From Decompression Melting to Mantle-Wedge Refertilization and Metamorphism: Insights from Peridotites of the Alag Khadny Accretionary Complex (SW Mongolia)
Abstract
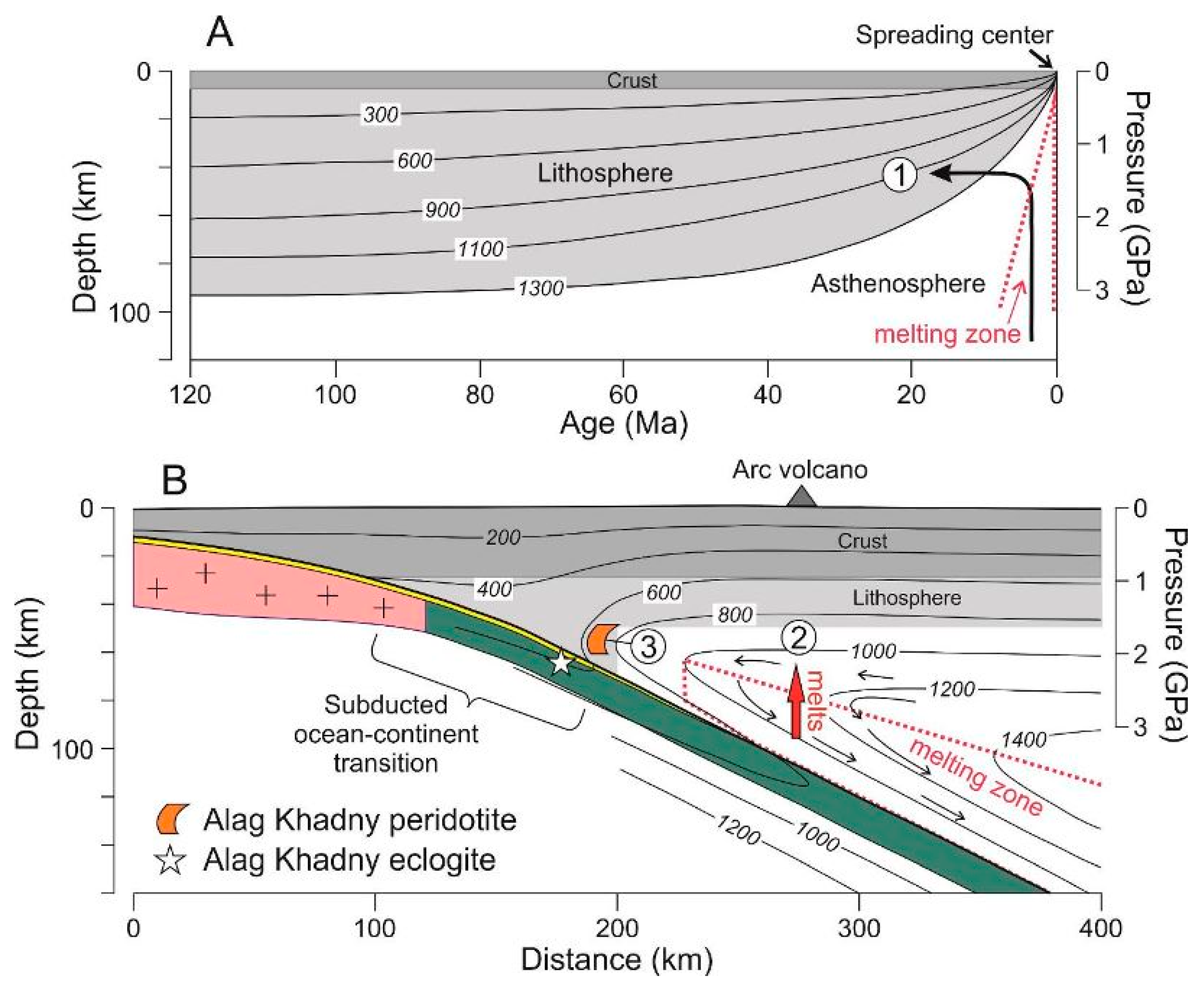
:1. Introduction
2. Geological Background
3. Materials and Methods
4. Results
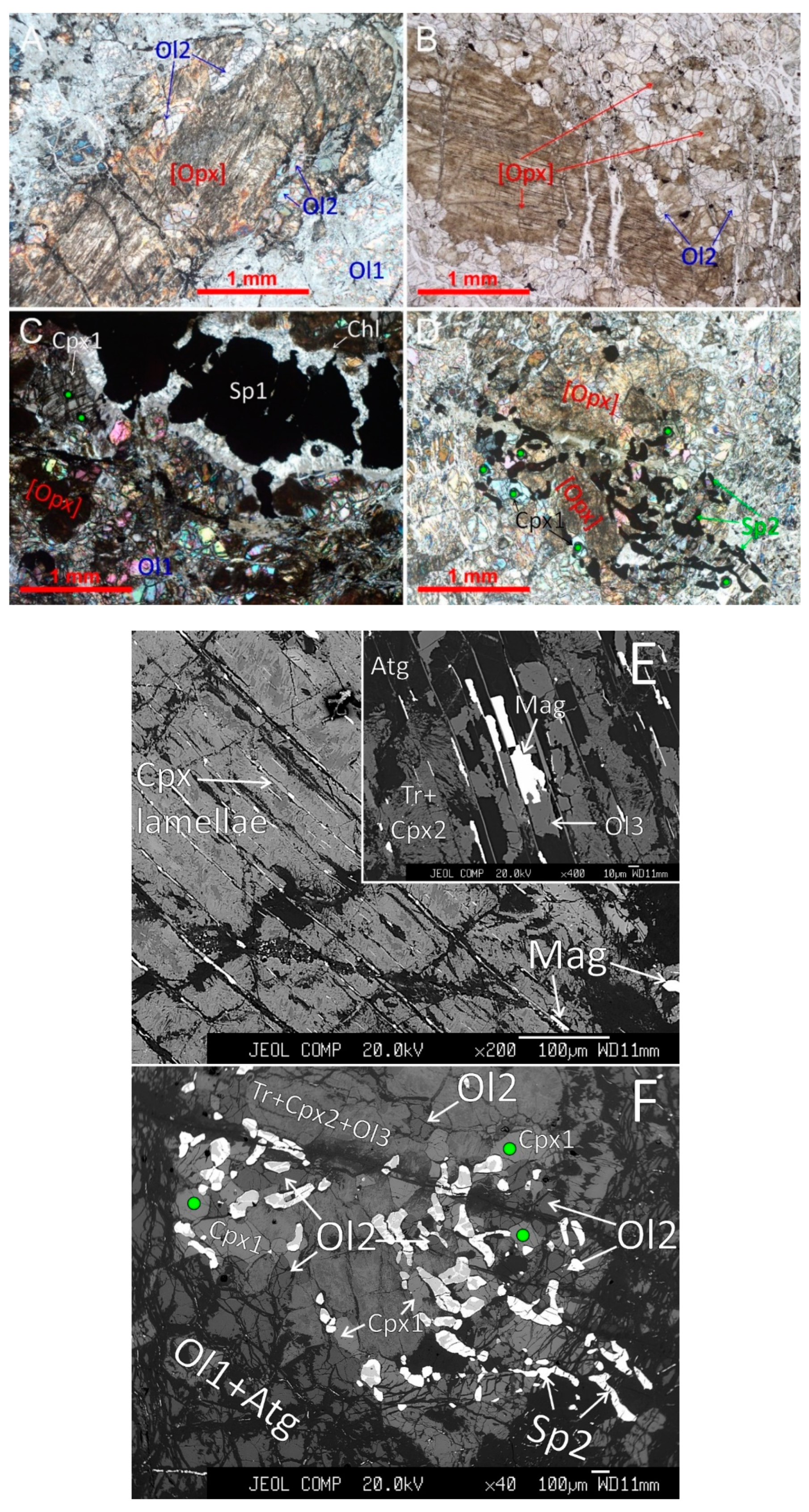
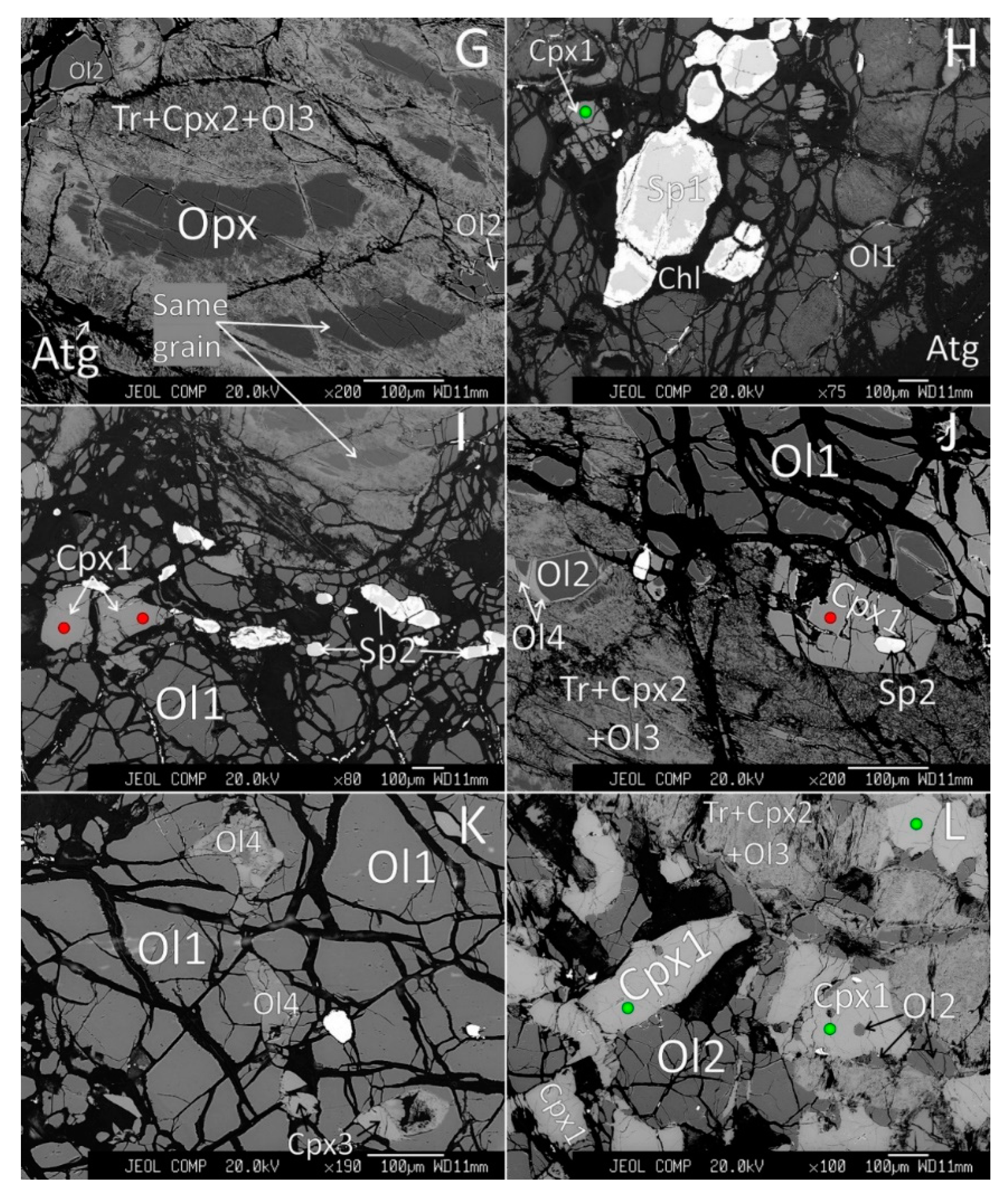
4.1. Petrography
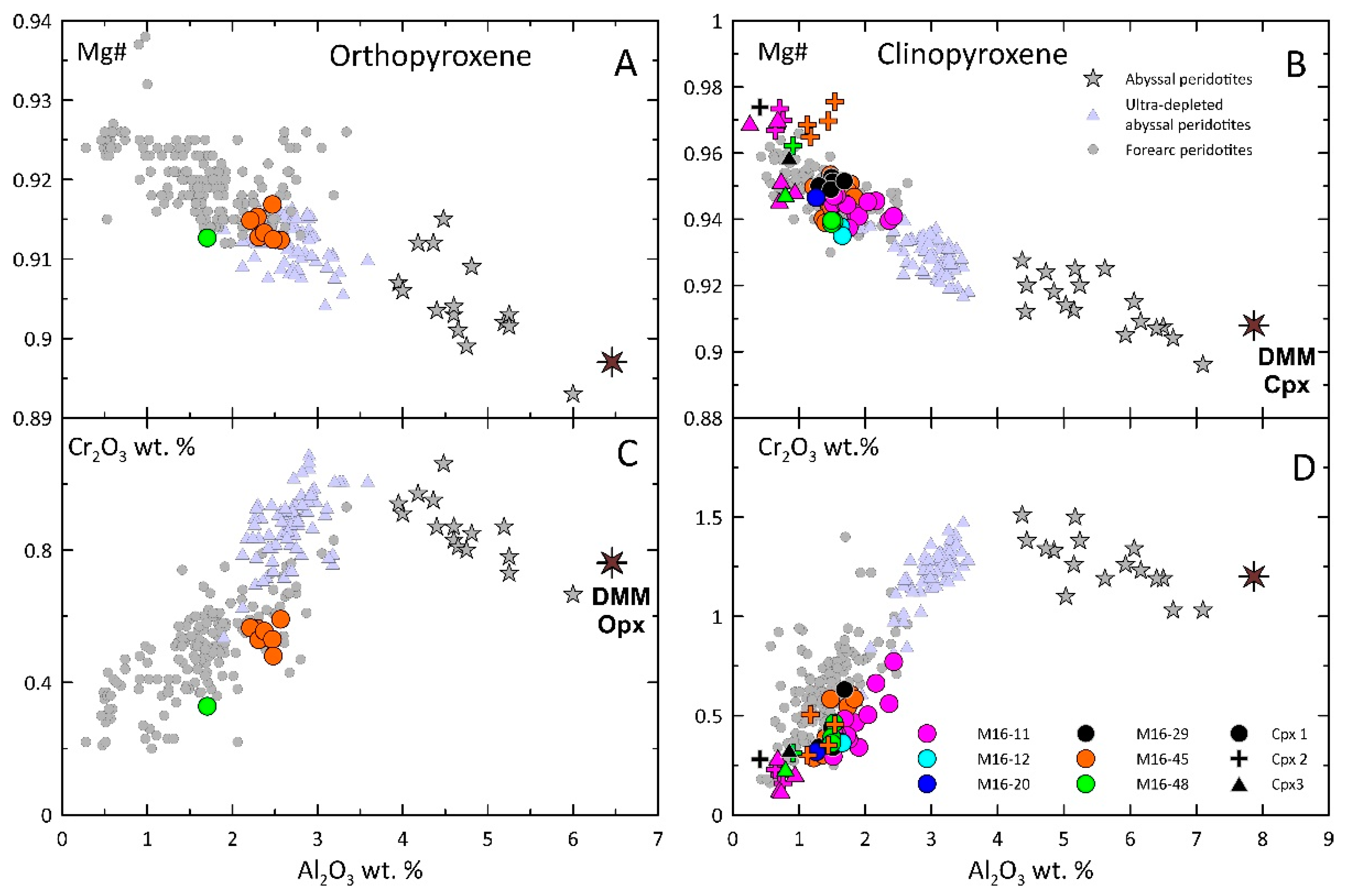
4.2. Mineral Compositions
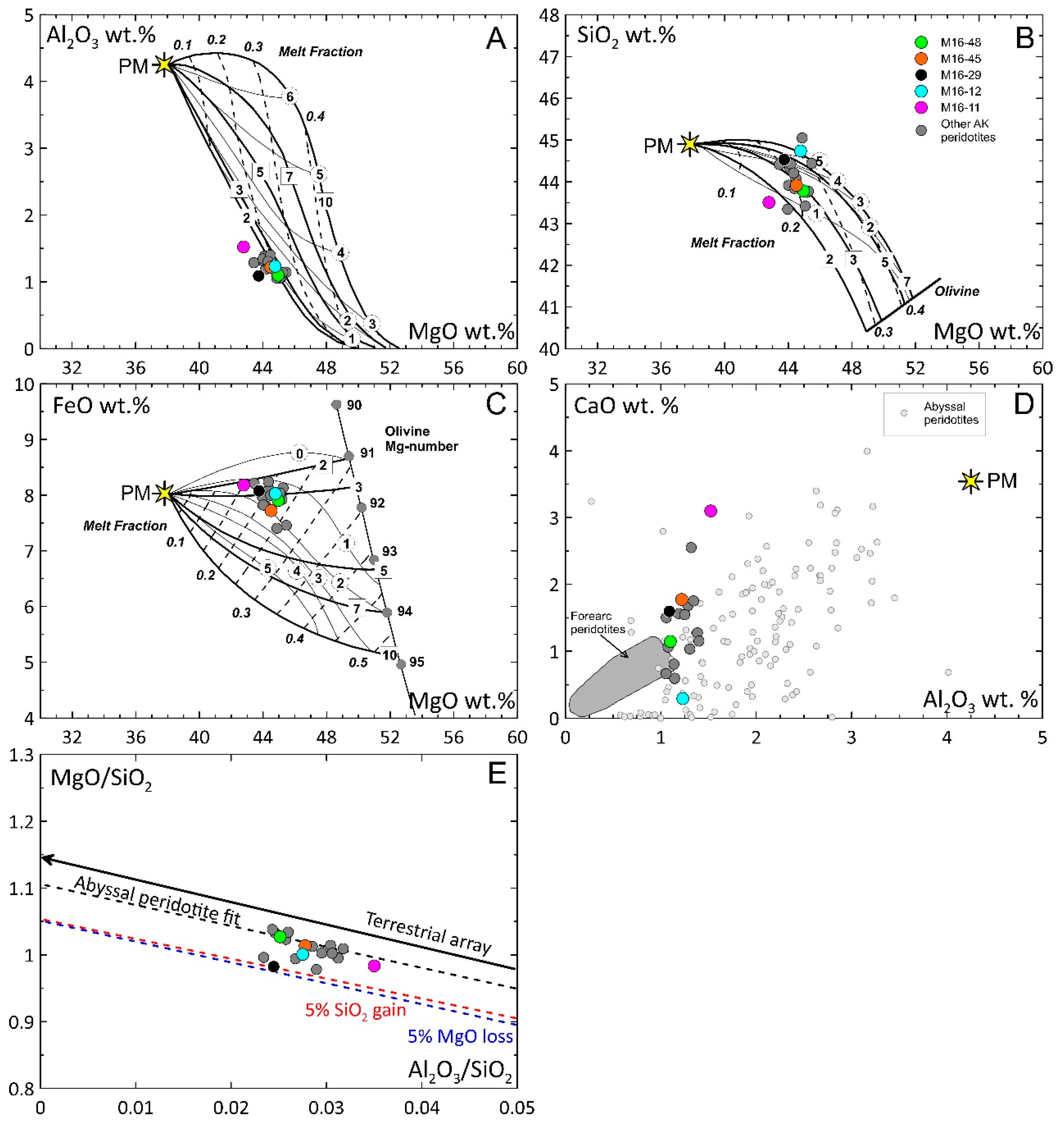
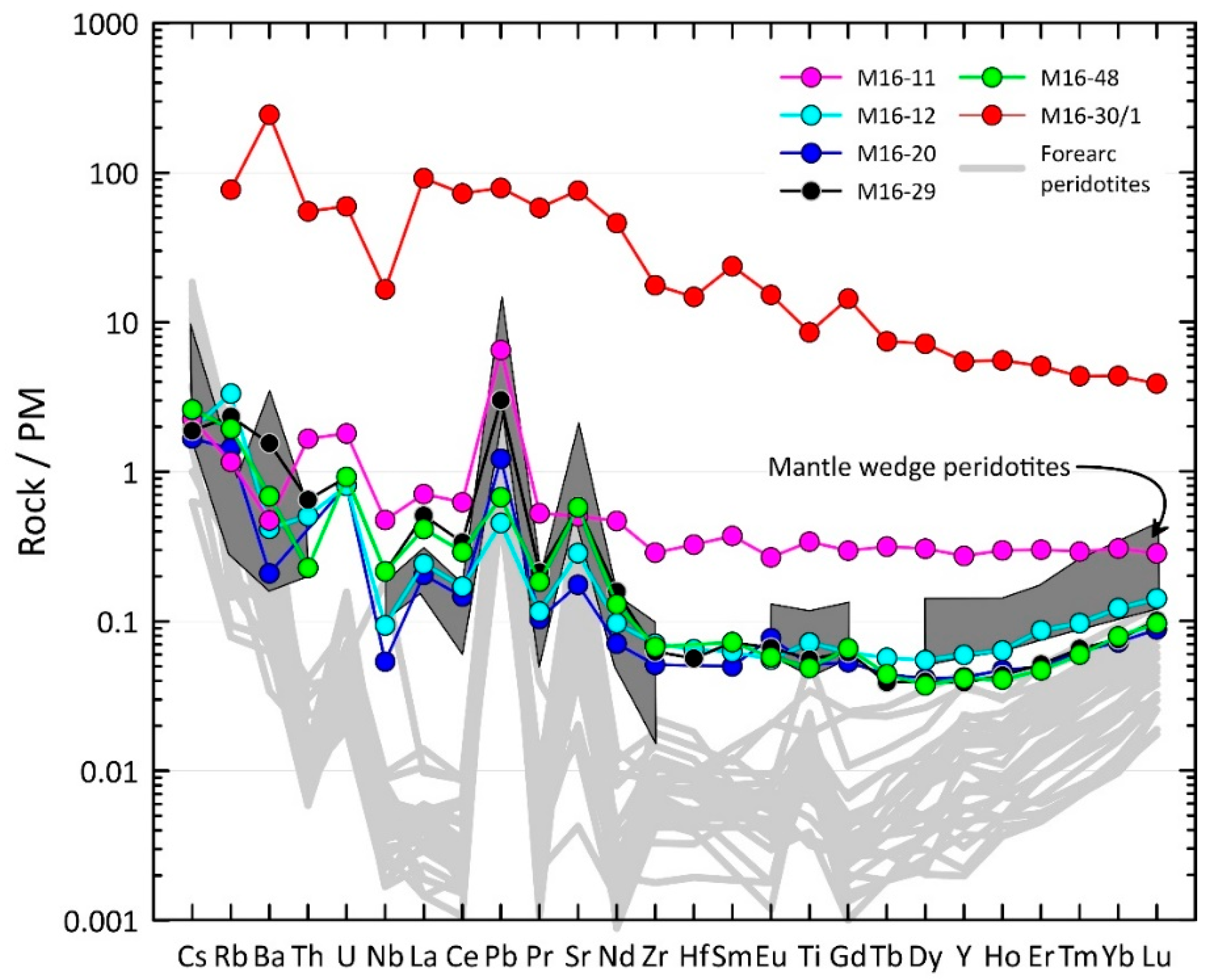
4.3. Bulk-Rock Compositions
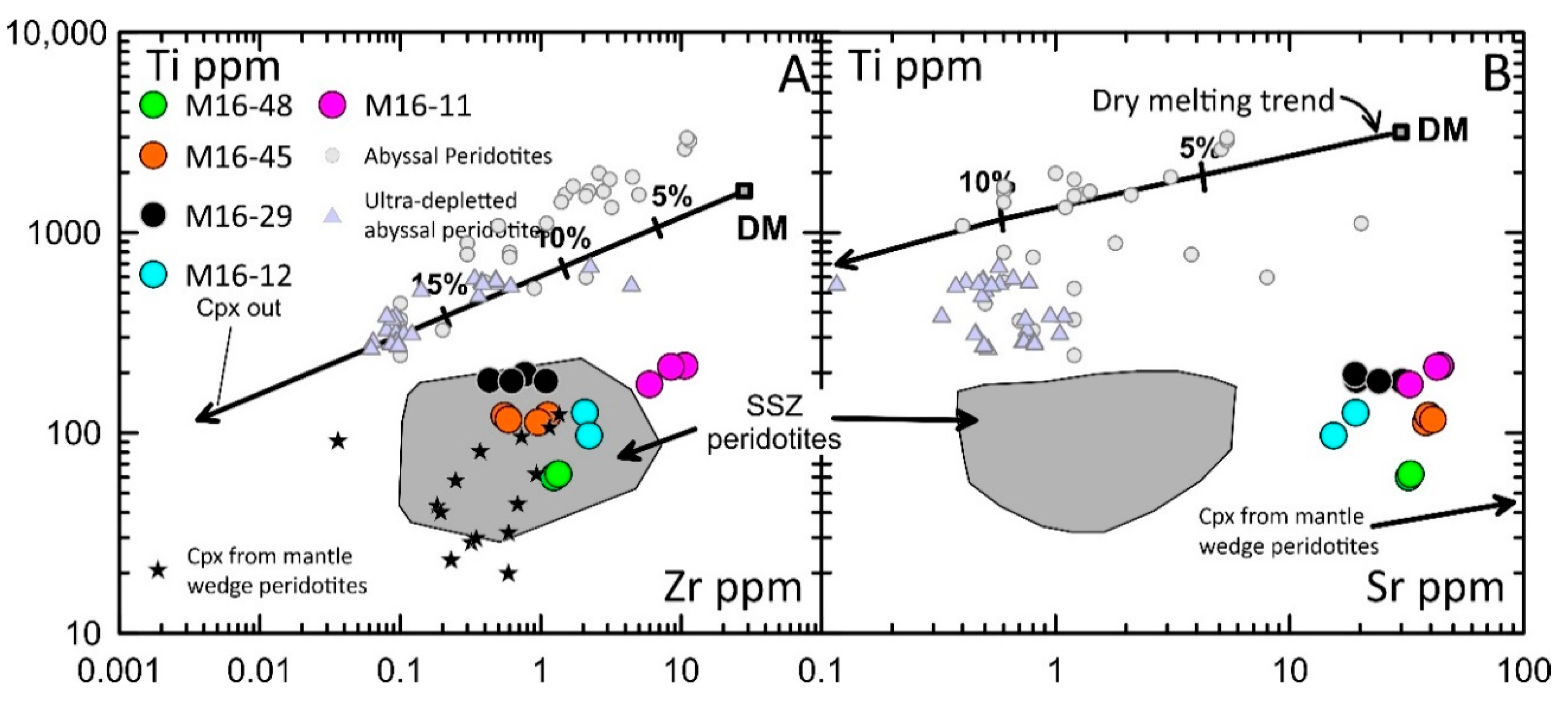
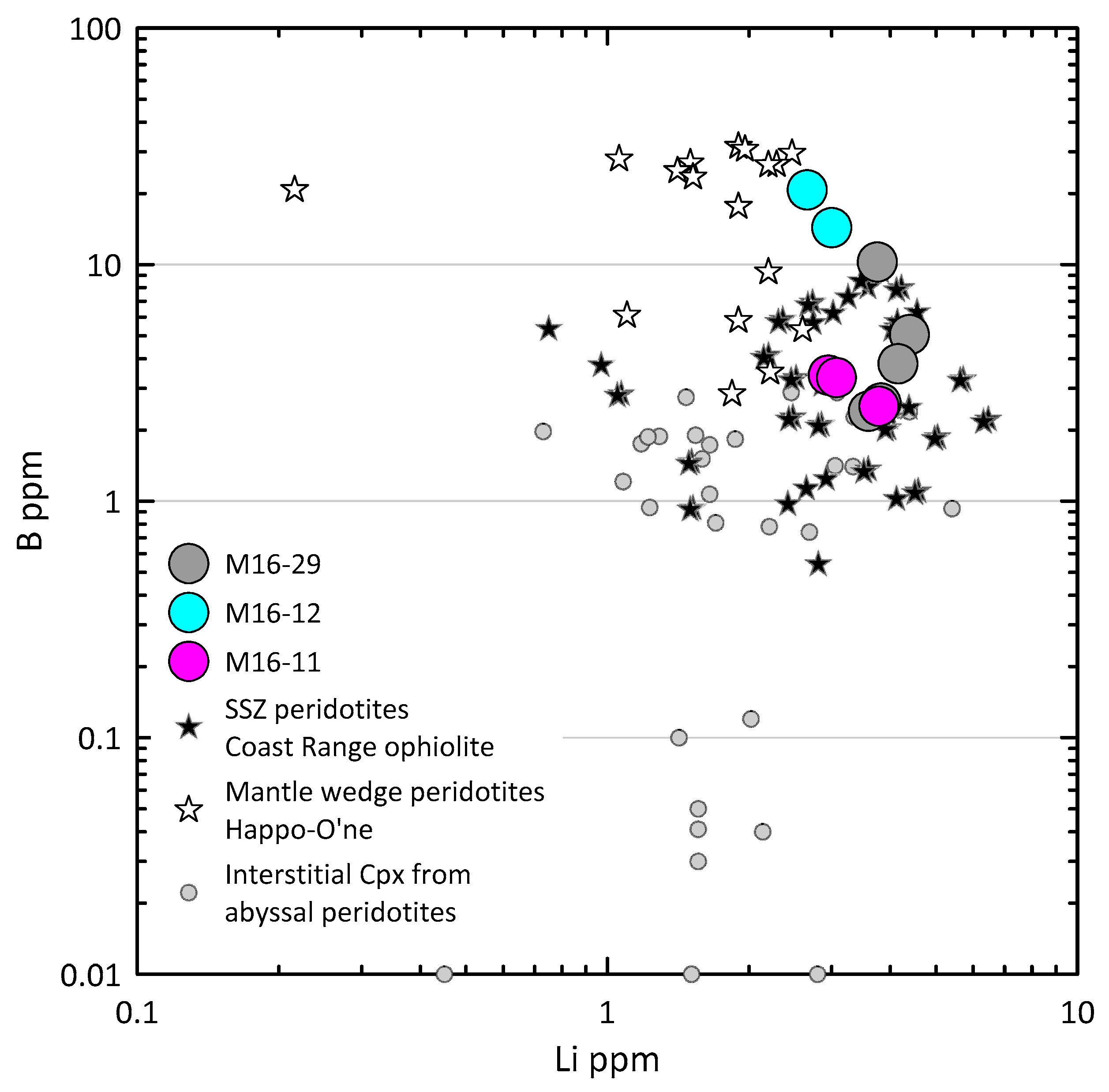
4.4. Trace Elements in Clinopyroxene
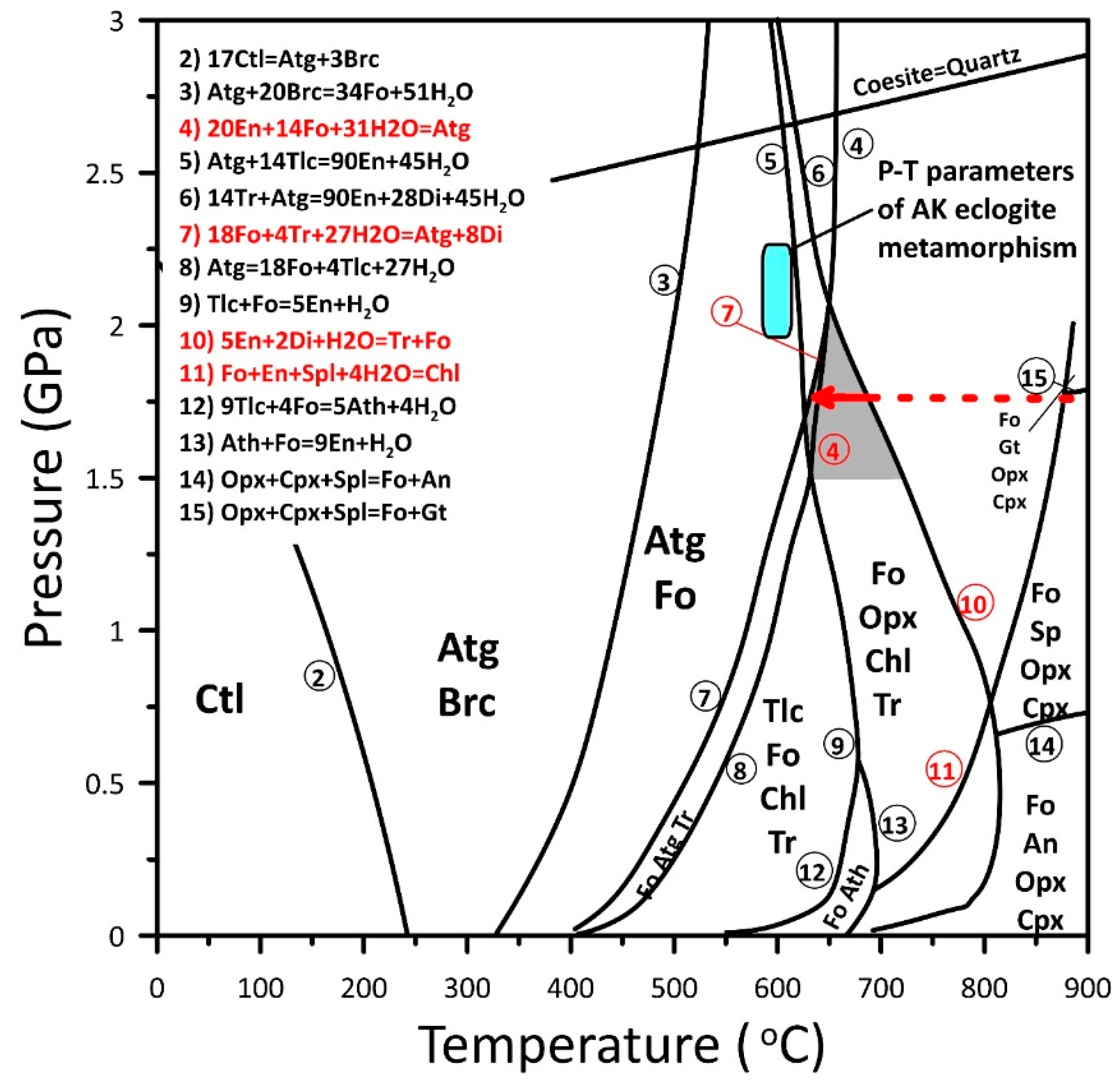
5. Discussion
5.1. The Origin of the Alag Khadny Peridotites
5.2. Modeling of Partial Melting and Refertilization
5.3. Metamorphism of the Peridotites
5.4. Implications to Regional Tectonics and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deschamps, F.; Godard, M.; Guillot, S.; Hattori, K. Geochemistry of subduction zone serpentinites: A review. Lithos 2013, 178, 96–127. [Google Scholar] [CrossRef]
- Spandler, C.; Pirard, C. Element recycling from subducting slabs to arc crust: A review. Lithos 2013, 170–171, 208–223. [Google Scholar] [CrossRef]
- Scambelluri, M.; Pettke, T.; Cannaò, E. Fluid-related inclusions in Alpine high-pressure peridotite reveal trace element recycling during subduction-zone dehydration of serpentinized mantle (Cima di Gagnone, Swiss Alps). Earth Planet. Sci. Lett. 2015, 429, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Kendrick, M.A.; Hémond, C.; Kamenetsky, V.S.; Danyushevsky, L.; Devey, C.W.; Rodemann, T.; Jackson, M.G.; Perfit, M.R. Seawater cycled throughout Earth’s mantle in partially serpentinized lithosphere. Nat. Geosci. 2017, 10, 222–228. [Google Scholar] [CrossRef]
- Scambelluri, M.; Pettke, T.; Rampone, E.; Godard, M.; Reusser, E. Petrology and Trace Element Budgets of High-pressure Peridotites Indicate Subduction Dehydration of Serpentinized Mantle (Cima di Gagnone, Central Alps, Switzerland). J. Petrol. 2014, 55, 459–498. [Google Scholar] [CrossRef] [Green Version]
- Bebout, G.E. Metamorphic chemical geodynamics of subduction zones. Earth Planet. Sci. Lett. 2007, 260, 373–393. [Google Scholar] [CrossRef]
- Guillot, S.; Schwartz, S.; Reynard, B.; Agard, P.; Prigent, C. Tectonic significance of serpentinites. Tectonophysics 2015, 646, 1–19. [Google Scholar] [CrossRef]
- Cannaò, E.; Agostini, S.; Scambelluri, M.; Tonarini, S.; Godard, M. B, Sr and Pb isotope geochemistry of high-pressure Alpine metaperidotites monitors fluid-mediated element recycling during serpentinite dehydration in subduction mélange (Cima di Gagnone, Swiss Central Alps). Geochim. Cosmochim. Acta 2015, 163, 80–100. [Google Scholar] [CrossRef]
