3.1. Results of the Optimization of Na-EtX Synthesis Parameters
In the experimental part of this paper, optimal parameters of sodium/potassium alkyl xanthate synthesis were determined following the reaction of alcohol, sodium/potassium hydroxide, and carbon disulfide in xylene as the reaction medium. The optimization of the synthesis process was made by determining the yield of the obtained products and the content of the active substance (concentration of the obtained product in the aqueous solution) depending on the reaction parameters: reaction time (
Table 1), temperature of the first and second reaction step (
Table 2), molar ratio of reactants (
Table 3), concentration of the reaction mixture suspension (
Table 4) (solvent used -xylene). The results of the optimization of the Na-EtX synthesis are presented in detail in this paper (
Table 1,
Table 2,
Table 3 and
Table 4), while that of the other synthesized xanthates are summarised in
Table 5.
The optimal reaction time of the Na-EtX synthesis was 4.5 h, when a yield of 87.71% was achieved. Extending the reaction time to 5 h does not provide a higher degree of conversion of the reactants into the product.
It is to be noted that the optimal temperature for the first step that represents alcoholate synthesis is 65 °C, while for the second step of xanthate synthesis in xylene in the form of a suspension of 35 °C, the yield is 87.72%. At lower temperatures, the yield decreases, and the resulting product is less pure. This is probably due to the formation of secondary products of tritiocarbonate, since the reaction of carbonate sulfide with sodium hydroxide is favored at a lower temperature. It is not recommended to increase the temperature of the reaction mixture in the second step of the reaction over 40 °C, as it includes easily evaporative and flammable reactants, and consequently, a significant increase in yield is not achieved.
Concerning the results of the dependence test of the yield of Na-EtX synthesis on the molar ratio of reactants (
Table 3), the highest conversion (87.70%) was achieved using the molar ratio of the reactants EtOH/NaOH/CS
2 = 1.1/1.1/1.0 Increasing the concentration of reactants does not improve yield due to the formation of the by-product and the residue of unreacted carbon disulfide.
The dependence of the Na-EtX yield on product suspension concentration (
Table 4), indicated that the optimal concentration of the suspension is 20%. A yield of 87.70% was achieved when reactants reacted in the present medium xylene in an amount yielding 20% suspension of the final product. Increasing the concentration of reactants in order to increase reactor productivity is not desirable due to the formation of a concentrated product suspension, which causes difficulties in mixing of the reaction mixture and by-product formation. In addition, reducing reactant concentrations does not result in satisfactory yields and product purity due to the lower probability of properly-oriented collisions of the particles that react, even with prolonged reaction times (Experiments 1 and 2).
3.2. Results of Optimization of the Conditions of Sodium/Potassium Alkyl Xanthate Synthesis
In an analogous manner, as per the optimal synthesis of the Na-EtX in laboratory conditions, a series of alkyl xanthates was synthesized: sodium isopropyl xanthate (Na-iPrX), sodium isobutyl xanthate (Na-iBuX), potassium ethyl xanthate (K-EtX), potassium izobutyl xanthate (K-iBX), and potassium amyl xanthate (K-AmX). The obtained results are presented in
Table 5.
Based on the experimental results of the optimization of the conditions of the sodium/potassium alkyl xanthates synthesis, the main conclusion is that the reaction time parameter for all the experiments shown is 4.5 to 5.0 h. The reaction time of 5.0 h is optimal in the synthesis of K-iBuX and K-AmX. The molecular ratio of the reactants is intended to be such that alcohols are used from 3% to 10% and carbon disulfide from 4% to 7% in excess. Further reactant addition does not lead to an increase in yield, but widens the possibility of side-effects (the formation of tritiocarbonate and sulfide). The optimum temperature for the first reaction step is 60–85 °C, and for the second 35–45 °C, with lower reaction temperatures for the synthesis of Na-EtX, Na-iPrX, and K-EtX. In all the experiments, the concentration of suspension of xanthates products is optimal, except in the synthesis of K-EtX, where the concentration of the suspension was 5%. Increasing the concentration of reactants in order to increase the productivity of the reactor does not result in a quality product due to the inability to efficiently mix the reaction mixture and perform the reaction. The highest yield achieved is in the synthesis of Na-EtX, and the lowest for Na-iBuX, which can be explained by the steric factor of the nucleophile in the reaction of the alkoxide ions to the carbon disulfide. Thus, the nucleophilic attack by the ethoxide ions on carbon disulfide is more efficient than that by isopropoxide and isobutoxide, because of more pronounced steric disturbances.
