1. Introduction
Nickel, as a national strategic reserve metal, is not only a threat to the sustainable development of the national economy but also a potential major threat to the national defense. As an important metal, nickel is widely used in stainless steel and new material industries. According to marketing observations, 66.2% of the total metal nickel is consumed by the stainless-steel industry. Rapid expansion of these industries, especially stainless-steel manufacture, has dramatically increased the demand for nickel in recent years. Of all the land nickel reserves, 30% exist as sulfide ores, with the balance comprised of oxide ores [
1]. Because of the low grade of nickel ore mined from mines, most of the nickel concentrate can be obtained after the beneficiation process before smelting. Nickel ores are usually divided into three types: Nickel sulfide, nickel oxide, and nickel arsenide. Nickel arsenide contains minerals, such as red nickel NiAs, arsenic NiAs
2, and nickel arsenide NiAsS. Only a small amount of these minerals are produced in Morocco, North Africa, and nickel extraction from nickel arsenide is currently limited to individual countries. About 70% of the world’s nickel production comes from nickel sulfide and 30% from nickel oxide. There are more than 60 known nickel minerals, and the following six kinds of sulfide minerals (Ni 1.0–2.5%) and three kinds of oxidized minerals (Ni 0.5–1.5%) have industrial value, including: Nickel-bearing laterite, (Fe,Ni)O(OH)·
nH
2O, genthite (Ni,Mg)
4·3SiO
2·6H
2O, garnierite H
2(Ni,Mg)SiO
4·
xH
2O, pentlandite (Fe,Ni)
9S
8, Ni-bearing pyrrhotite (Ni,Fe)
7S
8, millerite NiS, violarite (Fe,Ni)
2S
4, polydymite 3NiS·FeS
2, and hengleinite (Ni,Co)
3S
4 [
2,
3].
Laterite nickel deposit is formed by the weathering and leaching of nickel-bearing peridotite in tropical or subtropical regions. As a nickel ore, it was discovered by Garnier in New Caledonia in 1863, so the ore is called “garnierite”—a magnesium-based nickel silicate. Because the ore is red due to the oxidation of iron after weathering, it is called laterite. In fact, in the upper part of the deposit, as a result of weathering and leaching, more iron, and slightly higher cobalt as compared to lesser silicon, magnesium, and nickel, it appears red; in the lower part of the deposit, as a result of weathering and enrichment, nickel ore is more silicon, more magnesium, lesser iron, higher nickel, and lower cobalt, which is called magnesium garnierite laterite ore. Both ores, relative to nickel sulfide deposits, are called nickel oxide ores [
4,
5,
6].
Laterite nickel ore is the most abundant nickel ore resource in the world. The nickel in laterite is in the state of a disseminated structure, and the effect of traditional mineral processing is quite poor. Only the large gangue minerals with a low nickel content and low rate of decay can be removed by wet or dry beneficiation. So far, no effective physical beneficiation method has been found to enrich laterite nickel ore. Laterite ore can be divided into two types: One is the limonite type, which is contained in the surface of laterite ore, in which nickel occurs mainly in the solid melt state of nickel oxide and iron oxide, such as (Fe,Ni)O(OH)·
nH
2O; and the other type is the silicate-type nickel oxide, in which Ni, Fe,t and Co replace part of magnesium oxide in different proportions, especially serpentine Mg
6[Si
4O
10](OH)
8 [
7,
8,
9,
10].
At present, the pyrometallurgy process, hydrometallurgy process, and combination of the hydrometallurgy and pyrometallurgy processes are the three processing technologies of laterite nickel ore in the world. The pyrometallurgy process can also be divided into the process of producing ferro-nickel by reduction melting and the process of producing nickel-matte by reduction sulfurization melting. The hydrometallurgical process can be divided into the ammonia leaching process and acid leaching process according to the different leaching solutions. According to the different dressing methods after roasting, the combined process of hydrometallurgy and pyrometallurgy can be divided into reduction roasting–magnetic separation and reduction roasting–flotation. In the process of pyrometallurgy, it is difficult to prevent the reduction of iron oxides into metallic iron, so the products of pyrometallurgy are usually ferro-nickel alloys, also known as ferro-nickel. To purify nickel from ferronickel, it is very expensive to obtain pure metallic nickel. Nickel and iron are the main components of stainless steel. Therefore, the production of ferro-nickel in the form of nickel and iron has no impact on the stainless-steel industry [
11,
12,
13,
14,
15,
16].