- Guillot, S.; Hattori, K.H.; De Sigoyer, J.; Nägler, T.; Auzende, A.-L. Evidence of hydration of the mantle wedge and its role in the exhumation of eclogites. Earth Planet. Sci. Lett. 2001, 193, 115–127. [Google Scholar] [CrossRef]
- Bostock, M.G.; Hyndman, R.D.; Rondenay, S.; Peacock, S.M. An inverted continental Moho and serpentinization of the forearc mantle. Nature 2002, 417, 536–538. [Google Scholar] [CrossRef]
- Savov, I.P.; Ryan, J.G.; D’Antonio, M.; Kelley, K.; Mattie, P. Geochemistry of serpentinized peridotites from the Mariana Forearc Conical Seamount, ODP Leg 125: Implications for the elemental recycling at subduction zones: Serpentinized peridotites. Geochem. Geophys. Geosystems 2005, 6, 1–24. [Google Scholar] [CrossRef]
- Khedr, M.Z.; Arai, S. Hydrous peridotites with Ti-rich chromian spinel as a low-temperature forearc mantle facies: Evidence from the Happo-O’ne metaperidotites (Japan). Contrib. Mineral. Petrol. 2010, 159, 137–157. [Google Scholar] [CrossRef]
- Khedr, M.Z.; Arai, S.; Tamura, A.; Morishita, T. Clinopyroxenes in high-P metaperidotites from Happo-O’ne, central Japan: Implications for wedge-transversal chemical change of slab-derived fluids. Lithos 2010, 119, 439–456. [Google Scholar] [CrossRef]
- Shervais, J.W.; Jean, M.M. Inside the subduction factory: Modeling fluid mobile element enrichment in the mantle wedge above a subduction zone. Geochim. Cosmochim. Acta 2012, 95, 270–285. [Google Scholar] [CrossRef]
- Le Roux, V.; Liang, Y. Ophiolitic Pyroxenites Record Boninite Percolation in Subduction Zone Mantle. Minerals 2019, 9, 565. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, S.; Saikawa, Y.; Miura, M.; Parlak, O.; Arai, S. Decoding of Mantle Processes in the Mersin Ophiolite, Turkey, of End-Member Arc Type: Location of the Boninite Magma Generation. Minerals 2018, 8, 464. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Xiong, Q.; Zhao, Y.; Li, W. Subduction-zone peridotites and their records of crust-mantle interaction. Sci. China Earth Sci. 2019, 62, 1033–1052. [Google Scholar] [CrossRef]
- Angiboust, S.; Agard, P.; Raimbourg, H.; Yamato, P.; Huet, B. Subduction interface processes recorded by eclogite-facies shear zones (Monviso, W. Alps). Lithos 2011, 127, 222–238. [Google Scholar] [CrossRef] [Green Version]
- Hermann, J.; Müntener, O.; Scambelluri, M. The importance of serpentinite mylonites for subduction and exhumation of oceanic crust. Tectonophysics 2000, 327, 225–238. [Google Scholar] [CrossRef]
- Schwartz, S.; Allemand, P.; Guillot, S. Numerical model of the effect of serpentinites on the exhumation of eclogitic rocks: Insights from the Monviso ophiolitic massif (Western Alps). Tectonophysics 2001, 342, 193–206. [Google Scholar] [CrossRef]