3.3. The Results of the Flotation Efficiency of the Synthesized Sodium Isopropyl Xanthates and Formulated Products Na-iPrX/HMFA
Flotation efficiency of a synthesized flotoreagent was tested on real samples of copper ores with the aim to determine their efficiency. Results of the investigated flotation efficiency of synthetic alkyl xanthates are presented in
Table 6. The process of flotation is based on manipulating the surface properties of minerals such that the mineral of interest acquires a hydrophobic surface. When air bubbles are introduced, the hydrophobic minerals attach themselves to the bubbles and are carried to the surface and skimmed away. The mineral’s behavior during froth flotation is controlled by its surface properties, which are a function of its chemistry, structure, and surface species formed by reactions during processing. The nature of the surface products formed as a result of the xanthate chemisorption is still under question.
In the flotation pulp, the mineral substrate was preliminarily hydrophobized by the chemically attached xanthate or dixanthogen. Both forms of the physically sorbed collector are found on a hydrophobized substrate (mineral) in the form of separate dispersed particles and cannot increase its hydrophobicity [
16]. The highest flotation efficiency showed Na-iPrX (
Table 6) due to a balanced contribution of hydrophylic part and coordination power of anionic part.
The creation of a favourable chemical environment is of major importance for the success of the flotation process. However, due to the complexity of the process and a large number of influential factors and their interactions, it is extremely difficult to ascertain the contribution of each factor to the overall flotation performance. Hence, various reagents can be added to flotation pulps to manipulate the chemical environment in order to create favourable conditions for the separation of the desired mineral from the unwanted gangue. To investigate the flotation capability of synthesized bio-based potential flotation agent, an analysis of the flotation recovery of the formulated product based on xanthates combined with 10, 15, 20, 25, 30, and 35 mass % of LA, HMF-LA, and HMFA. The best flotation performances were obtained with HMFA, and thus, this material was used in a determination of the optimal ratio of Na-iPrX and HMFA (in wt.%). The results of the flotation efficiency of the formulated reagent Na-iPrX/HMFA depending on mass ratio of Na-iPrX and HMFA, are presented in
Table 7. Flotation experiments carried out with copper ores in laboratory conditions were performed in “Denver” flotation machines in volume V = 2.8 L, on samples weighing 1 kg, under identical test conditions (fineness of grounded material, flotation time, pH value). Although the Na-iPrX/HMFA flotoreagent is a novelty in its application, in addition to the literary known characteristics of Na-iPrX and HMFA, the applied study was estimated to perform a series of three experiments in the same manner; the results were presented as mean value.
According to the obtained results shown in
Table 6, significant flotation recovery of copper was estimated by application of the Na-iPrX flotation reagent synthesized by described laboratory procedure in this research. Namely, the total of floated copper using Na-iPrX represents 92.78%, while by application of Na-iPrX/HMFA, the maximum achieved value is 91.72% (
Table 7). This value of total floated copper of 91.72% is achieved by mass ratio Na-iPrX/HMFA = 70/30. Based on the obtained results of the copper flotation test using the Na-iPrX/HMFA reagent, shown in
Table 7, significant flotation effect is achieved in the extended flotation process, achieving the same time of flotation, and the highest content of separated copper was obtained by application of the HMFA reagent in concentration of 30 wt.%; while, with further increase of HMFA content, the flotation power significantly decreases, indicating the technological inefficiency and inability of its application in real conditions. The efficiency of the application of the Na-iPrX/HMFA flotation reagent may be affected by pulp distribution from the mill section, by the slide to the open channel, due to inappropriate mass distribution (70:30 wt.%), as well as different pulp characteristics in terms of solid phase fineness and density. These parameters should be considered, as each of these parameters can affect the efficiency of the flotation process.