In traditional nickel industries, about 60% of the metal nickel was produced from sulfide ores about 10 years ago. However, miners of nickel sulfide ores have been confronted with increasing challenges due to much deeper drilling requirements, higher production costs, and greater depletion of reserves. Therefore, nickel laterite ore has attracted greater attention in recent years, leading to about 50% of metal nickel being produced from laterite currently. China has become the largest nickel consumer in the world due to the soaring production of stainless steel. Laterite ores are imported into China because of the depletion of domestic nickel sulfide resources. Consequently, it is of great importance to the Chinese economy to effectively utilize low-grade nickel laterite ore [
17].
There are abundant nickel oxide ore resources in the Yuanjiang, Mojiang, and Mangshi areas of Yunnan Province in China, among which the nickel oxide ore in Mojiang area contained about 0.7% nickel, which is the nickel ore resource with the lowest grade among the three areas. Mojiang nickel ore belongs to the magnesium serpentine-type nickel silicate ore type. Due to the lower nickel grade, higher magnesium content, complex mineral composition of the ore, and fine-grained dissemination, it has not been properly developed and utilized up to now. Therefore, in this paper, the magnesium garnierite laterite ore in Mojiang area was taken as the object of study, and the valuable metal nickel in the ores was extracted by segregation roasting and magnetic separation, thus providing great practical significance for the development of nickel oxide ores in this area.
2. Materials and Methods
2.1. Sampling
The garnierite laterite ore was collected as the test research sample in the Mojiang area of China. The samples taken this time were powdery, most of which had a particle size of less than 10 mm. Meanwhile, the samples were relatively moist. Therefore, after the samples were dried by natural drying, the samples of garnierite were crushed by the jaw crusher and finely ground by the roller crusher, and then the disk crusher was used for dry grinding until the particle size was less than 0.1 mm, and fully mixed evenly. The garnierite laterite ore contained Ni 0.72%, Co 0.029%, Fe 8.65%, MgO 29.66%, and SiO2 37.86%. Garnierite (Ni,Mg)O·SiO2·nH2O, potash feldspar K2O·Al2O3·6SiO2, forsterite Mg2Si2O6, tremolite Ca2Mg5Si8O22(OH)2, halloysite Al2O3·2SiO2·4H2O, quartz SiO2, and kaolinite Al2Si2O5(OH)4 were the main minerals in the garnierite laterite ores. The iron concentrate containing Fe 61.22% was collected as the additive from a dressing pant by magnetic separation and floatation in Dahongshang area of China. The main minerals were goethite FeOOH, flogopite KMg3[AlSi3O10]·[F,OH]2, sodaclase Na2O·Al2O3·6SiO2, and quartz SiO2.
The main chemical composition analysis of the silicate nickel and iron concentrate is shown in
Table 1 and
Table 2, respectively. The nickel phase of the garnierite laterite ore is shown in
Table 3, and the X-ray diffraction (XRD) analysis of the silicate nickel and iron concentrate is shown in
Figure 1.
2.2. Chemical Reagent and Equipment
The main chemical reagent used in this test was calcium chloride with analytical purity from Guangzhou Chemical Regent, Co., Ltd., Guangzhou, China. The coke from Shanxi Coking Coal Group Co. Ltd. (Taiyuan, China) was crushed and divided into six particle sizes of −3 + 2.5 mm, −2.5 + 2 mm, −2 + 1.5 mm, −1.5 + 1 mm, −1 + 0.5 mm, and −0.5 + 0 mm as reducing agents in the process of segregation roasting.
