Metasomatic Evolution of Coesite-Bearing Diamondiferous Eclogite from the Udachnaya Kimberlite
Abstract
:1. Introduction
2. Analytical Methods
3. Geological Setting
4. Results
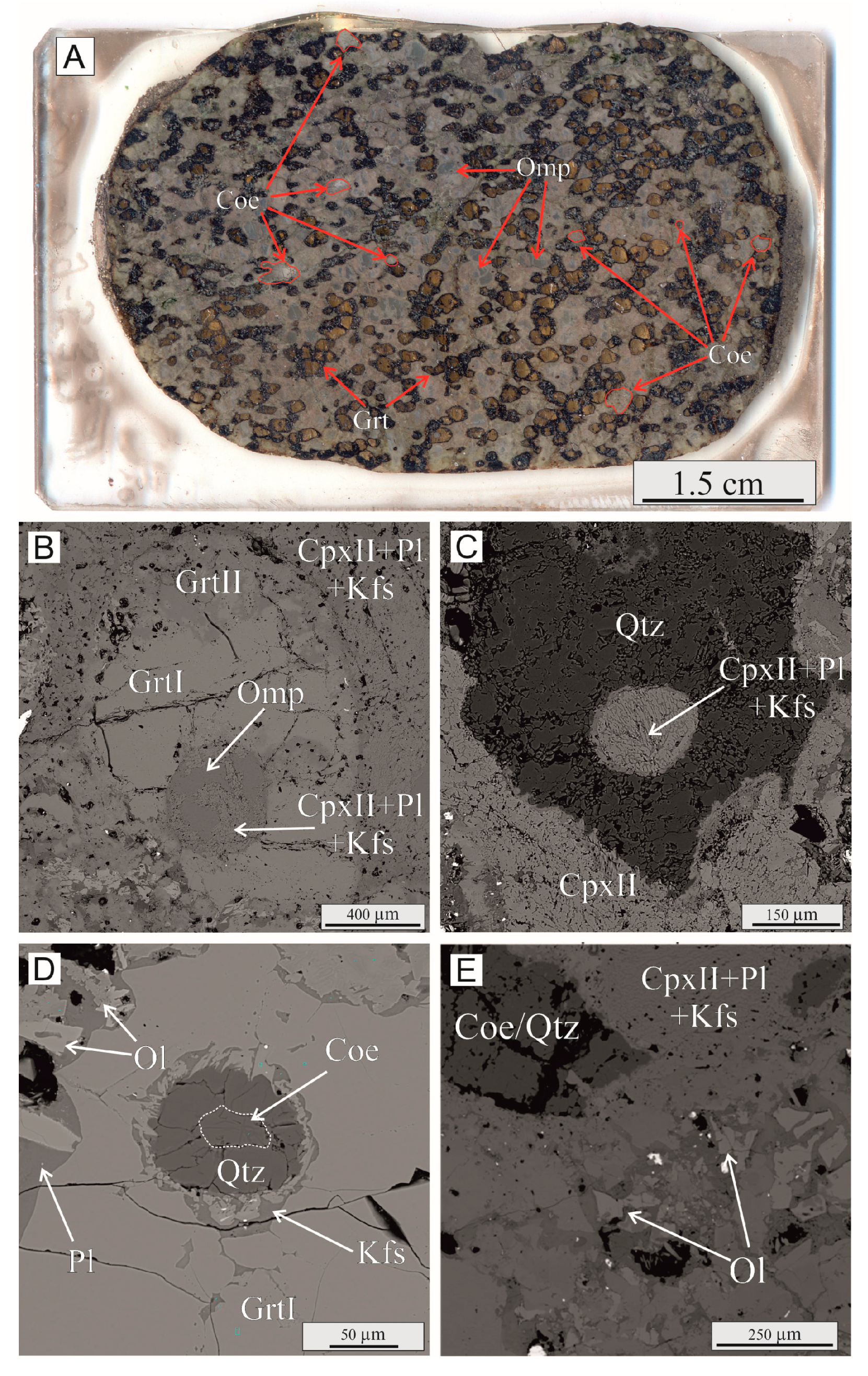
4.1. Petrography and Major-Element Composition
4.2. Trace-Element and Isotopic Composition
4.2.1. Clinopyroxene
4.2.2. Garnet
4.3. Geothermobarometry
4.4. Bulk Rock Composition
5. Discussion
5.1. Formation of a Mixed Paragenesis during Metasomatism
5.2. Petrogenesis of Coesite-bearing Eclogite Uv-537
5.2.1. Protolith Reconstruction and Metamorphic Evolution
5.2.2. Formation of Olivine and Associated Secondary Minerals
5.2.3. Formation of Heterogeneous Garnet
5.2.4. Metasomatic Reactions of Primitive Melt with Eclogite
6. Summary and Conclusions
- The reconstructed bulk composition of the diamond-coesite eclogite sample Uv-537 indicates its origin from common oceanic protoliths such as mafic gabbroic cumulates which typically form part of the lower crust in mid-ocean ridges. The unusually high contents of K2O in omphacitic clinopyroxene and Na2O in garnet most likely result as a combination of subsequent transport to high pressures (≥6 GPa) and metasomatism of eclogite by an alkaline fluid/melt.
- Omphacite from the Uv-537 eclogite has a high measured 87Sr/86Sr ratio (0.7036) that cannot be produced from the in situ decay of the low amount of Rb present (87Rb/86Sr of 0.0013) even for an assumed protolith age of 2.9 Ga. The 87Sr/86Sr is, however, similar to the lower end of 87Sr/86Sr determined for the host kimberlite and therefore suggested to reflect isotopic equilibration with, or Sr addition from, a melt with kimberlite affinity.
- Zoning in garnet is ascribed to reaction with a percolated and hybridized kimberlite melt shortly before eruption. The Zr/Y ratio (6 times greater in the rim than in the core of garnet grains) records precipitation of the garnet rim from a kimberlite-related melt having high Zr/Y. As mineral zoning cannot persist for extended timescales at upper mantle temperatures, it must reflect processes immediately preceding kimberlite eruption.