- Lehmann, J.; Schulmann, K.; Lexa, O.; Corsini, M.; Kroner, A.; Stipska, P.; Tomurhuu, D.; Otgonbator, D. Structural constraints on the evolution of the Central Asian Orogenic Belt in SW Mongolia. Am. J. Sci. 2010, 310, 575–628. [Google Scholar] [CrossRef]
- Štípská, P.; Schulmann, K.; Lehmann, J.; Corsini, M.; Lexa, O.; Tomurhuu, D. Early Cambrian eclogites in SW Mongolia: Evidence that the Palaeo-Asian Ocean suture extends further east than expected: Cambrian eclogites in SW Mongolia. J. Metamorph. Geol. 2010, 28, 915–933. [Google Scholar] [CrossRef] [Green Version]
- Skuzovatov, S.Y.; Shatsky, V.S.; Dril, S.I.; Perepelov, A.B. Elemental and isotopic (Nd–Sr–O) geochemistry of eclogites from the Zamtyn-Nuruu area (SW Mongolia): Crustal contribution and relation to Neoproterozoic subduction-accretion events. J. Asian Earth Sci. 2018, 167, 33–51. [Google Scholar] [CrossRef]
- Kozakov, I.K.; Sal’nikova, E.B.; Wang, T.; Didenko, A.N.; Plotkina, Y.V.; Podkovyrov, V.N. Early Precambrian crystalline complexes of the Central Asian microcontinent: Age, sources, tectonic position. Stratigr. Geol. Correl. 2007, 15, 121–140. [Google Scholar] [CrossRef]
- Buriánek, D.; Schulmann, K.; Hrdličková, K.; Hanžl, P.; Janoušek, V.; Gerdes, A.; Lexa, O. Geochemical and geochronological constraints on distinct Early-Neoproterozoic and Cambrian accretionary events along southern margin of the Baydrag Continent in western Mongolia. Gondwana Res. 2017, 47, 200–227. [Google Scholar] [CrossRef]
- Skuzovatov, S.Y.; Wang, K.-L.; Lee, H.-Y. Nature and (in-)coherent metamorphic evolution of subducted continental crust in the Neoproterozoic accretionary collage of SW Mongolia. Geosci. Front. 2020. under review. [Google Scholar]
- Kovalenko, V.I.; Yarmolyuk, V.V.; Kovach, V.P.; Kotov, A.B.; Kozakov, I.K.; Salnikova, E.B.; Larin, A.M. Isotope provinces, mechanisms of generation and sources of the continental crust in the Central Asian mobile belt: Geological and isotopic evidence. J. Asian Earth Sci. 2004, 23, 605–627. [Google Scholar] [CrossRef]
- Kröner, A.; Kovach, V.; Alexeiev, D.; Wang, K.-L.; Wong, J.; Degtyarev, K.; Kozakov, I. No excessive crustal growth in the Central Asian Orogenic Belt: Further evidence from field relationships and isotopic data. Gondwana Res. 2017, 50, 135–166. [Google Scholar] [CrossRef] [Green Version]
- Yarmolyuk, V.V.; Kovach, V.P.; Kovalenko, V.I.; Salnikova, E.B.; Kozlovskii, A.M.; Kotov, A.B.; Yakovleva, S.Z.; Fedoseenko, A.M. Composition, sources, and mechanism of continental crust growth in the Lake zone of the Central Asian Caledonides: I. Geological and geochronological data. Petrology 2011, 19, 55–78. [Google Scholar] [CrossRef]