The main equipment used in the experiment included a muffle furnace (≤1300 °C, Shanghai Shiyan Electric Furnace Co., Ltd. Shanghai, China), cone ball mill (XMQ—Φ240 × 90 mm, Jilin Exploration Machinery Plant, Changchun, China), disc grinder (Φ300 × 150 mm, Jilin Exploration Machinery Plant), drying box (Southwest Chengdu Experimental Equipment Co., Ltd. Chengdu, China), and vacuum filter (Φ300, Southwest Chengdu Experimental Equipment Co., Ltd.).
2.3. Roasting–Magnetic Separation Test
The garnierite laterite ores, calcium, hematite concentrate, and coke were mixed and put into the roasting furnace. These mixtures were heated to a certain temperature (950–1200 °C) under a neutral or weak reducing atmosphere, where the chlorinating agent was used to form volatile metal chlorides. For each magnetic separation test, segregation roasting ores were put into a 6.25 dm
3 Φ240 × 90 conical ball mill. The grinding density was set at 60%. The pulp was then placed in a XCGS−13 (Φ50, overall dimensions 1000 × 800 × 500 mm) Davis Magnetic Tube (Jilin Exploration Machinery Plant) with a special magnetic field intensity. The products were filtered, dried, weighed, sampled, and tested for further evaluation based on the grade and recovery of nickel and iron in the magnetic products. The calculation formulas of nickel recovery and iron recovery are shown in Equations (1) and (2):
where Q
1 is the weight of nickel concentrate, g; Q
0 is the weight of segregation roasting ores, g; β
Ni is the nickel grade of nickel concentrate, %; β
Fe is the iron grade of nickel concentrate, %; α
Ni is the nickel grade of segregation roasting ores, %; and α
Fe is the iron grade of segregation roasting ores, %.
2.4. Experiment Procedure
The garnierite laterite ores were crushed into <3.0 mm by a roll crusher and further ground to <0.1 mm by a disc mill as the segregation roasting sample. Garnierite laterite ores (a mass of 50 g for each test), calcium chloride, hematite concentrate, and coke were mixed and put into the muffle furnace for segregation roasting, which turned nickel into a new metal nickel mineral phase and iron into a new metal iron mineral phase. Metal nickel and metal iron are ferromagnetic metals. The specific magnetic susceptibility of nickel metal is greater than the magnetic susceptibility of magnetite. Nickel was recovered from segregation roasting ores after grinding with a magnetic separator. The process of segregation roasting–magnetic separation is shown in
Figure 2.
2.5. Analysis and Characterization
The chemical composition of solid materials was analyzed by a Z-2000 atomic absorption spectrophotometer (Hitachi Co., Ltd., Tokyo, Japan), the diffraction grating was Zenier-tana Type, 1800 lines/mm, the flash wavelength was 200 nm, the wavelength range was 190~900 nm, the automatic peak seeking setting, and the spectral bandwidth was divided into 4 grades (0.2, 0.4, 1.3, and 2.6 nm) for the analysis of the mineral chemical composition. The phase composition of solid substances was analyzed by X-ray diffraction (XRD, X Pert pro, Panaco, The Netherlands). The microstructure of the solid products was observed by SEM (S440, Hirschmann Laborgerate GmbH & Co. KG, Eberstadt, Germany) equipped with an energy dispersive X-ray spectroscopy (EDS) detector (UItra55, Carlzeiss NTS GmbH, Hirschmann Laborgerate GmbH & Co. KG, Eberstadt, Germany).
3. Results and Discussion
3.1. Effect of Calcium Chloride Dosage
The effect of different calcium chloride dosages on the extraction of nickel is shown in
Figure 3a. The nickel grade in ferric nickel decreased and the nickel recovery increased when the calcium chloride dosage increased. When the calcium chloride dosage exceeded 15%, the nickel grade decrease was more noticeable in nickel iron, and the increase of nickel recovery as smaller, which further shows that the amount of hydrogen chloride is advantageous to the nickel chloride increase by increasing the appropriate calcium chloride dosage. At the same time, other elements, such as magnesium, aluminum, iron, potassium, sodium, etc., can also react with hydrochloric gas and generate magnesium chloride, ferric chloride, potassium chloride, and sodium chloride. This leads to the consumption of a lot of calcium chloride and an increase of the chlorination agent cost. Therefore, a calcium chloride dosage of 15% is more appropriate; a ferronickel concentrate with a nickel grade of 4.01%, iron content of 12.16%, and nickel recovery of 51.82% was obtained.
