Metal-Containing Zinc Phosphate EDI Zeolites Synthesized by Sol–gel Assisted Hydrothermal Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
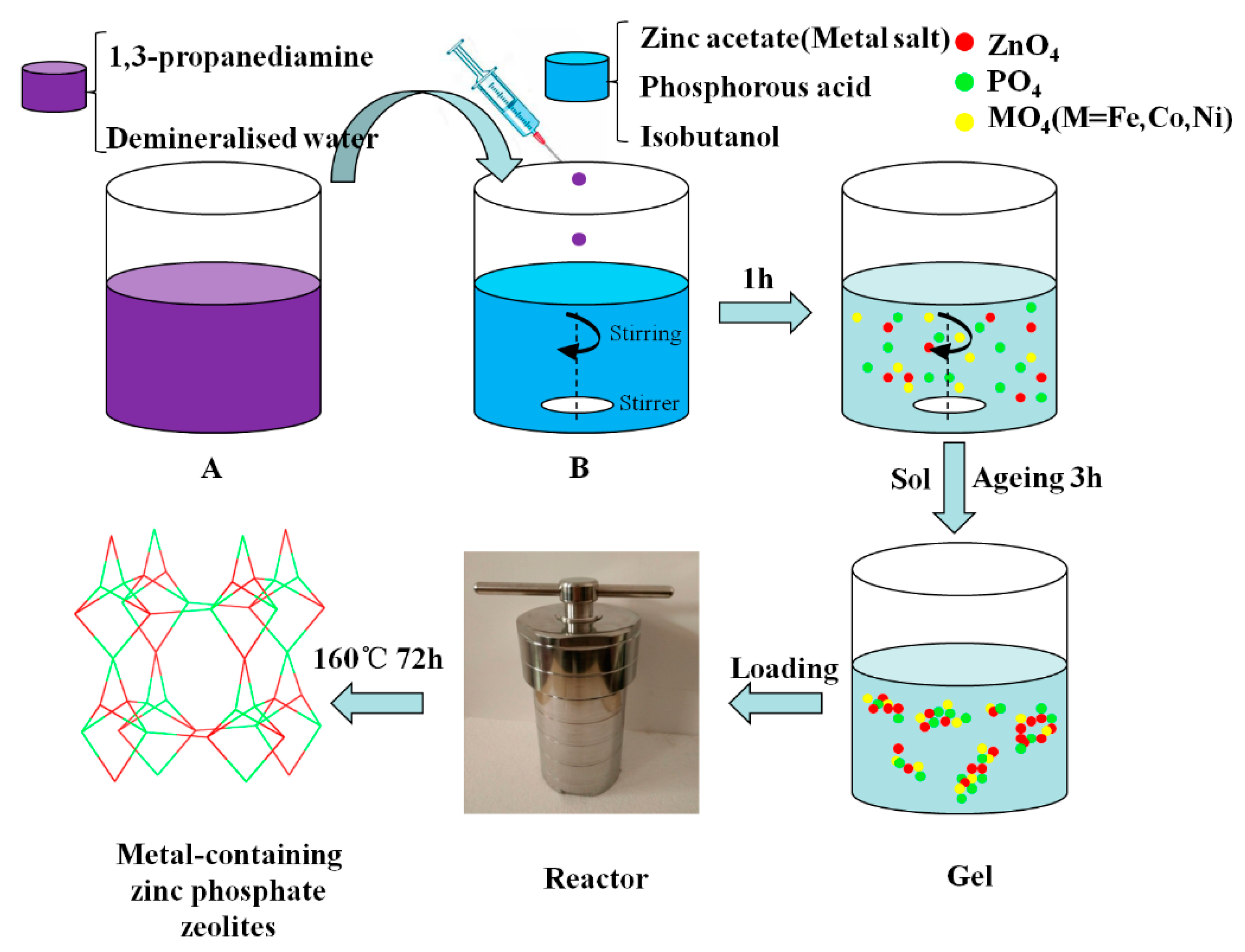
2.2. Synthesis
2.3. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheetham, A.K.; Férey, G.; Loiseau, T. Open-framework inorganic materials. Angew. Chem. Int. Ed. 1999, 38, 3268–3292. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Murugavel, R.; Choudhury, A.; Walawalkar, M.G.; Pothiraja, R.; Rao, C.N.R. Metal complexes of organophosphate esters and open-framework metal phosphates: Synthesis, structure, transformations, and applications. Chem. Rev. 2008, 108, 3549–3655. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef] [PubMed]
- Kragović, M.; Daković, A.; Marković, M.; Krstić, J.; Gatta, G.D.; Rotiroti, N. Characterization of lead sorption by the natural and Fe(III)-modified zeolite. Appl. Surf. Sci. 2013, 283, 764–774. [Google Scholar] [CrossRef]
- Yang, M.; Fan, D.; Wei, Y.; Tian, P.; Liu, Z. Recent Progress in Methanol-to-Olefins (MTO) Catalysts. Adv. Mater. 2019, 31, 1902181. [Google Scholar] [CrossRef]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate molecular sieves: A new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- MeurigáThomas, J. Synthesis and characterization of a novel extra large ring of aluminophosphate JDF-20. J. Chem. Soc. Chem. Commun. 1992, 12, 875–876. [Google Scholar]
- Wei, Y.; Tian, Z.; Gies, H.; Xu, R.; Ma, H.; Pei, R.; Zhang, W.; Xu, Y.; Wang, L.; Li, K. Ionothermal synthesis of an aluminophosphate molecular sieve with 20-ring pore openings. Angew. Chem. Int. Ed. 2010, 49, 5367–5370. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, S.; Jo, C.; Ryoo, R. Microporous aluminophosphate nanosheets and their nanomorphic zeolite analogues tailored by hierarchical structure-directing amines. J. Am. Chem. Soc. 2013, 135, 8806–8809. [Google Scholar] [CrossRef]
- Yuhas, B.D.; Mowat, J.P.; Miller, M.A.; Sinkler, W. AlPO-78: A 24-layer ABC-6 aluminophosphate synthesized using a simple structure-directing agent. Chem. Mater. 2018, 30, 582–586. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.; Shi, Z.; Bu, X.; Zhou, Y.; Xu, X.; Zhao, D. Hydrothermal synthesis of new pure beryllophosphate molecular sieve phases from concentrated amines. Chem. Mater. 2001, 13, 2042–2048. [Google Scholar] [CrossRef]
- Nenoff, T.M.; Harrison, W.T.; Gier, T.E.; Stucky, G.D. Room-temperature synthesis and characterization of new ZnPO and ZnAsO sodalite open frameworks. J. Am. Chem. Soc. 1991, 113, 378–379. [Google Scholar] [CrossRef]