3.4. DFT Predictions of the Reactivity of Na-iPrX and HMFA
The DFT methodology is a useful tool to predict the strength and mechanism of bonding interactions between flotation reagents and copper [
36]. The molecular electrostatic potential (MEP) extrema show the most reactive sites of a molecule, where maxima represent favorable sites for nucleophilic attack and minima represent sites where electrophile will interact with the molecule. The MEP maps of Na-iPrX and HMFA are shown in
Figure 2.
The favorable Lewis base sites on Na-iPrX are located at two S atoms, while HMFA is expected to donate electrons to d-orbitals on cooper surface via carboxylic and hydroxylic O atoms. The electron-accepting sites of Na-iPrX are localized at Na atom, and HMFA might accept electrons around 5-membered HMF ring and at the –COOH and –OH atoms.
The local reactivity of a molecule can also be predicted from HOMO and LUMO distribution. HOMO orbitals are involved in electron-donating reactions, while LUMO are associated with electron-accepting ability of a molecule. As can be seen from
Figure 3, two sulfur atoms of Na-iPrX are electron-donating sites. The flotation agent probably coordinates to Cu ions via donation of S-electrons to the empty
d-orbitals of metal. Back-bonding between
d-electrons of a metal surface and LUMO orbital of a flotation agent is less likely, as LUMO density is localized on a sodium atom, which is not a good electron-acceptor.
The HOMO and LUMO of HMFA are more delocalized, compared to Na-iPrX (
Figure 4).
The HOMO, LUMO energies, dipole moments, and polarizability of Na-iPrX and HMFA are listed in
Table 8.
Higher HOMO energy and more negative MEP minimum indicate higher Lewis basicity of Na-iPrX compared to HMFA and stronger interactions with empty
d-orbitals of the cooper surface. On the other hand, back-bonding with Cu surface is more feasible for HMFA as LUMO energy is lower. Similar study was performed by Ma et. al. [
36], showing that the sulfur atoms of S-benzoyl O-isobutyl xanthate (BIBX) are clearly the main electron-donating sites for collector adsorption on the mineral surface. In general, importance of the HOMO orbital from the sulfur atom is of utmost significance to the effective coordination of metal atom, i.e. increased flotation efficiency.
Another study related to the comparative study of thiohexanamide (THA), O-isopropyl-N-ethyl-thionocarbamate (IPETC), and sodium isobutyl xanthate (SIBX) using sulfide ores showed superior selectivity of THA to chalcopyrite [
37]. The MEP maps showed the location of most negative regions on C=S and the most positive region on N amide hydrogen atoms. The HOMO density located on sulfur atoms indicated that C=S and –SH groups are the most reactive sites of collectors toward electrophilic attack. In addition, it was shown that the ‒NH
2 also participates in the creation of the Cu‒N bond. The energy gap value (ΔE
HOMO-LUMO), as another parameter of chemical reactivity, was the lowest for THA, indicating its highest reactivity.
According to
Table 8, similar chemical reactivity of Na-iPrX and HMFA might be expected as ΔE
HOMO-LUMO is quite similar (−4.038 and −4.061 eV, respectively).
Compared to experimental results, it can be concluded that the flotation efficiency does not depend on a single factor such as ΔEHOMO-LUMO, and the effect of crystal structure, morphology, and surface properties of ore and processing conditions should be considered along with collector properties.
In conclusion, the obtained results offer the idea for future design of flotation agents and the selection of most active compounds to increase flotation efficiency.