3.2. Effect of Coke Dosage
The effect of different coke chloride dosages on the extraction of nickel is shown in
Figure 3b. In the process of segregation roasting, if the amount of reductant added increases, the water–gas reaction will easily occur, and then the in situ reduction of metal oxides, thus affecting the effect of segregation roasting. It is known from
Figure 3b that when the coke dosage increased to 20%, the nickel grade of ferronickel decreased to 2.76%. Compared with the coke dosage of 15%, the nickel grade decreased by 0.92% and the nickel recovery increased by 3.1%. This indicates that the coke dosage of 15% is optimal; a ferronickel concentrate with a nickel grade of 3.68%, iron content of 16.22%, and nickel recovery of 70.01% was obtained.
3.3. Effect of Roasting Temperature
The effect of different segregation roasting temperatures on the extraction of nickel is shown in
Figure 4a. The segregation roasting temperature is one of the key factors to determine whether the chlorination agent can decompose and whether the valuable metal iron can be chlorinated to form volatile metal chloride. Under the condition of the same surface tension, the vapor pressure of particles with a smaller radius of curvature is larger, so the process of particles growing up is the process of particles with a smaller radius of curvature melting and evaporating to the surface of particles with a larger radius of curvature condensation.
It is conducive to gradually increase the particles of ferronickel by increasing the roasting temperature, but the higher temperature will cause part of the ferronickel to re-enter the olivine lattice, reducing the yield of ferronickel. Upgrading the roasting temperature can improve the nickel iron nickel grade and nickel recovery. When the temperature exceeds 1100 °C, the nickel grade and recovery of a smaller increase in temperature may cause the energy costs to increase at the same time.
In the testing process, we found that the materials were partially melted at a temperature of 1100 °C, and the material was already in the melted state at a temperature of 1200 °C. Furthermore, the segregation roasting ores will be difficult to grind and affect the recovery of nickel and iron in the magnetic separation process. Therefore, the roasting temperature of 1100 °C is suitable; a ferronickel concentrate with a nickel grade of 4.52%, iron content of 19.22%, and nickel recovery of 70.01% was obtained.
3.4. Effect of Roasting Time
The effect of different roasting times on the extraction of nickel is shown in
Figure 4b. The chemical reactions in the process of segregation roasting are mainly divided into decomposition reaction, chlorination reaction, and reduction reaction. However, the reactions of decomposition, chlorination, and reduction are not only successive chemical reactions but also involve a complex phase change process. The segregation roasting time mainly affects the degree of the chemical reaction in the process of segregation roasting. If the roasting time is too short, the effective chemical reaction is not enough to achieve a better segregation effect. The results in
Figure 4b confirm that a roasting time of 90 min is suitable; a ferronickel concentrate with a nickel grade of 4.13%, iron content of 16.11%, and nickel recovery of 79.63% was obtained.
3.5. Effect of Coke Size
The effect of the coke size on the extraction of nickel is shown in
Figure 5a. During the segregation and roasting test, the particle size of the nickel silicate sample after crushing and dry grinding was <0.100 mm. In order to better promote the segregation and roasting effect, it is necessary to determine a reasonable coke particle size to match the particle size of the ore sample.
On the one hand, coke plays the role of atmosphere adjustment in the segregation roasting process. On the other hand, it also acts as an adsorption carrier for metal particles reduced by volatile chloride by hydrogen. Therefore, when coke is involved in the generation of hydrogen and carbon monoxide gas, it also needs to have a certain amount of surplus after achieving the segregation roasting reaction time.