- Yuan, H.-M.; Chen, J.-S.; Zhu, G.-S.; Li, J.-Y.; Yu, J.-H.; Yang, G.-D.; Xu, R.-R. The first organo-templated cobalt phosphate with a zeolite topology. Inorg. Chem. 2000, 39, 1476–1479. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Gan, L.; Wang, Z.; Yu, J.; Xu, R. Heteroatom-stabilized chiral framework of aluminophosphate molecular sieves. Angew. Chem. Int. Ed. 2009, 48, 314–317. [Google Scholar] [CrossRef]
- Estermann, M.; McCusker, L.; Baerlocher, C.; Merrouche, A.; Kessler, H. A Synthetic gallophosphate molecular sieve with a 20-tetrahedral-atom pore opening. Nature 1991, 352, 320–323. [Google Scholar] [CrossRef]
- Josien, L.; Simon-Masseron, A.; Gramlich, V.; Patarin, J.; Rouleau, L. Synthesis and crystal structure of IM-6, a new open framework cobalt–gallium phosphate with ten- and twelve-membered pore openings. Chemistry 2003, 9, 856–861. [Google Scholar] [CrossRef]
- Seo, S.; Yang, T.; Shin, J.; Jo, D.; Zou, X.; Hong, S. Two aluminophosphate molecular sieves built from Pairs of enantiomeric structural building units. Angew. Chem. Int. Ed. 2018, 130, 3789–3794. [Google Scholar] [CrossRef]
- Wang, B.; Mu, Y.; Zhang, H.; Shi, H.; Chen, G.; Yu, Y.; Yang, Z.; Li, J.; Yu, J. Red room-temperature phosphorescence of CDs@zeolite composites triggered by heteroatoms in zeolite frameworks. ACS Cent. Sci. 2019, 5, 349–356. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yan, Y.; Xu, J.; Guan, B.; Wang, Q.; Li, J.; Yu, J. ChemInform abstract: Luminescent carbon dots in a new magnesium aluminophosphate zeolite. Chem. Commun. 2013, 49, 9006–9008. [Google Scholar] [CrossRef]
- Wu, J.; Yan, Y.; Liu, B.; Wang, X.; Li, J.; Yu, J. Multifunctional open-framework zinc phosphate| C12H14N2|[Zn6(PO4)4(HPO4)(H2O)2]: Photochromic, photoelectric and fluorescent properties. Chem. Commun. 2013, 49, 4995–4997. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Wu, J.; Yan, Y.; Shi, C.; Li, J. A new methylviologen-templated zinc gallophosphate zeolite with photo-/thermochromism, fluorescent and photoelectric properties. Inorg. Chem. Front. 2016, 3, 541–546. [Google Scholar] [CrossRef]
- Gier, T.; Stucky, G. ChemInform abstract: Low-temperature synthesis of hydrated zinco(beryllo)phosphate and arsenate molecular sieves. Nature 1991, 349, 508–510. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Dong, Z.; Meng, C.; Li, X.; Hong, X.; Yang, S. The effects of different dimensional organic amines on synthetic zinc phosphites/phosphates. J. Porous. Mater. 2020, 27, 21–28. [Google Scholar] [CrossRef]
- Ke, Y.; He, G.; Li, J.; Zhang, Y.; Lu, S. A new mixed divalent metal phosphate with zeolite thomsonite framework topology. New J. Chem. 2001, 25, 1627–1630. [Google Scholar]