- The secondary mineral assemblage is formed by reaction of eclogite with derivates of the primary kimberlite melt. Formation of Mg-rich rims in garnet and Mg-rich cores in olivine results from reaction of alkali-rich kimberlite-related melt with eclogite rock residing in the mantle lithosphere beneath Udachnaya. Fe-rich olivine rims, quartz rim around coesite, symplectite after primary omphacite (secondary clinopyroxene, plagioclase, K-feldspar), djerfisherite, sodalite, possibly orthopyroxene and garnet kelyphitic rims were produced by the latest metasomatic reactions during eclogite transport by the kimberlite melt.
- The preservation of coesite, along with very fast ascent of the kimberlite melt, supports very low H2O contents in the latter. The water-poor kimberlite melt composition is confirmed by the lack of serpentine in the groundmass of a fresh kimberlite sample from the Udachnaya-East pipe [79,80,132,134].
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snyder, G.A.; Taylor, L.A.; Jerde, E.A.; Clayton, R.N.; Mayeda, T.K.; Deines, P.; Rossman, G.R.; Sobolev, N.V. Archean mantle heterogeneity and the origin of diamondiferous eclogites, Siberia: Evidence from stable isotopes and hydroxyl in garnet. Am. Mineral. 1995, 80, 799–809. [Google Scholar] [CrossRef]
- Jacob, D.E. Nature and origin of eclogite xenoliths from kimberlites. Lithos 2004, 77, 295–316. [Google Scholar] [CrossRef]
- Aulbach, S.; Jacob, D.E. Major- and trace-elements in cratonic mantle eclogites and pyroxenites reveal heterogeneous sources and metamorphic processing of low-pressure protoliths. Lithos 2016, 262, 586–605. [Google Scholar] [CrossRef]
- Griffin, W.L.; O’Reilly, S.Y. Cratonic lithospheric mantle: Is anything subducted? Episodes 2007, 30, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatton, C.J.; Gurney, J.J. Roberts Victor eclogites and their relation to the mantle. In Mantle Xenoliths; Nixon, P.H., Ed.; John Wiley & Sons: New York, NY, USA, 1987; pp. 453–463. [Google Scholar]
- Barth, M.G.; Rudnick, R.L.; Horn, I.; McDonough, W.F.; Spicuzza, M.J.; Valley, J.W.; Haggerty, S.E. Geochemistry of xenolithic eclogites from West Africa, Part I: A link between low MgO eclogites and Archean crust formation. Geochim. Cosmochim. Acta 2001, 65, 1499–1527. [Google Scholar] [CrossRef]
- Taylor, L.A.; Anand, M. Diamonds: Time capsules from the Siberian Mantle. Chem. Der Erde-Geochem. 2004, 64, 1–74. [Google Scholar] [CrossRef]
- Liu, Y.; Taylor, L.A.; Sarbadhikari, A.B.; Valley, J.W.; Ushikubo, T.; Spicuzza, M.J.; Kita, N.; Ketcham, R.A.; Carlson, W.; Shatsky, V. Metasomatic origin of diamonds in the world’s largest diamondiferous eclogite. Lithos 2009, 112, 1014–1024. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J. The origin of cratonic diamonds—Constraints from mineral inclusions. Ore Geol. Rev. 2008, 34, 5–32. [Google Scholar] [CrossRef]
- Stepanov, A.; Shatsky, V.; Zedgenizov, D.; Sobolev, N. Causes of variations in morphology and impurities of diamonds from the Udachnaya Pipe eclogite. Russ. Geol. Geophys. 2007, 48, 758–769. [Google Scholar] [CrossRef]
- Stachel, T.; Luth, R.W. Diamond formation—Where, when and how? Lithos 2015, 220, 200–220. [Google Scholar] [CrossRef]
- Sobolev, N.V. Deep-Seated Inclusions in Kimberlites and the Problem of the Composition of the Upper Mantle; Amer Geophysical Union: Washington, DC, USA, 1977. [Google Scholar]
- Meyer, H. Mantle Xenoliths; Wiley: Chichester, UK, 1987. [Google Scholar]
- Sobolev, N.; Taylor, L.; Zuev, V.; Bezborodov, S.; Snyder, G.; Sobolev, V.; Yefimova, E. The specific features of eclogitic paragenesis of diamonds from Mir and Udachnaya kimberlite pipes (Yakutia). Russ. Geol. Geophys. 1998, 39, 1667–1678. [Google Scholar]
- Schulze, D.J.; Valley, J.W.; Spicuzza, M.J. Coesite eclogites from the Roberts Victor kimberlite, South Africa. Lithos 2000, 54, 23–32. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Zedgenizov, D.A.; Pokhilenko, N.P.; Malygina, E.V.; Kuzmin, D.V.; Sobolev, A.V. Petrogenetic significance of minor elements in olivines from diamonds and peridotite xenoliths from kimberlites of Yakutia. Lithos 2009, 112, 701–713. [Google Scholar] [CrossRef]
- Prinz, M.; Mansoni, D.V.; Hlava, P.F.; Keil, K. Inclusions in diamonds: Garnet lherzolite and eclogite assemblages. In Physics and Chemistry of the Earth; Elsevier: Amsterdam, The Netherlands, 1975; pp. 797–815. [Google Scholar]
- Hall, A.; Smith, C.B. Lamproite diamonds-are they different. Kimberl. Occur. Orig. A Basis Concept. Models Explor. 1984, 8, 167–212. [Google Scholar]
- Moore, R.O.; Gurney, J.J. Mineral inclusions in diamonds from the Monastery kimberlite, South Africa. In Proceedings of the International Kimberlite Conference: Extended Abstracts, Perth, Australia, 11–15 August 1986; pp. 1406–1408. [Google Scholar]