3.5. Results of the Stability Test of the Synthesized Sodium/Potassium Alkyl Xanthates
In the literature [
31], the stability of xanthates in the function of storage time, pH, and temperature was investigated by monitoring the change in the absorption of the solution at a certain wavelength. It was found that with decreasing pH values from 10 to 5, prolongation of storage time and temperature increase resulted in xanthate decomposition. In our work, the stability of synthesized xanthates was studied by analyzing the content of the active substance, sulfide, and tritiocarbonate at certain time intervals at a constant pH of 12 and temperature of 20 °C. Synthesized alkaline alkyl xanthates in the form of aqueous solutions of certain concentrations represent practically final products, which can be used in the flotation process of ore. The obtained results give real conditions of final product storage in warehouses of ready-made goods or in mine warehouses where they are used. In addition to the pure water xanthate flotation reagents, two optimal composition of Na-iPrX/HMFA (70/30 wt.%) and K-iBuX/HMFA (70/30 wt.%) were also subjected to the stability test; the results obtained are given in
Table 9.
It can be noted that sodium ethyl xanthate and sodium isopropyl xanthate are stable and that their concentrations remained almost unchanged over a 60-day period. Thus, the investigated sodium ethyl xanthate and sodium isopropyl xanthate were practically not degrading under the test conditions for sixty days or more. This information is important in relation to the storage of products in the form of aqueous solutions at the site of application in mines. The concentration of sodium isobutyl xanthate showed the highest decrease in relation to other sodium xanthates, ranging from 41.0% to 38.6% over a 90-day interval. Results of the xanthate stability test showed that sodium xanthates are more stable than the potassium ones in the same time interval of testing and under the same conditions (temperature of 20 °C and pH in the range of 11.7 to 12.2). The concentration of potassium ethyl xanthate decreased from 5% to 12%, with a greater concentration decrease in more concentrated products. Thus, the concentration of product KEtX-1 decreases from 51.8% to 45.5%, and K-EtX-2 from 45.1% to 42.9%. The decrease in concentration of potassium xanthate K-iBuX and K-AmX amounts to 1.5% and 0.5% over a 90-day period. Thus, the examined potassium xanthates, KiBuX and KAmX, are more stable than KEtX in the same time interval of storage and under the same conditions.