- Hanžl, P.; Aichler, J.; Bolormaa, K.; Budil, P.; Buriánek, D.; Hrdličková, K.; Erban, V.; Gerdes, A.; Gilíková, H.; Holák, J.; et al. Geological Survey of the Mongolian Altay at a Scale 1:50.000 (Zamtyn Nuruu—50); Czech Geological Survey: Prague, Czech Republic, 2007; p. 376. [Google Scholar]
- Kröner, A.; Lehmann, J.; Schulmann, K.; Demoux, A.; Lexa, O.; Tomurhuu, D.; Stipska, P.; Liu, D.; Wingate, M.T.D. Lithostratigraphic and geochronological constraints on the evolution of the Central Asian Orogenic Belt in SW Mongolia: Early Paleozoic rifting followed by late Paleozoic accretion. Am. J. Sci. 2010, 310, 523–574. [Google Scholar] [CrossRef]
- Tomurtogoo, O. Geology of Ophiolitic Complex of Mongolia; Geological Funds of Mongolia: Ulanbaatar, Mongolia, 1980. [Google Scholar]
- Perfiliev, A.S.; Kheraskov, N.N. Diabase complexes and the problem of tectonic stratification of oceanic crust. In Tectonic Layering of Lithosphere; Nauka: Moscow, Russia, 1980; Volume 343, pp. 64–104. [Google Scholar]
- Gornova, M.A.; Enkhbat, D.; Karimov, A.A.; Belyaev, V.A.; Gerel, O.; Javkhlan, O. Petrography and mineralogy of retrograde metaperidotites from Alag Khadny accretionary wedge (SW Mongolia): Fluid modification in suprasubduction zone. Geodyn. Tectonophys. 2017, 8, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Demoux, A.; Kröner, A.; Badarch, G.; Jian, P.; Tomurhuu, D.; Wingate, M.T.D. Zircon Ages from the Baydrag Block and the Bayankhongor Ophiolite Zone: Time Constraints on Late Neoproterozoic to Cambrian Subduction- and Accretion-Related Magmatism in Central Mongolia. J. Geol. 2009, 117, 377–397. [Google Scholar] [CrossRef] [Green Version]
- Amosova, A.A.; Panteeva, S.V.; Tatarinov, V.V.; Chubarov, V.M.; Finkelstein, A.L. X-ray fluorescence determination of major rock forming elements in small samples 50 and 110 mg. Anal. Control. 2015, 19, 130–138. [Google Scholar]
- Bottazzi, P.; Ottolini, L.; Vanucci, R.; Zanetti, A. An accurate procedure for the quantification of rare earth elements in silicates. In Proceedings of the Secondary Ion Mass Spectrometry; Wiley: Yokohama, Japan, 1994; pp. 927–930. [Google Scholar]
- Mercier, J.-C.C.; Nicolas, A. Textures and Fabrics of Upper-Mantle Peridotites as Illustrated by Xenoliths from Basalts. J. Petrol. 1975, 16, 454–487. [Google Scholar] [CrossRef]
- Seyler, M.; Lorand, J.-P.; Dick, H.J.B.; Drouin, M. Pervasive melt percolation reactions in ultra-depleted refractory harzburgites at the Mid-Atlantic Ridge, 15° 20′ N: ODP Hole 1274A. Contrib. Mineral. Petrol. 2007, 153, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Dick, H.J.B.; Bullen, T. Chromian spinel as a petrogenetic indicator in abyssal and alpine-type peridotites and spatially associated lavas. Contrib. Mineral. Petrol. 1984, 86, 54–76. [Google Scholar] [CrossRef]