As can be seen from
Figure 5a, the reduced coke size is conducive to the improvement of the nickel grade and recovery of nickel iron. However, when the coke size decreased to −0.5 + 0 mm, the recovery rate of nickel decreased to 78.13%. Compared with the coke size of −1 + 0.5 mm, the nickel grade increased by 0.23% and the recovery rate decreased by 3.9%. It is further indicated that the coke size of −1 + 0.5 mm is more suitable; a ferronickel concentrate with a nickel grade of 4.25%, iron content of 17.02%, and nickel recovery of 82.03% was obtained.
3.6. Effect of Iron Concentrate Dosage
A certain amount of activator can be added in the roasting process to promote the favorable chemical reaction in the direction of the forward reaction and improve the metallurgical efficiency. In the segregation roasting process, the purpose of adding activator is to promote the conversion rate of valuable metal nickel, so as to realize the effective separation of nickel. Nickel and iron have strong magnetic properties. At higher temperatures, nickel is easily dissolved in iron oxides to form a solid solution. Different types of iron oxides have different solubility in nickel [
18,
19,
20].
In the iron-oxygen system, there are three oxides FeO, Fe
3O
4, and Fe
2O
3, and the theoretical oxygen content is 22.8%, 27.64%, and 30.06%, respectively. In the process of iron ore smelting, when the temperature is higher than 570 °C, according to the state phase diagram of the Fe-O system, FeO will be produced. Relevant studies believe that Fe
3O
4 is the solid solution in FeO. Fe
3O
4 is a molten compound with a melting point of 1597 °C. When the temperature is below 1100 °C, it is a pure substance, and at higher temperatures, the oxygen content changes. Before the temperature is higher than 1452 °C, as the temperature increases, the amount of dissolved Fe
2O
3 increases, so the oxygen content in the solid solution increases. Therefore, hematite concentrate was added as an activator in this experiment to investigate the extraction effect of nickel with different dosages [
21,
22,
23,
24,
25,
26].
The results in
Figure 5b reveal that the addition of iron concentrate has a very significant effect on the extraction of nickel, as the nickel grade of nickel iron can be significantly improved, and the iron content in nickel iron is also greatly increased. When 30% hematite concentrate is added, the highest nickel grade of ferronickel is 15.26% and the iron content is 72.65%. The 30% hematite concentrate was found to be the optimum condition for a better Ni grade and recovery rates. This shows that 30% hematite concentrate is ideal; a ferronickel concentrate with a nickel grade of 15.26%, iron content of 72.65%, and nickel recovery of 80.45% was obtained.
3.7. Effect of Magnetic Separation Conditions of Roasting Ores
3.7.1. Effect of Grinding Fineness
The effects of the grinding fineness of segregation roasting ores on the extraction of nickel are shown in
Figure 6a. Nickel silicate ore was processed by segregation roasting, and the valuable metal nickel was absorbed on the surface of coke by a chlorination reduction and the metal particles were small. It is necessary to basically dissociate the mineral after grinding before magnetic separation, and the fineness of grinding is an important index of the dissociation of the monomer. Improving the fineness of grinding and the degree of mineral dissociation is beneficial to the separation of nickel and iron in the magnetic separation process. However, it is easy to produce an over-grinding phenomenon when the grinding is too fine, and the energy consumption is said to be increased, which is not conducive to the recovery of nickel and iron.
Minerals are more affected by the mechanical external forces in the magnetic separation process with the decrease of the particle size. It is necessary to change the magnetic field strength to overcome the mechanical external forces. Taking all things into consideration, the grinding fineness of <0.045 mm occupying 90% is suitable; a ferronickel concentrate with a nickel grade of 18.23%, iron content of 75.22%, and nickel recovery of 78.91% was obtained.