- Otter, M.L.; Gurney, J.J. Mineral inclusions in diamonds from the Sloan diatremes, Colorado-Wyoming State Line kimberlite district, North America. Kimberl. Relat. Rocks 1989, 2, 1042–1053. [Google Scholar]
- Bulanova, G.P. The formation of diamond. J. Geochem. Explor. 1995, 53, 1–23. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib. Mineral. Petrol. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Wang, W. Formation of diamond with mineral inclusions of “mixed” eclogite and peridotite paragenesis. Earth Planet. Sci. Lett. 1998, 160, 831–843. [Google Scholar] [CrossRef]
- Ragozin, A.L.; Shatsky, V.S.; Zedgenizov, D.A.; Mityukhin, S.I. Evidence for evolution of diamond crystallization medium in eclogite xenolith from the Udachnaya kimberlite pipe, Yakutia. Dokl. Earth Sci. 2006, 407, 465–468. [Google Scholar] [CrossRef]
- Gubanov, N.; Zedgenizov, D.; Sharygin, I.; Ragozin, A. Origin and Evolution of High-Mg Carbonatitic and Low-Mg Carbonatitic to Silicic High-Density Fluids in Coated Diamonds from Udachnaya Kimberlite Pipe. Minerals 2019, 9, 734. [Google Scholar] [CrossRef] [Green Version]
- Yaxley, G.M.; Green, D.H.; Kamenetsky, V. Carbonatite metasomatism in the southeastern Australian lithosphere. J. Petrol. 1998, 39, 1917–1930. [Google Scholar] [CrossRef]
- Izraeli, E.S.; Harris, J.W.; Navon, O. Brine inclusions in diamonds: A new upper mantle fluid. Earth Planet. Sci. Lett. 2001, 187, 323–332. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Mantle fluid evolution—A tale of one diamond. Lithos 2004, 77, 243–253. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Fluid inclusions in diamonds from the Diavik mine, Canada and the evolution of diamond-forming fluids. Geochim. Cosmochim. Acta 2007, 71, 723–744. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Logvinova, A.M.; Schrauder, M.; Spetius, Z.V.; Weiss, Y.; Hauri, E.H.; Kaminsky, F.V.; Sobolev, N.V.; Navon, O. High-Mg carbonatitic microinclusions in some Yakutian diamonds—A new type of diamond-forming fluid. Lithos 2009, 112, 648–659. [Google Scholar] [CrossRef]
- Weiss, Y.; Kessel, R.; Griffin, W.; Kiflawi, I.; Klein-BenDavid, O.; Bell, D.; Harris, J.; Navon, O. A new model for the evolution of diamond-forming fluids: Evidence from microinclusion-bearing diamonds from Kankan, Guinea. Lithos 2009, 112, 660–674. [Google Scholar] [CrossRef]
- Zedgenizov, D.; Rege, S.; Griffin, W.; Kagi, H.; Shatsky, V. Composition of trapped fluids in cuboid fibrous diamonds from the Udachnaya kimberlite: LA-ICP-MS analysis. Chem. Geol. 2007, 240, 151–162. [Google Scholar] [CrossRef]
- Zedgenizov, D.; Ragozin, A.; Shatsky, V.; Araujo, D.; Griffin, W.L.; Kagi, H. Mg and Fe-rich carbonate–Silicate high-density fluids in cuboid diamonds from the Internationalnaya kimberlite pipe (Yakutia). Lithos 2009, 112, 638–647. [Google Scholar] [CrossRef]
- Zedgenizov, D.; Ragozin, A.; Shatsky, V.; Griffin, W. Diamond formation during metasomatism of mantle eclogite by chloride-carbonate melt. Contrib. Mineral. Petrol. 2018, 173, 84. [Google Scholar] [CrossRef]
- Sobolev, N.; Kaminsky, F.; Griffin, W.; Yefimova, E.; Win, T.; Ryan, C.; Botkunov, A. Mineral inclusions in diamonds from the Sputnik kimberlite pipe, Yakutia. Lithos 1997, 39, 135–157. [Google Scholar] [CrossRef]
- Leost, I.; Stachel, T.; Brey, G.; Harris, J.; Ryabchikov, I. Diamond formation and source carbonation: Mineral associations in diamonds from Namibia. Contrib. Mineral. Petrol. 2003, 145, 15–24. [Google Scholar] [CrossRef]
- Kaminsky, F.; Wirth, R.; Matsyuk, S.; Schreiber, A.; Thomas, R. Nyerereite and nahcolite inclusions in diamond: Evidence for lower-mantle carbonatitic magmas. Mineral. Mag. 2009, 73, 797–816. [Google Scholar] [CrossRef] [Green Version]
- Kaminsky, F. Mineralogy of the lower mantle: A review of ‘super-deep’mineral inclusions in diamond. Earth-Sci. Rev. 2012, 110, 127–147. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Wirth, R.; Schreiber, A. A microinclusion of lower-mantle rock and other mineral and nitrogen lower-mantle inclusions in a diamond. Can. Mineral. 2015, 53, 83–104. [Google Scholar] [CrossRef]
- Logvinova, A.; Wirth, R.; Zedgenizov, D.; Taylor, L. Carbonate–Silicate–Sulfide Polyphase Inclusion in Diamond from the Komsomolskaya Kimberlite Pipe, Yakutia. Geochem. Int. 2018, 56, 283–291. [Google Scholar] [CrossRef]
- Ionov, D.A.; Dupuy, C.; O’Reilly, S.Y.; Kopylova, M.G.; Genshaft, Y.S. Carbonated peridotite xenoliths from Spitsbergen: Implications for trace element signature of mantle carbonate metasomatism. Earth Planet. Sci. Lett. 1993, 119, 283–297. [Google Scholar] [CrossRef]