- Parkinson, I.J.; Pearce, J.A. Peridotites from the Izu–Bonin–Mariana Forearc (ODP Leg 125): Evidence for Mantle Melting and Melt–Mantle Interaction in a Supra-Subduction Zone Setting. J. Petrol. 1998, 39, 1577–1618. [Google Scholar] [CrossRef]
- Evans, B.; Frost, R. Chrome-spinel in progressive metamorphism—A preliminary analysis. Geochim. Cosmochim. Acta 1975, 39, 959–972. [Google Scholar] [CrossRef]
- Arai, S. Characterization of spinel peridotites by olivine-spinel compositional relationships: Review and interpretation. Chem. Geol. 1994, 113, 191–204. [Google Scholar] [CrossRef]
- Hellebrand, E.; Snow, J.E.; Dick, H.J.B.; Hofmann, A.W. Coupled major and trace elements as indicators of the extent of melting in mid-ocean-ridge peridotites. Nature 2001, 410, 677–681. [Google Scholar] [CrossRef]
- Takahashi, E.; Uto, K.; Schilling, J.-G. Primary Magma Compositions and Mg/Fe Ratios of Their Mantle Residues along Mid Atlantic Ridge 29 N to73 N; Okayama University: Okayama, Japan, 1987; pp. 1–4. [Google Scholar]
- Workman, R.K.; Hart, S.R. Major and trace element composition of the depleted MORB mantle (DMM). Earth Planet. Sci. Lett. 2005, 231, 53–72. [Google Scholar] [CrossRef]
- Seyler, M.; Cannat, M.; Mével, C. Evidence for major-element heterogeneity in the mantle source of abyssal peridotites from the Southwest Indian Ridge (52° to 68° E): Major-element heterogenity. Geochem. Geophys. Geosystems 2003, 4, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Hellebrand, E. Garnet-field Melting and Late-stage Refertilization in “Residual” Abyssal Peridotites from the Central Indian Ridge. J. Petrol. 2002, 43, 2305–2338. [Google Scholar] [CrossRef]
- Ishii, T.; Robinson, P.T.; Maekava, H.; Fiske, R. Petrological studies of peridotites from diapiric serpentinite seamounts in the Izu-Ogasawara-Mariana Forearc, Leg 125. In Proceedings of the Ocean Drilling Program, 125 Scientific Results; Fryer, P., Pearce, J.A., Stokking, L.B., Ali, J.R., Arculus, R., Balotti, D., Burke, M.M., Ciampo, G., Haggerty, J.A., Haston, R.B., et al., Eds.; Ocean Drilling Program: College Station, TX, USA, 1992; Volume 125, pp. 445–485. [Google Scholar] [CrossRef]
- Herzberg, C. Geodynamic Information in Peridotite Petrology. J. Petrol. 2004, 45, 2507–2530. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y. Bulk-rock Major and Trace Element Compositions of Abyssal Peridotites: Implications for Mantle Melting, Melt Extraction and Post-melting Processes Beneath Mid-Ocean Ridges. J. Petrol. 2004, 45, 2423–2458. [Google Scholar] [CrossRef] [Green Version]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Jagoutz, E.; Palme, H.; Baddenhausen, H.B.; Cendales, M.; Dreibus, G. The abundances of major, minor and trace elements in the earth’s mantle as derived from primitive ultramafic nodules. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 19–23 March 1979; Volume 2, pp. 2031–2050. [Google Scholar]