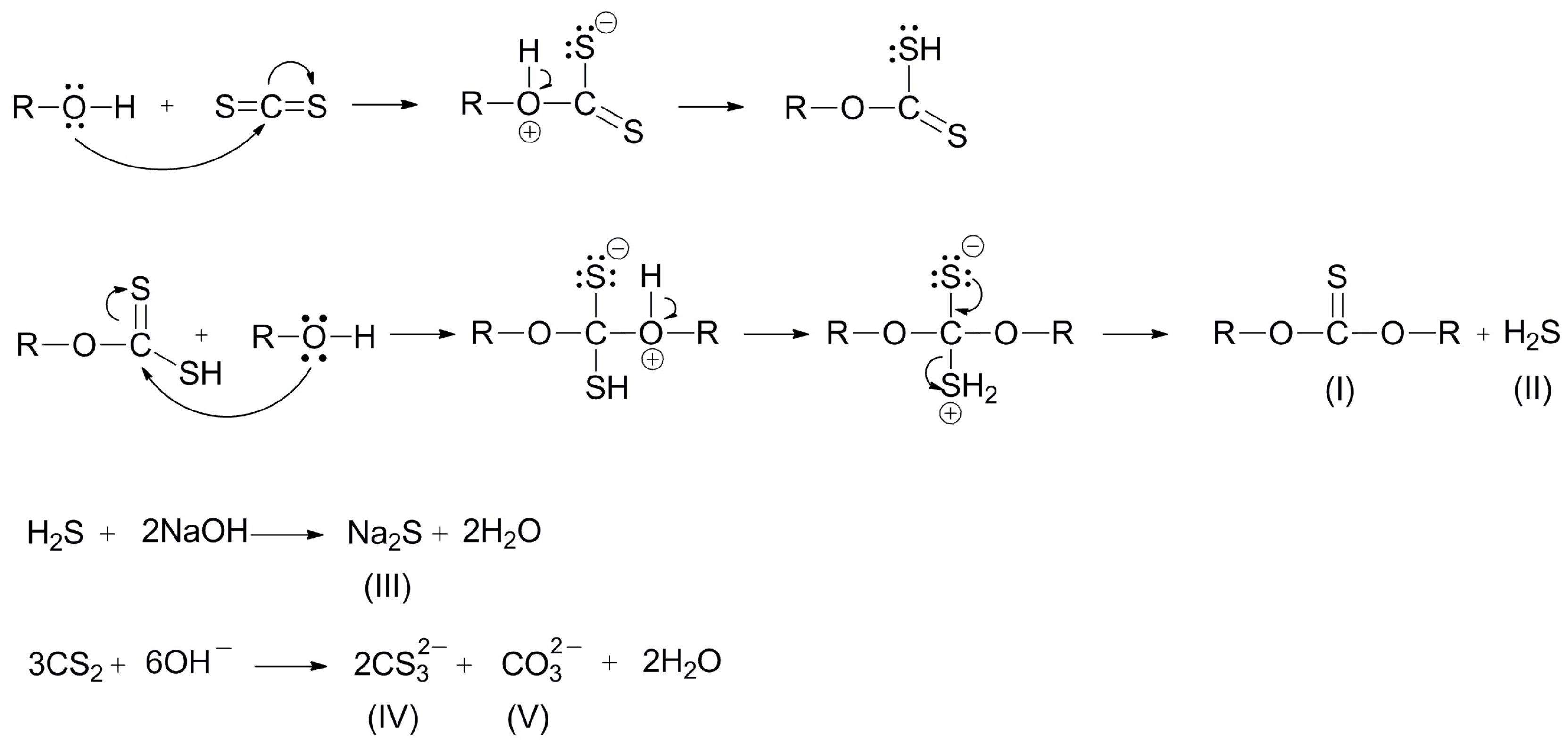
The concentrations of the present sulfides and tritiocarbonates in the final product are in the range of 1.4% to 1.8% for sulfide and from 0.30% to 0.43% for tritiocarbonate. These by-products are formed by reaction of carbon disulfide and alkali present in excess. The developed method, i.e. appropriate control of reaction condition/procedure, minimizes the side-reaction, which generates trithiocarbonate. Namely, if alcohol reacts more readily with alkoxide, then carbon disulfide is mostly consumed in the reaction. Otherwise, if there is a greater amount of free alkalis, the formation of trithiocarbonates will occur, formed by the reaction of the present alkali with carbon disulfide, followed by water release during the xanthate synthesis:
In the initial step of addition of CS2 to the reaction mixture, xanthate is formed, which is dissolved in the water added at the beginning of the synthesis in the dissolution of sodium hydroxide step and the water formed during the alcohol production step. The obtained alcohol is dissolved in xylene, which is present in the reaction mixture, and the resulting xanthate, insoluble in xylene, is dissolved in water present in the reaction mixture. The resulting alkoxide reacts with carbon disulfide, whereby xanthate is formed, which dissolves in water; thus, further reaction is successively carried out with maximum conversion. In this way, the equilibrium reaction between alcohol and sodium hydroxide is shifted to the formation of alkoxide. As the reaction of CS2 and the resulting alkoxide in the first stage of synthesis continues, the second step of the xanthate formation virtually moves the balance of the first reaction step to the right. The maximum conversion of the reactants to the xanthate product is achieved and obtained in the form of a suspension in the xylene reaction medium. By adding a certain amount of water to the reaction mixture in order to obtain an aqueous solution, the resulting xanthate is dissolved, and the solvent xylene is separated and used for a new synthesis. Unless xanthates are to be obtained in the form of an aqueous solution of defined concentration, the reaction mixture is cooled down and filtrated after synthesis. The synthesized xanthate is isolated as a filtration cake, and filtrate-xylene is used again for new synthesis reactions.