- Helliwell, M.; Helliwell, J.; Kaucic, V.; Zabukovec Logar, N.; Barba, L.; Busetto, E.; Lausi, A. Determination of the site of incorporation of cobalt in CoZnPO-CZP by multiple-wavelength anomalous-dispersion crystallography. Acta Crystallogr. Sec. B Struct. Sci. 1999, 55, 327–332. [Google Scholar] [CrossRef]
- Ng, H.Y.; Harrison, W.T.A. Monoclinic NaZnPO4-ABW, a new modification of the zeolite ABW structure type containing elliptical eight-ring channels. Microporous Mesoporous Mater. 1998, 23, 197–202. [Google Scholar] [CrossRef]
- Chippindale, A.; Cowley, A.; Peacock, K. Synthesis and characterisation of [TH][ZnGaP2O8] (T = CN3H5 and C4NH9); Two microporous zinc-gallium phosphates with the gismondine structure. Microporous Mesoporous Mater. 1998, 24, 133–141. [Google Scholar] [CrossRef]
- Bu, X.; Gier, T.; Feng, P.; Stucky, G. Template control of framework topology and charge in new phosphate- and arsenate-based sodalite analogs. Microporous Mesoporous Mater. 1998, 20, 371–379. [Google Scholar] [CrossRef]
- Tusar, N.N.; Kaucic, V.; Geremia, S.; Vlaic, G. A zinc-rich CHA-type aluminophosphate. Zeolites 1995, 15, 708–713. [Google Scholar] [CrossRef]
- Ng, E.P.; Ghoy, J.P.; Awala, H.; Vicente, A.; Adnan, R.; Ling, T.C.; Mintova, S. Ionothermal synthesis of FeAPO-5 in the presence of phosphorous acid. Crystengcomm 2016, 18, 257–265. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Yu, J.; Xu, R. Divalent-Metal-Stabilized Aluminophosphates Exhibiting a New Zeolite Framework Topology. Inorg. Chem. 2012, 51, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Dell’Era, A.; Pasquali, M.; Bauer, E.; Vecchio, S.; Scaramuzzo, F.; Lupi, C. Synthesis, Characterization, and Electrochemical Behavior of LiMnxFe(1 − x)PO4 Composites Obtained from Phenylphosphonate-Based Organic-Inorganic Hybrids. Materials 2017, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Tranquillo, E.; Dell’Era, A.; Tuffi, R.; Vecchio, S. Thermal behavior and structural study of ZrO2/poly(ε-caprolactone) hybrids synthesized via sol–gel route. Ceram. Int. 2018, 45, 2771–2778. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Barrino, F.; Blanco, I.; Dal Poggetto, F.; Naviglio, D. Drug release of hybrid materials containing Fe(II) citrate synthesized by sol–gel technique. Materials 2018, 11, 2270. [Google Scholar] [CrossRef]
- Chen, D.; Huang, F.; Bing, C.; Caruso, R. Mesoporous Anatase TiO2 Beads With High Surface Areas and Controllable Pore Sizes: A Superior Candidate for High-Performance Dye-Sensitized Solar Cells. Adv. Mater. 2009, 21, 2206–2210. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, P.; Yan, W.; Boronat, M.; Li, X.; Su, J.-H.; Wang, J.; Li, Y.; Corma, A.; Xu, R.; et al. Accelerated crystallization of zeolites via hydroxyl free radicals. Science 2016, 351, 1188–1191. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Yang, S.; Yan, H.; Hong, X.; Dong, W.; Liu, Y.; Zhang, B.; Wen, Z. Interlayer space regulating of NiMn layered double hydroxides for supercapacitors by controlling hydrothermal reaction time. Electrochim. Acta 2019, 295, 1–6. [Google Scholar] [CrossRef]