- Malvoisin, B. Mass transfer in the oceanic lithosphere: Serpentinization is not isochemical. Earth Planet. Sci. Lett. 2015, 430, 75–85. [Google Scholar] [CrossRef]
- Marchesi, C.; Garrido, C.J.; Proenza, J.A.; Hidas, K.; Varas-Reus, M.I.; Butjosa, L.; Lewis, J.F. Geochemical record of subduction initiation in the sub-arc mantle: Insights from the Loma Caribe peridotite (Dominican Republic). Lithos 2016, 252–253, 1–15. [Google Scholar] [CrossRef]
- Johnson, K.T.M.; Dick, H.J.B.; Shimizu, N. Melting in the oceanic upper mantle: An ion microprobe study of diopsides in abyssal peridotites. J. Geophys. Res. 1990, 95, 2661–2678. [Google Scholar] [CrossRef] [Green Version]
- Bizimis, M.; Salters, V.J.M.; Bonatti, E. Trace and REE content of clinopyroxenes from supra-subduction zone peridotites. Implications for melting and enrichment processes in island arcs. Chem. Geol. 2000, 165, 67–85. [Google Scholar] [CrossRef]
- Parkinson, I.J.; Johnson, K.T.M.; Ingram, G. Trace element geochemistry of peridotites from the Izu-Bonin-Mariana Forearc, Leg 125. In Proceedings of the Ocean Drilling Program, 125 Scientific Results, Tokyo, Japan, 15 February–17 April 1989; Fryer, P., Pearce, J.A., Stokking, L.B., Ali, J.R., Arculus, R., Balotti, D., Burke, M.M., Ciampo, G., Haggerty, J.A., Haston, R.B., et al., Eds.; Ocean Drilling Program: College Station, TX, USA, 1992; Volume 125, pp. 487–506. [Google Scholar] [CrossRef]
- Liu, X.; Neil, H.S.C. The Effect of Cr2O3 on the Partial Melting of Spinel Lherzolite in the System CaO-MgO–Al2O3–SiO2–Cr2O3 at 1.1 GPa. J. Petrol. 2004, 45, 2261–2286. [Google Scholar] [CrossRef]
- Brunelli, D.; Seyler, M.; Cipriani, A.; Ottolini, L.; Bonatti, E. Discontinuous Melt Extraction and Weak Refertilization of Mantle Peridotites at the Vema Lithospheric Section (Mid-Atlantic Ridge). J. Petrol. 2006, 47, 745–771. [Google Scholar] [CrossRef]
- Deschamps, F.; Guillot, S.; Godard, M.; Chauvel, C.; Andreani, M.; Hattori, K. In situ characterization of serpentinites from forearc mantle wedges: Timing of serpentinization and behavior of fluid-mobile elements in subduction zones. Chem. Geol. 2010, 269, 262–277. [Google Scholar] [CrossRef]
- Murata, K.; Maekawa, H.; Yokose, H.; Yamamoto, K.; Fujioka, K.; Ishii, T.; Chiba, H.; Wada, Y. Significance of serpentinization of wedge mantle peridotites beneath Mariana forearc, western Pacific. Geosphere 2009, 5, 90–104. [Google Scholar] [CrossRef]