The products produced in this synthesis are carbonates, tritiocarbonates, and sulfides.
Figure 5 shows the reaction of the formation of the by-product in the reaction mixture. If the reaction takes place in excess of the alcohol or the alcohol is a reactant and a reaction medium at the same time, it will react with carbon disulfide to give dialkyl thiocarbonate (I) with the separation of hydrogen sulfide (II). This reaction was favorable to a small extent, confirmed by a special study on the reaction of alkyl xanthic acid with alcohol. The reaction of neutralizing xanthic acid with sodium hydroxide is incomparably faster. Hydrogen sulfide reacted with the present alkali in the reaction mixture with the separation of alkaline sulfide (III). Carbon disulfide reacts simultaneously with the alkali, which is contained in the reaction mixture, resulting in the formation of trithiocarbonate (IV) and carbonate (V).
According to the described optimized laboratory procedure for the synthesis of alkali alkyl xanthates in aqueous solutions, the possibility of producing by-products is minimized. Additionally, the design of the reactor is such that it allows intensive mixing, which is necessary for maximizing the number of collisions of properly oriented reacting molecules. The well-known reactors in which these industrial-scale synthesis reactions take place are called "Malaxers". They comprise of horizontal cylinders with a wrapper that are used for the passage of heating or cooling fluid, with an axially mounted shaft on which the iron rods are located. By rotating the shaft with a gear motor, the rods stir the reaction material and, at the end of the reaction, grind the material during drying. This kind of reactor design is not efficient enough to perform a synthesis reaction due to the formation of dead corners where reaction does not occur, as well as poor mixing. Under these reaction conditions, the obtained product is of lower yield and purity (82–85%). These xanthates are less efficient in the flotation process and they are used in higher quantity because of the impurities present. In contrast, the procedure described in this work, after filtration of the reaction mixture and drying, produces xanthates in solid state with significantly higher content of active substance and purity of 92–95%, or in the form of water solution of appropriate concentration.
The reactor used in this synthesis with ideal mixing enables much better phase contact and more efficient mixing, and thus providing better yield and product quality. Furthermore, in this paper, it has been demonstrated that with the extension of reaction time, a larger product yield is provided. Namely, trithiocarbonate (IV), which is produced as a by-product, reacts with the alcohol present in the reaction mixture, separating the xanthate (VI) and releasing sodium hydrosulfide (VII), which reacts with the hydroxide present to produce sulfide (III). The resulting sulfide reacts with the minimum amount of dialkyl thiocarbonate (I) present, which is also formed in the reaction of this synthesis, producing the desired xanthate (VI) and alcoholate (VIII). These reactions are presented in
Figure 6.
The proposed mechanism of alkyl xanthate synthesis helped in the definition of experimental condition (design) to achieve the optimal ratio of reactants and conditions that provide the highest yield and purity of the desired product. Moreover, the results of laboratory analyses of sulfide and trithiocarbonate content in the product show higher quantities of present trithiocarbonate sulfides. The reactions presented in
Figure 5 and
Figure 6 show that the resulting trithiocarbonates are consumed in the reaction with the excess of alcohol present in the reaction mixture, which is the reason for their presence in the final product to a lesser extent.
Selectivity depends on the structure of the xanthates hydrocarbon chain, which affects flotation recovery. An optimized laboratory procedure for the synthesis of sodium/potassium alkyl xanthates in the form of aqueous solutions, the reaction of alkoxide obtained in the reaction of alcohol, and sodium/potassium hydroxide (step I reaction) and carbon disulfide, was performed to obtain the corresponding sodium/potassium alkyl xanthate (II reaction step). Xylene is used as a solvent since it dissolves alkoxide, while the xanthate product builds a suspension. By adding water to the reaction mixture at the end of the reaction, the resulting xanthates is dissolved and the layers are separated. Xanthate dissolves in water; therefore, the suspension disappears, and xylene can be separated from xanthate water dispersion as an upper organic layer and returned to the synthesis process.