- Harrison, W. [H3N(CH2)3NH3]0.5[ZnPO4], an organically templated zincophosphate analogue of the aluminosilicate zeolite edingtonite. Acta Crystallogr. E Struct. Pep. Online 2001, 57, 248–250. [Google Scholar] [CrossRef]
- Wang, K.C.; Li, T.; Zeng, H.M.; Zou, G.H.; Zhang, Q.H.; Lin, Z.E. Ionothermal synthesis of open-framework metal phosphates using a multifunctional ionic liquid. Inorg. Chem. 2018, 57, 8726–8729. [Google Scholar] [CrossRef]
- Lin, Z.E.; Nayek, H.P.; Dehnen, S. Flux synthesis of three-dimensional open-framework zinc phosphite and manganese phosphite-oxalate with 12-ring channels. Microporous Mesoporous Mater. 2009, 126, 95–100. [Google Scholar] [CrossRef]






| Element | (1) | Range | SD | (2) | Range | SD | (3) | Range | SD |
|---|---|---|---|---|---|---|---|---|---|
| Zn | 8.03 | 7.98–8.07 | 0.03 | 10.78 | 10.72–10.81 | 0.04 | 10.82 | 10.75–10.85 | 0.03 |
| M | 23.76 | 23.52–23.86 | 0.05 | 20.12 | 20.01–20.28 | 0.05 | 20.89 | 20.73–20.97 | 0.04 |
| P | 15.94 | 15.78–16,03 | 0.06 | 15.89 | 15.78–15.95 | 0.03 | 15.90 | 15.76–16.03 | 0.06 |
| C | 9.36 | 9.25–9.43 | 0.04 | 9.21 | 9.10–9.32 | 0.04 | 9.25 | 9.16–9.33 | 0.03 |
| H | 3.28 | 3.24–3.30 | 0.02 | 3.09 | 3.01–3.13 | 0.02 | 3.11 | 3.03–3.18 | 0.02 |
| N | 7.38 | 7.31–7.42 | 0.03 | 7.18 | 7.02–7.32 | 0.04 | 7.20 | 7.08–7.26 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Dong, Z.; Wang, Q.; Meng, C.; Zhuang, W.; Liu, J.; Song, Y.; Jin, Y.; Yang, S. Metal-Containing Zinc Phosphate EDI Zeolites Synthesized by Sol–gel Assisted Hydrothermal Method. Minerals 2020, 10, 391. https://doi.org/10.3390/min10050391
Wang X, Dong Z, Wang Q, Meng C, Zhuang W, Liu J, Song Y, Jin Y, Yang S. Metal-Containing Zinc Phosphate EDI Zeolites Synthesized by Sol–gel Assisted Hydrothermal Method. Minerals. 2020; 10(5):391. https://doi.org/10.3390/min10050391
Chicago/Turabian StyleWang, Xuelei, Zhaojun Dong, Qiufeng Wang, Chao Meng, Weibin Zhuang, Jiyuan Liu, Ying Song, Yuxin Jin, and Shaobin Yang. 2020. "Metal-Containing Zinc Phosphate EDI Zeolites Synthesized by Sol–gel Assisted Hydrothermal Method" Minerals 10, no. 5: 391. https://doi.org/10.3390/min10050391
APA StyleWang, X., Dong, Z., Wang, Q., Meng, C., Zhuang, W., Liu, J., Song, Y., Jin, Y., & Yang, S. (2020). Metal-Containing Zinc Phosphate EDI Zeolites Synthesized by Sol–gel Assisted Hydrothermal Method. Minerals, 10(5), 391. https://doi.org/10.3390/min10050391