- Kogarko, L.; Henderson, C.; Pacheco, H. Primary Ca-rich carbonatite magma and carbonate-silicate-sulphide liquid immiscibility in the upper mantle. Contrib. Mineral. Petrol. 1995, 121, 267–274. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Kamenetsky, V.; Green, D.H.; Falloon, T.J. Glasses in mantle xenoliths from western Victoria, Australia and their relevance to mantle processes. Earth Planet. Sci. Lett. 1997, 148, 433–446. [Google Scholar] [CrossRef]
- Laurora, A.; Mazzucchelli, M.; Rivalenti, G.; Vannucci, R.; Zanetti, A.; Barbieri, M.A.; Cingolani, C.A. Metasomatism and melting in carbonated peridotite xenoliths from the mantle wedge: The Gobernador Gregores case (Southern Patagonia). J. Petrol. 2001, 42, 69–87. [Google Scholar] [CrossRef]
- Misra, K.C.; Anand, M.; Taylor, L.A.; Sobolev, N.V. Multi-stage metasomatism of diamondiferous eclogite xenoliths from the Udachnaya kimberlite pipe, Yakutia, Siberia. Contrib. Mineral. Petrol. 2004, 146, 696–714. [Google Scholar] [CrossRef]
- Moine, B.; Grégoire, M.; O’Reilly, S.Y.; Delpech, G.; Sheppard, S.; Lorand, J.; Renac, C.; Giret, A.; Cottin, J. Carbonatite melt in oceanic upper mantle beneath the Kerguelen Archipelago. Lithos 2004, 75, 239–252. [Google Scholar] [CrossRef]
- Ionov, D.A.; Doucet, L.S.; Xu, Y.; Golovin, A.V.; Oleinikov, O.B. Reworking of Archean mantle in the NE Siberian craton by carbonatite and silicate melt metasomatism: Evidence from a carbonate-bearing, dunite-to-websterite xenolith suite from the Obnazhennaya kimberlite. Geochim. Cosmochim. Acta 2018, 224, 132–153. [Google Scholar] [CrossRef]
- Ionov, D.A.; Qi, Y.-H.; Kang, J.-T.; Golovin, A.V.; Oleinikov, O.B.; Zheng, W.; Anbar, A.D.; Zhang, Z.-F.; Huang, F. Calcium isotopic signatures of carbonatite and silicate metasomatism, melt percolation and crustal recycling in the lithospheric mantle. Geochim. Cosmochim. Acta 2019, 248, 1–13. [Google Scholar] [CrossRef]
- McGetchin, T.R.; Besancon, J. Carbonate inclusions in mantle-derived pyropes. Earth Planet. Sci. Lett. 1973, 18, 408–410. [Google Scholar] [CrossRef]
- Smith, D. Genesis of carbonate in pyrope from ultramafic diatremes on the Colorado Plateau, southwestern United States. Contrib. Mineral. Petrol. 1987, 97, 389–396. [Google Scholar] [CrossRef]
- Rezvukhin, D.I.; Malkovets, V.G.; Sharygin, I.S.; Tretiakova, I.G.; Griffin, W.L.; O’Reilly, S.Y. Inclusions of crichtonite-group minerals in Cr-pyropes from the Internatsionalnaya kimberlite pipe, Siberian Craton: Crystal chemistry, parageneses and relationships to mantle metasomatism. Lithos 2018, 308, 181–195. [Google Scholar] [CrossRef]
- Araújo, D.; Griffin, W.L.; O’Reilly, S.Y. Mantle melts, metasomatism and diamond formation: Insights from melt inclusions in xenoliths from Diavik, Slave Craton. Lithos 2009, 112, 675–682. [Google Scholar] [CrossRef]
- Giuliani, A.; Kamenetsky, V.S.; Phillips, D.; Kendrick, M.; Wyatt, B.; Goemann, K. Nature of alkali-carbonate fluids in the sub-continental lithospheric mantle. Geology 2012, 40, 967–970. [Google Scholar] [CrossRef]
- Golovin, A.V.; Sharygin, I.S.; Korsakov, A.V. Origin of alkaline carbonates in kimberlites of the Siberian craton: Evidence from melt inclusions in mantle olivine of the Udachnaya-East pipe. Chem. Geol. 2017, 455, 357–375. [Google Scholar] [CrossRef]
- Golovin, A.V.; Sharygin, I.S.; Kamenetsky, V.S.; Korsakov, A.V.; Yaxley, G.M. Alkali-carbonate melts from the base of cratonic lithospheric mantle: Links to kimberlites. Chem. Geol. 2018, 483, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Golovin, A.; Sharygin, I.; Korsakov, A.; Kamenetsky, V.; Abersteiner, A. Can primitive kimberlite melts be alkali-carbonate liquids: Composition of the melt snapshots preserved in deepest mantle xenoliths. J. Raman Spectrosc. 2019, in press. [Google Scholar] [CrossRef]
- van Achterbergh, E.; Griffin, W.L.; Ryan, C.G.; O’Reilly, S.Y.; Pearson, N.J.; Kivi, K.; Doyle, B.J. Subduction signature for quenched carbonatites from the deep lithosphere. Geology 2002, 30, 743–746. [Google Scholar] [CrossRef]
- Bussweiler, Y.; Stone, R.S.; Pearson, D.G.; Luth, R.W.; Stachel, T.; Kjarsgaard, B.A.; Menzies, A. The evolution of calcite-bearing kimberlites by melt-rock reaction: Evidence from polymineralic inclusions within clinopyroxene and garnet megacrysts from Lac de Gras kimberlites, Canada. Contrib. Mineral. Petrol. 2016, 171, 65. [Google Scholar] [CrossRef]
- Abersteiner, A.; Kamenetsky, V.S.; Goemann, K.; Golovin, A.V.; Sharygin, I.S.; Giuliani, A.; Rodemann, T.; Spetsius, Z.V.; Kamenetsky, M. Djerfisherite in kimberlites and their xenoliths: Implications for kimberlite melt evolution. Contrib. Mineral. Petrol. 2019, 174, 8. [Google Scholar] [CrossRef]
