Record of Environmental and Microbial Signatures in Ancient Microbialites: The Terminal Carbonate Complex from the Neogene Basins of Southeastern Spain. Minerals. 2020; 10(3):276. https://doi.org/10.3390/min10030276
Chicago/Turabian StyleBourillot, Raphaël, Emmanuelle Vennin, Christophe Dupraz, Aurélie Pace, Anneleen Foubert, Jean-Marie Rouchy, Patricia Patrier, Philippe Blanc, Dominique Bernard, Julien Lesseur, and et al. 2020. "The Record of Environmental and Microbial Signatures in Ancient Microbialites: The Terminal Carbonate Complex from the Neogene Basins of Southeastern Spain" Minerals 10, no. 3: 276. https://doi.org/10.3390/min10030276
APA StyleBourillot, R., Vennin, E., Dupraz, C., Pace, A., Foubert, A., Rouchy, J.-M., Patrier, P., Blanc, P., Bernard, D., Lesseur, J., & Visscher, P. T. (2020). The Record of Environmental and Microbial Signatures in Ancient Microbialites: The Terminal Carbonate Complex from the Neogene Basins of Southeastern Spain. Minerals, 10(3), 276. https://doi.org/10.3390/min10030276