- Batanova, V.G. Mantle Lherzolites from Troodos Ophiolites: Mineralogy and Ion Probe Geochemistry of Clinopyroxenes. Mineral. Mag. 1994, 58A, 57–58. [Google Scholar] [CrossRef]
- Ozawa, K.; Shimizu, N. Open-system melting in the upper mantle: Constraints from the Hayachine-Miyamori ophiolite, northeastern Japan. J. Geophys. Res. Solid Earth 1995, 100, 22315–22335. [Google Scholar] [CrossRef]
- Pagé, P.; Bédard, J.H.; Tremblay, A. Geochemical variations in a depleted fore-arc mantle: The ordovician thetford mines ophiolite. Lithos 2009, 113, 21–47. [Google Scholar] [CrossRef]
- Morishita, T.; Dilek, Y.; Shallo, M.; Tamura, A.; Arai, S. Insight into the uppermost mantle section of a maturing arc: The Eastern Mirdita ophiolite, Albania. Lithos 2011, 124, 215–226. [Google Scholar] [CrossRef]
- Churikova, T.; Dorendorf, F.; Wörner, G. Sources and Fluids in the Mantle Wedge below Kamchatka, Evidence from Across-arc Geochemical Variation. J. Petrol. 2001, 42, 1567–1593. [Google Scholar] [CrossRef] [Green Version]
- Vils, F.; Pelletier, L.; Kalt, A.; Müntener, O.; Ludwig, T. The Lithium, Boron and Beryllium content of serpentinized peridotites from ODP Leg 209 (Sites 1272A and 1274A): Implications for lithium and boron budgets of oceanic lithosphere. Geochim. Cosmochim. Acta 2008, 72, 5475–5504. [Google Scholar] [CrossRef] [Green Version]
- Godard, M.; Bodinier, J.-L.; Vasseur, G. Effects of mineralogical reactions on trace element redistributions in mantle rocks during percolation processes: A chromatographic approach. Earth Planet. Sci. Lett. 1995, 133, 449–461. [Google Scholar] [CrossRef]
- Vernières, J.; Godard, M.; Bodinier, J.-L. A plate model for the simulation of trace element fractionation during partial melting and magma transport in the Earth’s upper mantle. J. Geophys. Res. Solid Earth 1997, 102, 24771–24784. [Google Scholar] [CrossRef]
- Zou, H. Trace element fractionation during modal and nonmodal dynamic melting and open-system melting: A mathematical treatment. Geochim. Cosmochim. Acta 1998, 62, 1937–1945. [Google Scholar] [CrossRef]
- Ersoy, E.Y. PETROMODELER (Petrological Modeler): A Microsoft® Excel© spreadsheet program for modelling melting, mixing, crystallization and assimilation processes in magmatic systems. Turk. J. Earth Sci. 2013, 22, 115–125. [Google Scholar] [CrossRef]
- Gaetani, G.A.; Grove, T.L. The influence of water on melting of mantle peridotite. Contrib. Mineral. Petrol. 1998, 131, 323–346. [Google Scholar] [CrossRef]
- Tamura, A.; Arai, S.; Ishimaru, S.; Andal, E.S. Petrology and geochemistry of peridotites from IODP Site U1309 at Atlantis Massif, MAR 30° N: Micro- and macro-scale melt penetrations into peridotites. Contrib. Mineral. Petrol. 2008, 155, 491–509. [Google Scholar] [CrossRef]