- Bussweiler, Y. Polymineralic Inclusions in Megacrysts as Proxies for Kimberlite Melt Evolution—A Review. Minerals 2019, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Abersteiner, A.; Kamenetsky, V.S.; Goemann, K.; Golovin, A.V.; Sharygin, I.S.; Pearson, D.G.; Kamenetsky, M.; Gornova, M.A. Polymineralic inclusions in kimberlite-hosted megacrysts: Implications for kimberlite melt evolution. Lithos 2019, 336, 310–325. [Google Scholar] [CrossRef]
- Agashev, A.; Ionov, D.; Pokhilenko, N.; Golovin, A.; Cherepanova, Y.; Sharygin, I. Metasomatism in lithospheric mantle roots: Constraints from whole-rock and mineral chemical composition of deformed peridotite xenoliths from kimberlite pipe Udachnaya. Lithos 2013, 160, 201–215. [Google Scholar] [CrossRef]
- Aulbach, S.; Griffin, W.L.; Pearson, N.J.; O’Reilly, S.Y. Nature and timing of metasomatism in the stratified mantle lithosphere beneath the central Slave craton (Canada). Chem. Geol. 2013, 352, 153–169. [Google Scholar] [CrossRef]
- Rudnick, R.L.; McDonough, W.F.; Chappell, B.W. Carbonatite metasomatism in the northern Tanzanian mantle: Petrographic and geochemical characteristics. Earth Planet. Sci. Lett. 1993, 114, 463–475. [Google Scholar] [CrossRef]
- Shu, Q.; Brey, G.P. Ancient mantle metasomatism recorded in subcalcic garnet xenocrysts: Temporal links between mantle metasomatism, diamond growth and crustal tectonomagmatism. Earth Planet. Sci. Lett. 2015, 418, 27–39. [Google Scholar] [CrossRef]
- Wallace, M.E.; Green, D.H. An experimental determination of primary carbonatite magma composition. Nature 1988, 335, 343. [Google Scholar] [CrossRef]
- Dalton, J.A.; Wood, B.J. The compositions of primary carbonate melts and their evolution through wallrock reaction in the mantle. Earth Planet. Sci. Lett. 1993, 119, 511–525. [Google Scholar] [CrossRef]
- Safonov, O.G.; Perchuk, L.L.; Litvin, Y.A. Melting relations in the chloride–carbonate–silicate systems at high-pressure and the model for formation of alkalic diamond–forming liquids in the upper mantle. Earth Planet. Sci. Lett. 2007, 253, 112–128. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; McDonough, W.F.; Spiegelman, M.; Withers, A.C. Trace element partitioning between garnet lherzolite and carbonatite at 6.6 and 8.6 GPa with applications to the geochemistry of the mantle and of mantle-derived melts. Chem. Geol. 2009, 262, 57–77. [Google Scholar] [CrossRef]
- Dasgupta, R.; Mallik, A.; Tsuno, K.; Withers, A.C.; Hirth, G.; Hirschmann, M.M. Carbon-dioxide-rich silicate melt in the Earth’s upper mantle. Nature 2013, 493, 211. [Google Scholar] [CrossRef]
- Litasov, K.D.; Shatskiy, A.; Ohtani, E.; Yaxley, G.M. Solidus of alkaline carbonatite in the deep mantle. Geology 2013, 41, 79–82. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Sharygin, I.S.; Ohtani, E. Composition of primary kimberlite melt in a garnet lherzolite mantle source: Constraints from melting phase relations in anhydrous Udachnaya-East kimberlite with variable CO2 content at 6.5 GPa. Gondwana Res. 2017, 45, 208–227. [Google Scholar] [CrossRef]
- Jacob, D.; Jagoutz, E.; Lowry, D.; Mattey, D.; Kudrjavtseva, G. Diamondiferous eclogites from Siberia: Remnants of Archean oceanic crust. Geochim. Cosmochim. Acta 1994, 58, 5191–5207. [Google Scholar] [CrossRef]
- Spetsius, Z.V.; Taylor, L.A. Partial Melting in Mantle Eclogite Xenoliths: Connections with Diamond Paragenesis. Int. Geol. Rev. 2002, 44, 973–987. [Google Scholar] [CrossRef]
- Lavrent’ev, Y.G.; Karmanov, N.; Usova, L. Electron probe microanalysis of minerals: Microanalyzer or scanning electron microscope? Russ. Geol. Geophys. 2015, 56, 1154–1161. [Google Scholar] [CrossRef]
- Aulbach, S.; Viljoen, K. Eclogite xenoliths from the Lace kimberlite, Kaapvaal craton: From convecting mantle source to palaeo-ocean floor and back. Earth Planet. Sci. Lett. 2015, 431, 274–286. [Google Scholar] [CrossRef]
- Aulbach, S.; Gerdes, A.; Viljoen, K. Formation of diamondiferous kyanite–eclogite in a subduction mélange. Geochim. Cosmochim. Acta 2016, 179, 156–176. [Google Scholar] [CrossRef]
- Kharkiv, A.; Zuenko, V.; Zinchuk, N.; Kryuchkov, A.; Ukhanov, V.; Bogatykh, M. Petrochemistry of kimberlites; Nedra: Moscow, Russia, 1991; 304p. [Google Scholar]
- Kamenetsky, V.S.; Golovin, A.V.; Maas, R.; Giuliani, A.; Kamenetsky, M.B.; Weiss, Y. Towards a new model for kimberlite petrogenesis: Evidence from unaltered kimberlites and mantle minerals. Earth-Sci. Rev. 2014, 139, 145–167. [Google Scholar] [CrossRef] [Green Version]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Golovin, A.V.; Sharygin, V.V.; Maas, R. Ultrafresh salty kimberlite of the Udachnaya–East pipe (Yakutia, Russia): A petrological oddity or fortuitous discovery? Lithos 2012, 152, 173–186. [Google Scholar] [CrossRef]
- Morimoto, N. Nomenclature of pyroxenes. Mineral. Petrol. 1988, 39, 55–76. [Google Scholar] [CrossRef]
