Geochemical Behavior of Potentially Toxic Elements in Riverbank-Deposited Weathered Tailings and Their Environmental Effects: Weathering of Pyrite and Manganese Pyroxene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Analysis
3. Results
3.1. Sample pH
3.2. Mineral Compositions
3.3. Chemical Compositions of Major Elements
3.4. Chemical Compositions of Potentially Toxic Elements
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pagnanelli, F.; Moscardini, E.; Giuliano, V.; Toro, L. Sequential extraction of heavy metals in river sediments of an abandoned pyrite mining area: Pollution detection and affinity series. Environ. Pollut. 2004, 132, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, D.; Hudson-Edwards, K.A.; Dubbin, W.E.; Alfredsson, M. Major and trace metal mobility during weathering of mine tailings: Implications for flood plain soils. Appl. Geochem. 2012, 27, 562–576. [Google Scholar] [CrossRef]
- Rico, M.; Benito, G.; Salgueiro, A.R.; Díez-Herrero, A.; Pereira, H.G. Reported tailings dam failures A review of the European incidents in the worldwide context. J. Hazard. Mater. 2008, 152, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimalt, J.O.; Ferrer, M.; Macpherson, E. The mine tailing accident in Aznalcollar. Sci. Total Environ. 1999, 242, 3–11. [Google Scholar] [CrossRef]
- Benito, G.; Benito-Calvo, A.; Gallart, F.; Martín-Vide, J.P.; Regües, D.; Bladé, E. Hydrological and geomorphological criteria to evaluate the dispersion risk of waste sludge generated by the Aznalcollar mine spill (SW Spain). Environ. Geol. 2001, 40, 417–428. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef] [Green Version]
- García-Carmona, M.; García-Robles, H.; Turpín Torrano, C.; Fernández Ondoño, E.; Lorite Moreno, J.; Sierra Aragón, M.; Martín Peinado, F.J. Residual pollution and vegetation distribution in amended soils 20 years after a pyrute mine tailings spill (Aznalcóllar, Spain). Sci. Total Environ. 2019, 650, 933–940. [Google Scholar] [CrossRef]
- Ji, S.; Kim, S.; Ko, J. The status of the passive treatment systems for acid mine drainage in South Korea. Environ. Geol. 2008, 55, 1181–1194. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, Y. A quantitative estimation of factors affecting pH changes using simple geochemical data from acid mine drainage. Environ. Geol. 2008, 55, 65–75. [Google Scholar] [CrossRef]
- Fukushi, K.; Sato, T.; Yanase, N. Solid-solution reaction in As(V) sorption by schwertmannite. Environ. Sci. Technol. 2003, 37, 3581–3586. [Google Scholar] [CrossRef]
- Fukushi, K.; Sato, T.; Yanase, N.; Minato, J.; Yamada, H. Arsenate sorption on schwertmannite. Am. Mineral. 2004, 89, 1728–1734. [Google Scholar] [CrossRef]