3.6. Synthesis of Alkyl Xanthates at the Industrial Level
Based on the defined parameters of the process on optimization of the laboratory procedure for the synthesis of alkali alkyl xanthates in the form of aqueous solutions of certain concentrations, a trial production was conducted in industrial conditions. The technological scheme of the trial industrial production is presented in
Figure 7.
In the 5 m3 reactor (1), 60.0 kg (1.1 kmol) of 98% potassium hydroxide and 130 L of water were placed; the heating was switched on in a manner that the temperature of the reactor mass was kept at 65 °C. Then, 60.8 L (1.1 kmol) of ethanol from dose (5) was added and mixing was initiated; 3.25 m3 xylene was added from dosage unit (4). The reaction mixture was stirred at a temperature of 60 °C for 1.5 h. Thereafter, the reaction mixture was cooled to about 35 °C, and 61.5 L (1.0 kmol) of carbon disulfide were added from dosage unit (3). The addition of carbon disulfide was carried out for 2.5 h, providing a constant temperature of 35 °C. Then, 134 L of water was added and the reaction mixture was stirred for 5 min. Afterwards, the reaction mixture was transferred to the separator (2), where the upper layer of xylene were separated from the lower aqueous portion, which is an aqueous solution of synthesized potassium ethyl xanthate. The resulting product was analyzed for content of the active substance, i.e. concentration of aqueous xanthate solution (50.1%), trithiocarbonate content (1.3%), and sulfide (0.3%).
At the facility in Chemical Industry “Župa“Kruševac, a trial production of three batches of aqueous xanthate solution was done according to the procedure described above. The obtained products were analyzed for content of the active substance, i.e., the concentration of aqueous xanthate solution, the content of trithiocarbonate, and sulfide. The results of the analysis of the synthesized alkali alkyl xanthates under industrial conditions are given in
Table 10.
Based on the obtained results from trial alkali xanthates production in the industrial conditions presented in
Table 10, it can be seen that satisfactory yields have been achieved, and the obtained products in the form of aqueous solutions show a high degree of purity.
3.7. Application of Innovated Product of Mine Sample Flotation at Laboratory and Industrial Levels
Comparative flotation analysis of the powdery Na-iPrX produced at the Chemical Industry “Župa” Kruševac by conventional industrial process and those obtained by the described innovative process using liquid Na-iPrX are presented in
Table 11 and
Table 12. Flotation behavior of sulfide ores was investigated by bench-scale tests, which are described in detail in the
Supplementary Materials Section.
Results from
Table 10 and
Table 11 imply that better effects of copper concentration in ore flotation are obtained using Na-iPrX in the form of a water solution produced by the innovative industrial process (93.59%,
Table 12), compared to Na-iPrX from a conventional industrial process (89.88%,
Table 11) applied under the same technological parameters. The optimized technological procedure for the synthesis of xanthates, presented in this paper, is economically justified, in comparison to the classical methods due to lower energy costs (no drying phase), higher conversion rate, cleaner product being obtained, and lower consumption of the obtained products in the flotation process.
The results demonstrated that compared to other known collectors, NaiPr exhibited high recovery efficiency towards copper. According to previous literature data, the recoveries of chalcopyrite were 90.84%, 85.83%, 84.65%, 83.39%, and 82.49%, for S-benzoyl O-isobutyl xanthate (BIBX), sodium isopropyl xanthate (Na-iPrX), thiohexanamide (THA), sodium isobutyl xanthate (SIBH), and O-isopropyl-N-ethylthionocarbamate (IPETC), respectively [
36,
37], while for Na-iPrX in the form of a water solution produced by the innovative industrial process, recovery was 93.59%, and 89.88% for Na-iPrX with the conventional industrial process. Additionally, selectivity determined by Hassanzadeh et al. [
38] is similar to that obtained in this work, i.e.,
KMCu and
KMFe values are 42.2 and 6.23, respectively. This result indicates good separation feasibility of the Na-iPr collector.