- Taylor, L.A.; Neal, C.R. Eclogites with Oceanic Crustal and Mantle Signatures from the Bellsbank Kimberlite, South Africa, Part I: Mineralogy, Petrography and Whole Rock Chemistry. J. Geol. 1989, 97, 551–567. [Google Scholar] [CrossRef]
- Coleman, R.G.; Lee, D.E.; Beatty, L.B.; Brannock, W.W. Eclogites and eclogites: Their differences and similarities. Geol. Soc. Am. Bull. 1965, 76, 483–508. [Google Scholar] [CrossRef]
- Nasdala, L.; Steger, S.; Reissner, C. Raman study of diamond-based abrasives and possible artefacts in detecting UHP microdiamond. Lithos 2016, 265, 317–327. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Berry, A.J.; Kamenetsky, V.S.; Woodland, A.B.; Golovin, A.V. An oxygen fugacity profile through the Siberian Craton—Fe K-edge XANES determinations of Fe3+/∑ Fe in garnets in peridotite xenoliths from the Udachnaya East kimberlite. Lithos 2012, 140, 142–151. [Google Scholar] [CrossRef]
- Ionov, D.A.; Doucet, L.S.; von Strandmann, P.A.P.; Golovin, A.V.; Korsakov, A.V. Links between deformation, chemical enrichments and Li-isotope compositions in the lithospheric mantle of the central Siberian craton. Chem. Geol. 2017, 475, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Doucet, L.S.; Ionov, D.A.; Golovin, A.V. The origin of coarse garnet peridotites in cratonic lithosphere: New data on xenoliths from the Udachnaya kimberlite, central Siberia. Contrib. Mineral. Petrol. 2013, 165, 1225–1242. [Google Scholar] [CrossRef]
- Sobolev, N.; Sobolev, A.; Tomilenko, A.; Batanova, V.; Tolstov, A.; Logvinova, A.; Kuz’min, D. Unique compositional peculiarities of olivine phenocrysts from the post flood basalt diamondiferous Malokuonapskaya kimberlite pipe, Yakutia. Dokl. Earth Sci. 2015, 463, 828. [Google Scholar] [CrossRef]
- Sobolev, N.; Sobolev, A.; Tomilenko, A.; Kuz’min, D.; Grakhanov, S.; Batanova, V.; Logvinova, A.; Bul’bak, T.; Kostrovitskii, S.; Yakovlev, D. Prospects of search for diamondiferous kimberlites in the northeastern Siberian Platform. Russ. Geol. Geophys. 2018, 59, 1365–1379. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Sobolev, A.V.; Golovin, A.V.; Demouchy, S.; Faure, K.; Sharygin, V.V.; Kuzmin, D.V. Olivine in the Udachnaya-East kimberlite (Yakutia, Russia): Types, compositions and origins. J. Petrol. 2007, 49, 823–839. [Google Scholar] [CrossRef]
- Sobolev, N.; Sobolev, A.; Tomilenko, A.; Kovyazin, S.; Batanova, V.; Kuz’min, D. Paragenesis and complex zoning of olivine macrocrysts from unaltered kimberlite of the Udachnaya-East pipe, Yakutia: Relationship with the kimberlite formation conditions and evolution. Russ. Geol. Geophys. 2015, 56, 260–279. [Google Scholar] [CrossRef]
- Orlov, U.L. The Mineralogy of the Diamond; John Wiley & Sons: Hoboken, NJ, USA, 1977. [Google Scholar]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Perk, N.W.; Coogan, L.A.; Karson, J.A.; Klein, E.M.; Hanna, H.D. Petrology and geochemistry of primitive lower oceanic crust from Pito Deep: Implications for the accretion of the lower crust at the Southern East Pacific Rise. Contrib. Mineral. Petrol. 2007, 154, 575–590. [Google Scholar] [CrossRef]
- Casey, J.F. Comparison of major-and trace-element geochemistry of abyssal peridotites and mafic plutonic rocks with basalts from the MARK region of the Mid-Atlantic Ridge. Proc. Ocean Drill. Program Sci. Results 1997, 153, 181–241. [Google Scholar]
- Safonov, O.G.; Bindi, L.; Vinograd, V.L. Potassium-bearing clinopyroxene: A review of experimental, crystal chemical and thermodynamic data with petrological applications. Mineral. Mag. 2011, 75, 2467–2484. [Google Scholar] [CrossRef]
- Kushiro, I.; Erlank, A. Stability of potassic richterite. Carnegie Inst. Wash. Yearb. 1970, 68, 231–233. [Google Scholar]
- Xiong, X.; Keppler, H.; Audétat, A.; Gudfinnsson, G.; Sun, W.; Song, M.; Xiao, W.; Yuan, L. Experimental constraints on rutile saturation during partial melting of metabasalt at the amphibolite to eclogite transition, with applications to TTG genesis. Am. Mineral. 2009, 94, 1175–1186. [Google Scholar] [CrossRef]
- Klemme, S.; Prowatke, S.; Hametner, K.; Günther, D. Partitioning of trace elements between rutile and silicate melts: Implications for subduction zones. Geochim. Cosmochim. Acta 2005, 69, 2361–2371. [Google Scholar] [CrossRef]
- Safonov, O.G.; Perchuk, L.L.; Litvin, Y.A. Equilibrium K-bearing clinopyroxene-melt as a model for barometry of mantle-derived mineral assemblages. Russ. Geol. Geophys. 2005, 46, 1318–1334. [Google Scholar]
- Ellis, D.; Green, D. An experimental study of the effect of Ca upon garnet-clinopyroxene Fe-Mg exchange equilibria. Contrib. Mineral. Petrol. 1979, 71, 13–22. [Google Scholar] [CrossRef]
- Beyer, C.; Frost, D.; Miyajima, N. Experimental calibration of a garnet–clinopyroxene geobarometer for mantle eclogites. Contrib. Mineral. Petrol. 2015, 169, 18. [Google Scholar] [CrossRef]