- Li, X.-P.; Rahn, M.; Bucher, K. Serpentinites of the Zermatt-Saas ophiolite complex and their texture evolution. J. Metamorph. Geol. 2004, 22, 159–177. [Google Scholar] [CrossRef]
- Berman, R.G.; Engi, M.; Greenwood, H.J.; Brown, T.H. Derivation of Internally-Consistent Thermodynamic Data by the Technique of Mathematical Programming: A Review with Application the System MgO–SiO2–H2O. J. Petrol. 1986, 27, 1331–1364. [Google Scholar] [CrossRef]
- Berman, R.G. Internally-Consistent Thermodynamic Data for Minerals in the System Na2O–K2O–CaO–MgO–FeO–Fe2O3–Al2O3–SiO2–TiO2–H2O–CO2. J. Petrol. 1988, 29, 445–522. [Google Scholar] [CrossRef]
- Savelieva, G.N.; Raznitsin, Y.N.; Merkulova, M.V. Metamorphism of peridotites in the mantle wedge above the subduction zone: Hydration of the lithospheric mantle. Dokl. Earth Sci. 2016, 468, 438–440. [Google Scholar] [CrossRef]
- Davis, E.E.; Chapman, D.S. Lithosphere, Oceanic: Thermal Structure. Encycl. Solid Earth Geophys. 2011, 709–716. [Google Scholar] [CrossRef]
- Peacock, S.M. Seismic Consequences of Warm Versus Cool Subduction Metamorphism: Examples from Southwest and Northeast Japan. Science 1999, 286, 937–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grove, T.L.; Till, C.B.; Lev, E.; Chatterjee, N.; Médard, E. Kinematic variables and water transport control the formation and location of arc volcanoes. Nature 2009, 459, 694–697. [Google Scholar] [CrossRef]
- Agard, P.; Vitale-Brovarone, A. Thermal regime of continental subduction: The record from exhumed HP–LT terranes (New Caledonia, Oman, Corsica). Tectonophysics 2013, 601, 206–215. [Google Scholar] [CrossRef]
- Guillot, S.; Hattori, K.H.; De Sigoyer, J. Mantle wedge serpentinization and exhumation of eclogites: Insights from eastern Ladakh, northwest Himalaya. Geology 2000, 28, 199–202. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornova, M.; Karimov, A.; Skuzovatov, S.; Belyaev, V. From Decompression Melting to Mantle-Wedge Refertilization and Metamorphism: Insights from Peridotites of the Alag Khadny Accretionary Complex (SW Mongolia). Minerals 2020, 10, 396. https://doi.org/10.3390/min10050396
Gornova M, Karimov A, Skuzovatov S, Belyaev V. From Decompression Melting to Mantle-Wedge Refertilization and Metamorphism: Insights from Peridotites of the Alag Khadny Accretionary Complex (SW Mongolia). Minerals. 2020; 10(5):396. https://doi.org/10.3390/min10050396
Chicago/Turabian StyleGornova, Marina, Anas Karimov, Sergei Skuzovatov, and Vasiliy Belyaev. 2020. "From Decompression Melting to Mantle-Wedge Refertilization and Metamorphism: Insights from Peridotites of the Alag Khadny Accretionary Complex (SW Mongolia)" Minerals 10, no. 5: 396. https://doi.org/10.3390/min10050396