- Burgos, W.D.; Borch, T.; Troyer, L.D.; Luan, F.; Larson, L.N.; Brown, J.F.; Lambson, J.; Shimizu, M. Schwermannite and Fe oxides formed by biological lo-pH Fe(II) oxidation versus abiotic neutralization: Impact on trace metal sequestration. Geochim. Cosmochim. Acta 2012, 76, 29–44. [Google Scholar] [CrossRef]
- Baleeiro, A.; Fiol, S.; Otero-Fariña, A.; Antelo, J. Surface chemistry of iron oxides formed by neutralization of acidic mine waters: Removal of trace metals. Appl. Geochem. 2018, 89, 129–137. [Google Scholar] [CrossRef]
- Kim, Y. Effects of different oxyanions in solution on the precipitation of jarosite at room temperature. J. Hazard. Mater. 2018, 353, 118–126. [Google Scholar]
- Hajji, S.; Montes-Hernandez, G.; Sarret, G.; Tordo, A.; Morin, G.; Ona-Nguema, G.; Bureau, S.; Turki, T.; Mzoughi, N. Arsenite and chromate sequestration onto ferrihydrite, siderite and goethite nanostructured minerals: Isotherms from flow-through Reactor Experiments and XAS measurements. J. Hazard. Mater. 2019, 362, 358–367. [Google Scholar] [CrossRef]
- Mascaro, I.; Benvenuti, B.; Corsini, F.; Costagliola, P.; Lattanzi, P.; Parrini, P.; Tanelli, G. Mine wastes at the polymetallic deposit of Fenice Capanne (southern Tuscany, Italy), Mineralogy, geochemistry, and environmental impact. Environ. Geol. 2001, 41, 417–429. [Google Scholar]
- Meza-Figueroa, D.; Maier, R.M.; de la O-Villanueva, M.; Gómez-Alvarez, A.; Morenzo-Zazueta, A.; Rivera, J.; Campillo, A.; Grandlic, C.J.; Anaya, R.; Palafox-Reyes, J. The impact of unconfined mine tailings in residential areas from a mining town in a semi-arid environment: Nacozari, Sonora, Mexico. Chemosphere 2009, 77, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Li, X.D.; Coles, B.J.; Ramsey, M.H.; Thornton, I. Sequential extraction of soils for multi-element analysis by ICP-AES. Chem. Geol. 1995, 124, 109–123. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Schaller, W.T. Johansennite, a new manganese pyroxene. Am. Mineral. 1938, 23, 575–582. [Google Scholar]
- Martínez, C.E.; McBride, M.B. Coprecipitates of Cd, Cu, Pb and Zn in iron oxides: Solid phase transformation and metal solubility after aging and thermal treatment. Clays Clay Miner. 1998, 46, 537–545. [Google Scholar] [CrossRef]
- Martínez, C.E.; McBride, M.B. Cd, Cu, Pb, and Zn coprecipitates in Fe oxide formed at different pH: Aging effects on metal solubility and extractability by citrate. Environ. Toxicol. Chem. 2001, 20, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Bigham, J.M.; Faure, G. Removal of trace metals by coprecioitation with Fe, Al and Mn from natural eaters contaminated with acid mine drainage in the Bucktown mining district, Tennessee. Appl. Geochem. 2002, 17, 569–581. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Q.; Sun, J.; Borkiewicz, O.J.; Huang, R.; Saad, E.M.; Fields, B.; Chen, S.; Zhu, M.; Tang, Y. Effect of Zn coprecipitation on the structure of layererd Mn oxides. Chem. Geol. 2018, 493, 234–245. [Google Scholar] [CrossRef]