3.7.2. Effect of Magnetic Field Intensity
In order to further improve the separation index of ferronickel concentrate, it is necessary to investigate the extraction and separation effect of ferronickel with a different magnetic field intensity. The results in
Figure 6b show that the nickel grade decreases, and the nickel recovery increases with the magnetic field intensity increasing. The magnetic field intensity of
H = 0.10 T was selected, and a ferronickel concentrate with nickel grade of 15.56%, iron content of 73.22%, and nickel recovery of 89.52% was obtained.
3.8. Scale-Up Test of Roasting–Magnetic Separation
To further investigate the process conditions for repeatability, a scale-up test was carried out under the comprehensive condition used: Segregation roasting temperature of 1100 °C, segregation roasting time of 90 min, calcium chloride dosage of 15%, iron concentrate dosage of 30%, coke dosage of 15%, coke size of −1 + 0.5 mm, magnetic separation grinding fineness of <45 μm occupying 90%, and magnetic separation magnetic field intensity of H = 0.10 T.
A mass of 500 g was used for each scale-up repeat test, and the results are shown in
Table 4. These results show that the index of the repeated experiments is superior to the conditions of the results of the extraction index of nickel is better than the condition test index. Main element analysis also reports the ferronickel concentrate (
Table 5), and the valuable cobalt was enriched to 0.42% in the nickel iron product. Nickel, iron, and cobalt will be respectively extracted by a subsequent smelting process.
3.9. Nickel and Iron Phase Transformation Mechanism in the Roasting Process
According to the mineral analysis of the garnierite laterite ore, the main mineral phases in the garnierite laterite ores were K
2O·Al
2O
3·6SiO
2, Mg
2Si
2O
6, Al
2O
3·2SiO
2·4H
2O, Al
2Si
2O
5(OH)
4, FeOOH, Ca
2Mg
5Si
8O
22(OH)
2, and (Ni,Mg)O·SiO
2·
nH
2O. The main mineral phases in the iron concentrate were Fe
2O
3 and SiO
2, The main chemical reactions that may occur in the segregation roasting system are shown in Equations (3)–(19):
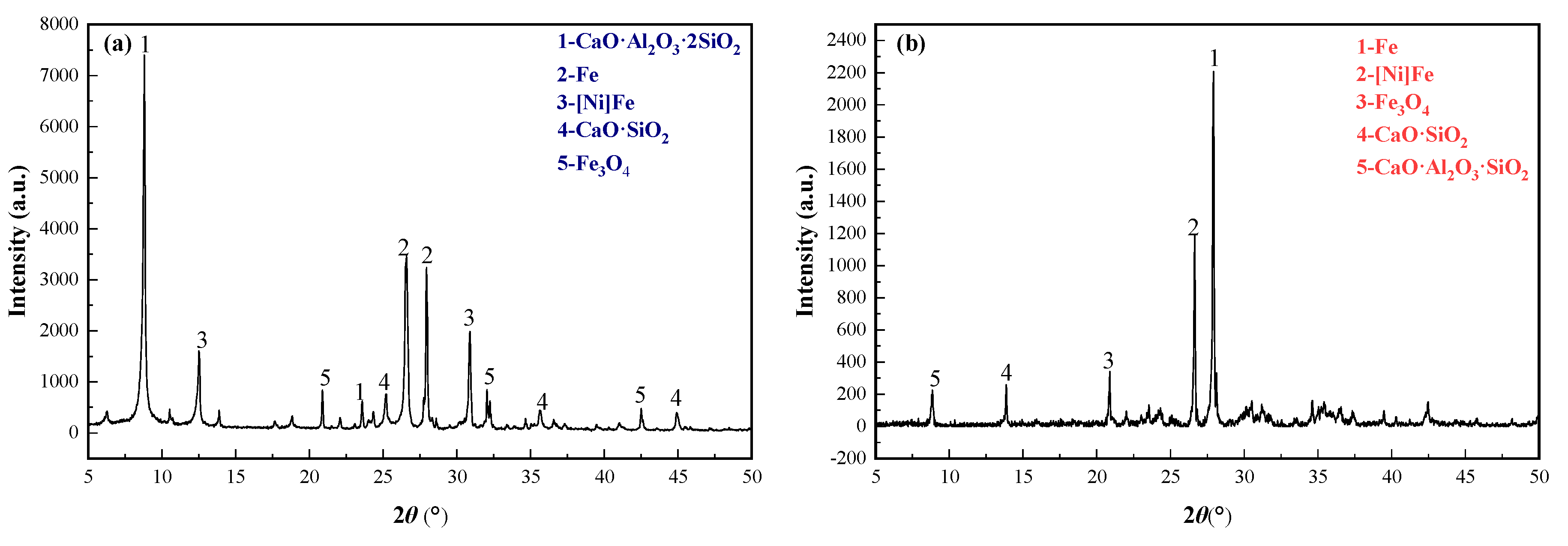
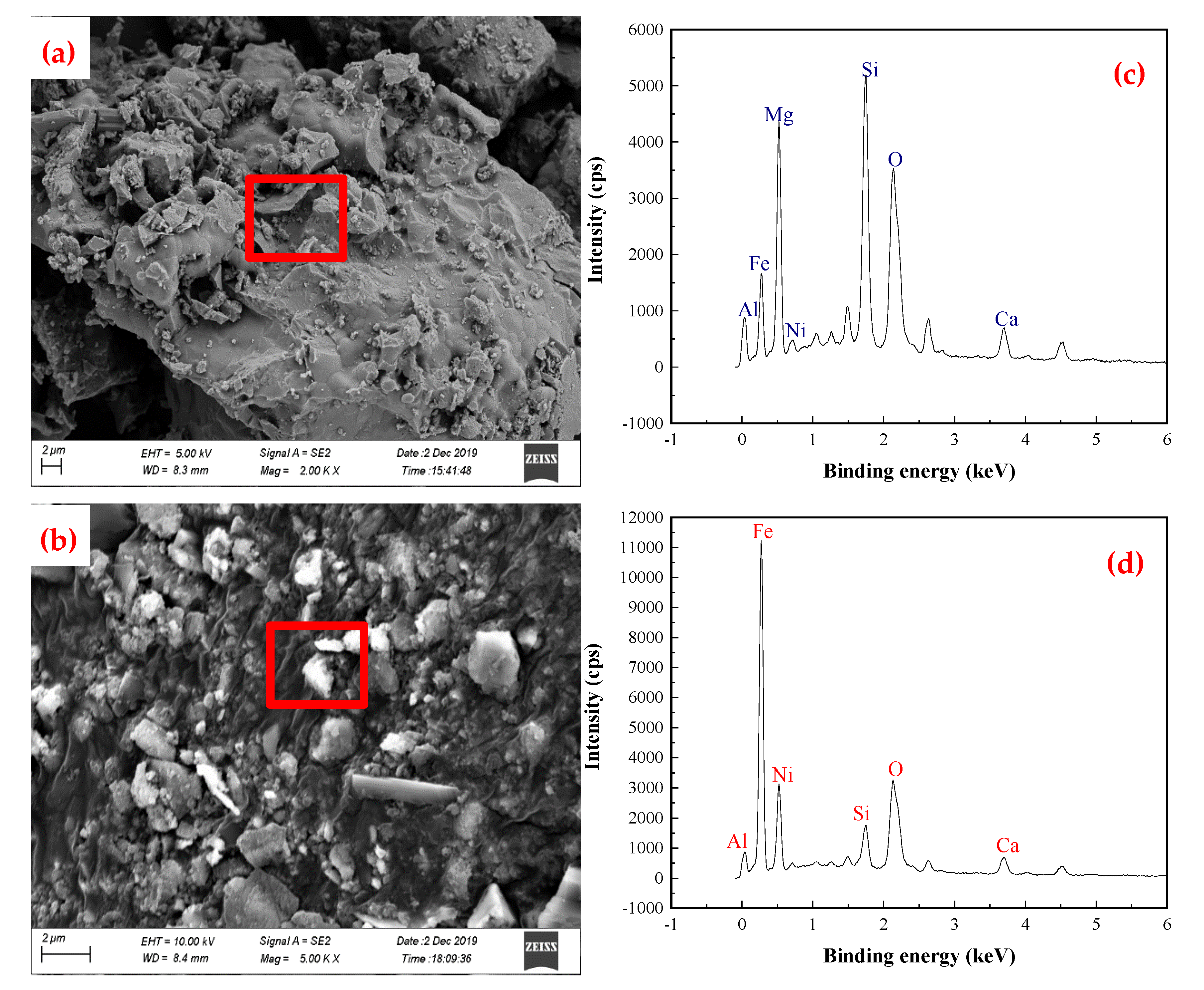
X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive X-ray spectroscopy (EDS) were used to characterize the segregation roasting ores and ferronickel, and analyzing the phase transformation of nickel and iron in the segregation roasting process. The XRD analysis results of the segregation roasting ores and ferronickel concentrate are shown in
Figure 7, and the SEM-EDS analysis results of the segregation roasting ores and ferronickel are shown in
Figure 8.