- Pollack, H.N.; Chapman, D.S. On the regional variation of heat flow, geotherms and lithospheric thickness. Tectonophysics 1977, 38, 279–296. [Google Scholar] [CrossRef] [Green Version]
- Heaman, L.M.; Creaser, R.A.; Cookenboo, H.O.; Chacko, T. Multi-stage modification of the Northern Slave mantle lithosphere: Evidence from zircon-and diamond-bearing eclogite xenoliths entrained in Jericho kimberlite, Canada. J. Petrol. 2006, 47, 821–858. [Google Scholar] [CrossRef]
- Jerde, E.A.; Taylor, L.A.; Crozaz, G.; Sobolev, N.V.; Sobolev, V.N. Diamondiferous eclogites from Yakutia, Siberia: Evidence for a diversity of protoliths. Contrib. Mineral. Petrol. 1993, 114, 189–202. [Google Scholar] [CrossRef]
- Benoit, M.; Polvé, M.; Ceuleneer, G. Trace element and isotopic characterization of mafic cumulates in a fossil mantle diapir (Oman ophiolite). Chem. Geol. 1996, 134, 199–214. [Google Scholar] [CrossRef]
- Kopylova, M.G.; Gurney, J.J.; Daniels, L.R. Mineral inclusions in diamonds from the River Ranch kimberlite, Zimbabwe. Contrib. Mineral. Petrol. 1997, 129, 366–384. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.-L. Carbonation and melting reactions in the system CaO–MgO–SiO2–CO2 at mantle pressures with geophysical and petrological applications. Contrib. Mineral. Petrol. 1976, 54, 79–107. [Google Scholar] [CrossRef]
- Schrauder, M.; Navon, O. Solid carbon dioxide in a natural diamond. Nature 1993, 365, 42. [Google Scholar] [CrossRef]
- Frey, F.; Green, D.; Roy, S. Integrated models of basalt petrogenesis: A study of quartz tholeiites to olivine melilitites from south eastern Australia utilizing geochemical and experimental petrological data. J. Petrol. 1978, 19, 463–513. [Google Scholar] [CrossRef]
- Mallmann, G.; O’Neill, H.S.C. The crystal/melt partitioning of V during mantle melting as a function of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). J. Petrol. 2009, 50, 1765–1794. [Google Scholar] [CrossRef]
- Pernet-Fisher, J.F.; Howarth, G.H.; Liu, Y.; Barry, P.H.; Carmody, L.; Valley, J.W.; Bodnar, R.J.; Spetsius, Z.V.; Taylor, L.A. Komsomolskaya diamondiferous eclogites: Evidence for oceanic crustal protoliths. Contrib. Mineral. Petrol. 2014, 167, 981. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Green, D.H. Reactions between eclogite and peridotite: Mantle refertilisation by subduction of oceanic crust. Schweiz. Miner. Petrogr. Mitt 1998, 78, 243–255. [Google Scholar]
- Kopylova, M.; Russell, J.; Cookenboo, H. Petrography and chemistry of the Jericho kimberlite (Slave craton, northern Canada). In Proceedings of the 7th International Kimberlite Conference, Cape Town, South African Republic, 13–18 April 1998; pp. 449–451. [Google Scholar]
- Fung, A. Petrochemistry of upper mantle eclogites from the Grizzly, Leslie, Pigeon and Sable kimberlites in the Slave Province, Canada. In Proceedings of the 7th International Kimberlite Conference, Cape Town, South African Republic, 13–18 April 1998; pp. 230–232. [Google Scholar]
- Safonov, O.G.; Butvina, V.G. Indicator reactions of K and Na activities in the upper mantle: Natural mineral assemblages, experimental data and thermodynamic modeling. Geochem. Int. 2016, 54, 858–872. [Google Scholar] [CrossRef]
- Clarke, D. Synthesis of nickeloan djerfisherites and the origin of potassic sulphides at the Frank Smith Mine. Mantle Sample Incl. Kimberl. Other Volcan. 1979, 16, 300–308. [Google Scholar]
- Gorbachev, N.S.; Nekrasov, I.J. Features of the formation of synthetic and natural potassium sulfides. Dokl. Akad. Nauk SSSR 1980, 251, 682–685. [Google Scholar]
- Sharp, Z.; Helffrich, G.; Bohlen, S.R.; Essene, E.J. The stability of sodalite in the system NaAlSiO4-NaCl. Geochim. Cosmochim. Acta 1989, 53, 1943–1954. [Google Scholar] [CrossRef] [Green Version]
- Dawson, J.B.; Harley, S.L. Some post-equilibrium reactions in kimberlite-derived eclogites Paper A-00081. Lithos 2009, 112, 1025–1031. [Google Scholar] [CrossRef]
- Schulze, D.J. Low-Ca garnet harzburgites from Kimberley, South Africa: Abundance and bearing on the structure and evolution of the lithosphere. J. Geophys. Res. Solid Earth 1995, 100, 12513–12526. [Google Scholar] [CrossRef]
- Griffin, W.L.; Shee, S.R.; Ryan, C.G.; Win, T.T.; Wyatt, B.A. Harzburgite to lherzolite and back again: Metasomatic processes in ultramafic xenoliths from the Wesselton kimberlite, Kimberley, South Africa. Contrib. Mineral. Petrol. 1999, 134, 232–250. [Google Scholar] [CrossRef]
- Griffin, W.L.; Smith, D.; Boyd, F.R.; Cousens, D.R.; Ryan, C.G.; Sie, S.H.; Suter, G.F. Trace-element zoning in garnets from sheared mantle xenoliths. Geochim. Cosmochim. Acta 1989, 53, 561–567. [Google Scholar] [CrossRef]