The results in
Figure 7 and
Figure 8 show that after the segregation roasting of garnierite laterite ore, nickel changes from (Ni,Mg)O·SiO
2·
nH
2O to a new nickel mineral phase dominated by an [Ni]Fe solid solution; and iron changes from Fe
2O
3 and FeOOH to a new iron mineral phase dominated by metal Fe and Fe
3O
4. Nickel and iron belong to ferromagnetic metals. Magnetite belongs to strong magnetic minerals, and their specific magnetization coefficients are different to some extent. The main minerals in the ferronickel concentrate obtained from the segregation roasting ore after grinding were Fe, [Ni]Fe, Fe
3O
4, and SiO
2. In the process of magnetic separation, a small amount of gangue minerals, such as CaO·SiO
2 and CaO·Al
2O
3·SiO
2, entered into the ferronickel concentrate as a result of mechanical entrainment. In the segregation roasting system, the relationship between the difficulties in the reduction of metal nickel and metal iron chloride is FeCl
3 < NiCl
2, and nickel is a kind of iron-soluble oxide. FeCl
3 itself is a kind of chlorinating agent, promoting the chlorination and segregation of nickel, and forms the solid solution of [Ni]Fe.
4. Conclusions
Based on the results obtained in this work, we drew the following conclusions:
(1) The garnierite laterite ore sample was collected in the Mojiang area of China. The garnierite laterite ore contained Ni 0.72%, Co 0.029%, Fe 8.65%, MgO 29.66%, and SiO2 37.86%. Garnierite was the Ni-bearing nickel mineral in the garnierite laterite ore. Potash feldspar, forsterite, tremolite, halloysite, quartz, and kaolinite are the other gangue minerals in the garnierite laterite ore.
(2) A process of segregation roasting–magnetic separation was used to process the garnierite laterite ore. The ferronickel concentrate with Ni of 16.16%, Fe 73.67%, and nickel recovery of 90.33% was obtained under the conditions used: A segregation roasting temperature of 1100 °C, a segregation roasting time of 90 min, a calcium chloride dosage of 15%, an iron concentrate dosage of 30%, a coke dosage of 15%, a coke size of −1 + 0.5 mm, a magnetic separation grinding fineness of <45 μm occupying 90%, and a magnetic separation magnetic field intensity of H = 0.10 T.
(3) The results of the nickel and iron phase transformations show that nickel is transformed from (Ni,Mg)O·SiO2·nH2O to a new nickel mineral phase dominated by the [Ni]Fe solid solution. Iron changed from Fe2O3 and FeOOH to a new iron mineral phase dominated by metal Fe and Fe3O4 after segregation roasting.
(4) XRD, SEM, and EDS analysis of the segregation roasting ores and ferronickel concentrate show that the main minerals in ferronickel concentrate are Fe, [Ni]Fe, and a small amount of Fe3O4; a small amount of gangue minerals, such as CaO·SiO2 and CaO·Al2O3·SiO2, are present in the ferronickel concentrate because of mechanical entrainment in the magnetic separation process.