- Griffin, W.L.; Smith, D.; Ryan, C.G.; O’Reilly, S.Y.; Win, T.T. Trace-element zoning in mantle minerals; metasomatism and thermal events in the upper mantle. Can. Mineral. 1996, 34, 1179–1193. [Google Scholar]
- Jollands, M.C.; Hanger, B.J.; Yaxley, G.M.; Hermann, J.; Kilburn, M.R. Timescales between mantle metasomatism and kimberlite ascent indicated by diffusion profiles in garnet crystals from peridotite xenoliths. Earth Planet. Sci. Lett. 2018, 481, 143–153. [Google Scholar] [CrossRef]
- Walter, M.J. Melting of garnet peridotite and the origin of komatiite and depleted lithosphere. J. Petrol. 1998, 39, 29–60. [Google Scholar] [CrossRef]
- Workman, R.K.; Hart, S.R. Major and trace element composition of the depleted MORB mantle (DMM). Earth Planet. Sci. Lett. 2005, 231, 53–72. [Google Scholar] [CrossRef]
- Pearson, D.; Snyder, G.; Shirey, S.; Taylor, L.; Carlson, R.; Sobolev, N. Archaean Re–Os age for Siberian eclogites and constraints on Archaean tectonics. Nature 1995, 374, 711. [Google Scholar] [CrossRef]
- Aulbach, S.; Heaman, L.M.; Jacob, D.E.; Viljoen, K. Ages and sources of mantle eclogites: ID-TIMS and in situ MC-ICPMS Pb-Sr isotope systematics of clinopyroxene. Chem. Geol. 2019, 503, 15–28. [Google Scholar] [CrossRef]
- Maas, R.; Kamenetsky, M.B.; Sobolev, A.V.; Kamenetsky, V.S.; Sobolev, N.V. Sr, Nd and Pb isotope evidence for a mantle origin of alkali chlorides and carbonates in the Udachnaya kimberlite, Siberia. Geology 2005, 33, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Kamenetsky, M.B.; Sobolev, A.V.; Kamenetsky, V.S.; Maas, R.; Danyushevsky, L.V.; Thomas, R.; Pokhilenko, N.P.; Sobolev, N.V. Kimberlite melts rich in alkali chlorides and carbonates: A potent metasomatic agent in the mantle. Geology 2004, 32, 845–848. [Google Scholar] [CrossRef]
- Golovin, A.; Sharygin, V.; Pokhilenko, N. Melt inclusions in olivine phenocrysts in unaltered kimberlites from the Udachnaya-East pipe, Yakutia: Some aspects of kimberlite magma evolution during late crystallization stages. Petrology 2007, 15, 168–183. [Google Scholar] [CrossRef]
- Abersteiner, A.; Kamenetsky, V.S.; Golovin, A.V.; Kamenetsky, M.; Goemann, K. Was crustal contamination involved in the formation of the serpentine-free Udachnaya-East kimberlite? New insights into parental melts, liquidus assemblage and effects of alteration. J. Petrol. 2018, 59, 1467–1492. [Google Scholar] [CrossRef]
- Mosenfelder, J.L.; Schertl, H.-P.; Smyth, J.R.; Liou, J.G. Factors in the preservation of coesite: The importance of fluid infiltration. Am. Mineral. 2005, 90, 779–789. [Google Scholar] [CrossRef]








| Grt | Grt (core) | Grt (core) | Grt (rim) | Grt (rim) | Omp | Omp |
|---|---|---|---|---|---|---|
| SiO2 | 40.9 | 41.2 | 42.1 | 42.0 | 55.8 | 55.9 |
| TiO2 | 0.68 | 0.72 | 0.42 | 0.43 | 0.73 | 0.73 |
| Al2O3 | 21.8 | 21.9 | 22.1 | 22.3 | 7.9 | 8.0 |
| Cr2O3 | 0.04 | 0.03 | 0.08 | 0.06 | 0.04 | 0.04 |
| FeO | 16.0 | 16.1 | 12.4 | 12.7 | 5.5 | 5.4 |
| MnO | 0.46 | 0.46 | 0.38 | 0.40 | 0.11 | 0.08 |
| MgO | 13.1 | 13.3 | 16.8 | 16.6 | 10.8 | 10.7 |
| CaO | 6.8 | 6.8 | 5.8 | 5.8 | 14.5 | 14.4 |
| Na2O | 0.23 | 0.22 | 0.13 | 0.13 | 3.9 | 3.7 |
| K2O | 0.00 | 0.01 | 0.00 | 0.00 | 0.80 | 1.1 |
| Total | 100.1 | 100.8 | 100.2 | 100.4 | 100.2 | 100.1 |
| Si | 3.0 | 3.0 | 3.0 | 3.0 | 2.0 | 2.0 |
| Ti | 0.04 | 0.04 | 0.02 | 0.02 | 0.02 | 0.02 |
| Al | 1.9 | 1.9 | 1.9 | 1.9 | 0.33 | 0.34 |
| Cr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fetot | 0.93 | 0.93 | 0.66 | 0.69 | 0.16 | 0.16 |
| Mn | 0.03 | 0.03 | 0.02 | 0.02 | 0.00 | 0.00 |
| Mg | 1.4 | 1.5 | 1.8 | 1.8 | 0.58 | 0.57 |
| Ca | 0.54 | 0.53 | 0.45 | 0.45 | 0.56 | 0.55 |
| Na | 0.03 | 0.03 | 0.02 | 0.02 | 0.27 | 0.26 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.05 |
| Total | 8.0 | 8.0 | 8.0 | 8.0 | 4.0 | 4.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailenko, D.; Golovin, A.; Korsakov, A.; Aulbach, S.; Gerdes, A.; Ragozin, A. Metasomatic Evolution of Coesite-Bearing Diamondiferous Eclogite from the Udachnaya Kimberlite. Minerals 2020, 10, 383. https://doi.org/10.3390/min10040383
Mikhailenko D, Golovin A, Korsakov A, Aulbach S, Gerdes A, Ragozin A. Metasomatic Evolution of Coesite-Bearing Diamondiferous Eclogite from the Udachnaya Kimberlite. Minerals. 2020; 10(4):383. https://doi.org/10.3390/min10040383
Chicago/Turabian StyleMikhailenko, Denis, Alexander Golovin, Andrey Korsakov, Sonja Aulbach, Axel Gerdes, and Alexey Ragozin. 2020. "Metasomatic Evolution of Coesite-Bearing Diamondiferous Eclogite from the Udachnaya Kimberlite" Minerals 10, no. 4: 383. https://doi.org/10.3390/min10040383
APA StyleMikhailenko, D., Golovin, A., Korsakov, A., Aulbach, S., Gerdes, A., & Ragozin, A. (2020). Metasomatic Evolution of Coesite-Bearing Diamondiferous Eclogite from the Udachnaya Kimberlite. Minerals, 10(4), 383. https://doi.org/10.3390/min10040383