- Jönsson, J.; Persson, P.; Sjöberg, S.; Lövgren, L. Schwertmannite precipitated from acid mine drainage: Phase transformation, sulphate release and surface properties. Appl. Geochem. 2005, 20, 179–191. [Google Scholar] [CrossRef]
- Schroth, A.W.; Parnell, R.A. Trace metal retention through the schwertmannite to goethite transformation as observed in a field setting, Alta Mine, MT. Appl. Geochem. 2005, 20, 907–917. [Google Scholar] [CrossRef]
- Lee, P.K.; Touray, J.C. Characteristics of a Polluted Artificial Soil located along a Motorway and Effects of Acidification on the leaching Behavior of heavy metals (Pb, Zn, Cd). Water Res. 1998, 32, 3425–3435. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.; Choo, C.O. The effect of mineralogy on the mobility of heavy metals in mine tailings: A case study in the Samsanjeil mine, Korea. Environ. Earth Sci. 2014, 71, 3429–3441. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y. Mineralogical changes and distribution of heavy metals caused by the weathering of hydrothermally altered, pyrite-rich andesite. Environ. Earth Sci. 2016, 75, 1125. [Google Scholar] [CrossRef]
- Jurjovec, J.; Ptacek, C.J.; Blowes, D.W. Acid neutralization mechanisms and metal release in mine tailings: A laboratory column experiment. Geochim. Cosmochim. Acta 2002, 66, 1511–1523. [Google Scholar] [CrossRef]
- Heikkinen, P.M.; Räisänen, M.L. Mineralogical and geochemical alteration of Hitura sulphide mine tailings with emphasis on nickel mobility and retention. J. Geochem. Explor. 2008, 97, 1–20. [Google Scholar] [CrossRef]
- Jiang, W.; Lv, J.; Luo, L.; Yang, K.; Lin, Y.; Hu, F.; Zhang, J.; Zhang, S. Arsenate and cadmium co-adsorption and co-precipitation on goethite. J. Hazard. Mater. 2013, 262, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Dold, B.; Fontboté, L. A mineralogical and geochemical study of element mobility in sulfide mine tailings of Fe oxide Cu-Au deposits from the Punta del Cobre belt, northern Chile. Chem. Geol. 2002, 189, 135–163. [Google Scholar] [CrossRef]
- Carlsson, E.; Thunberg, J.; Ohlander, B.; Holmström, H. Sequential extraction of sulfide-rich tailings remediated by the application of till cover, Kristineberg mine, northern Sweden. Sci. Total Environ. 2002, 299, 207–226. [Google Scholar] [CrossRef]
- Caraballo, M.A.; Rotting, T.S.; Nieto, J.M.; Ayora, C. Sequential extraction and DXRD applicability to poorly crystalline Fe- and Al-phase characterization from an acid mine water passive remediation system. Am. Mineral. 2009, 94, 1029–1038. [Google Scholar] [CrossRef]
- Kim, Y. Mineral phases and mobilities of trace metals in white precipitates found in acid mine drainage. Chemosphere 2015, 119, 803–811. [Google Scholar] [CrossRef]

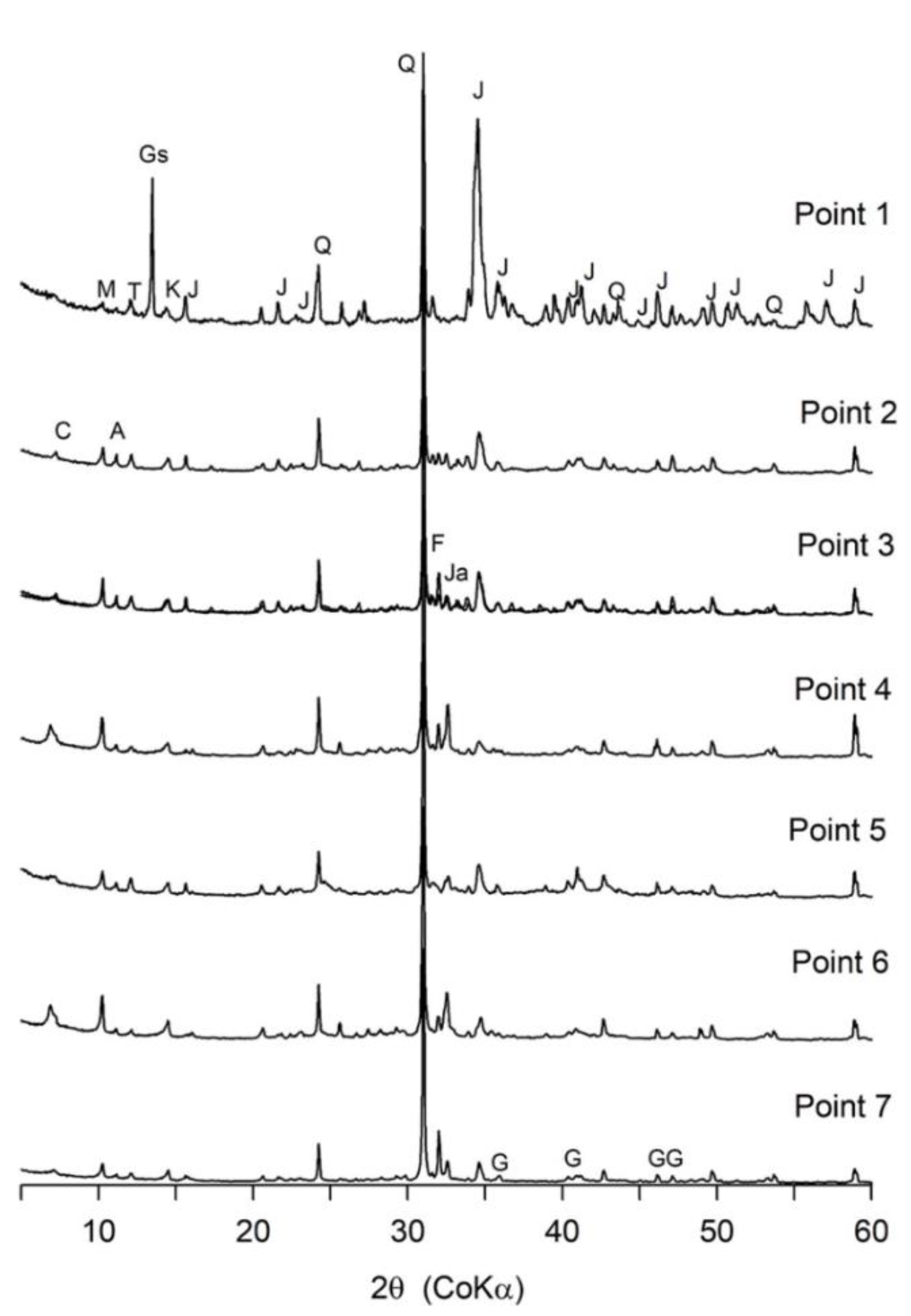
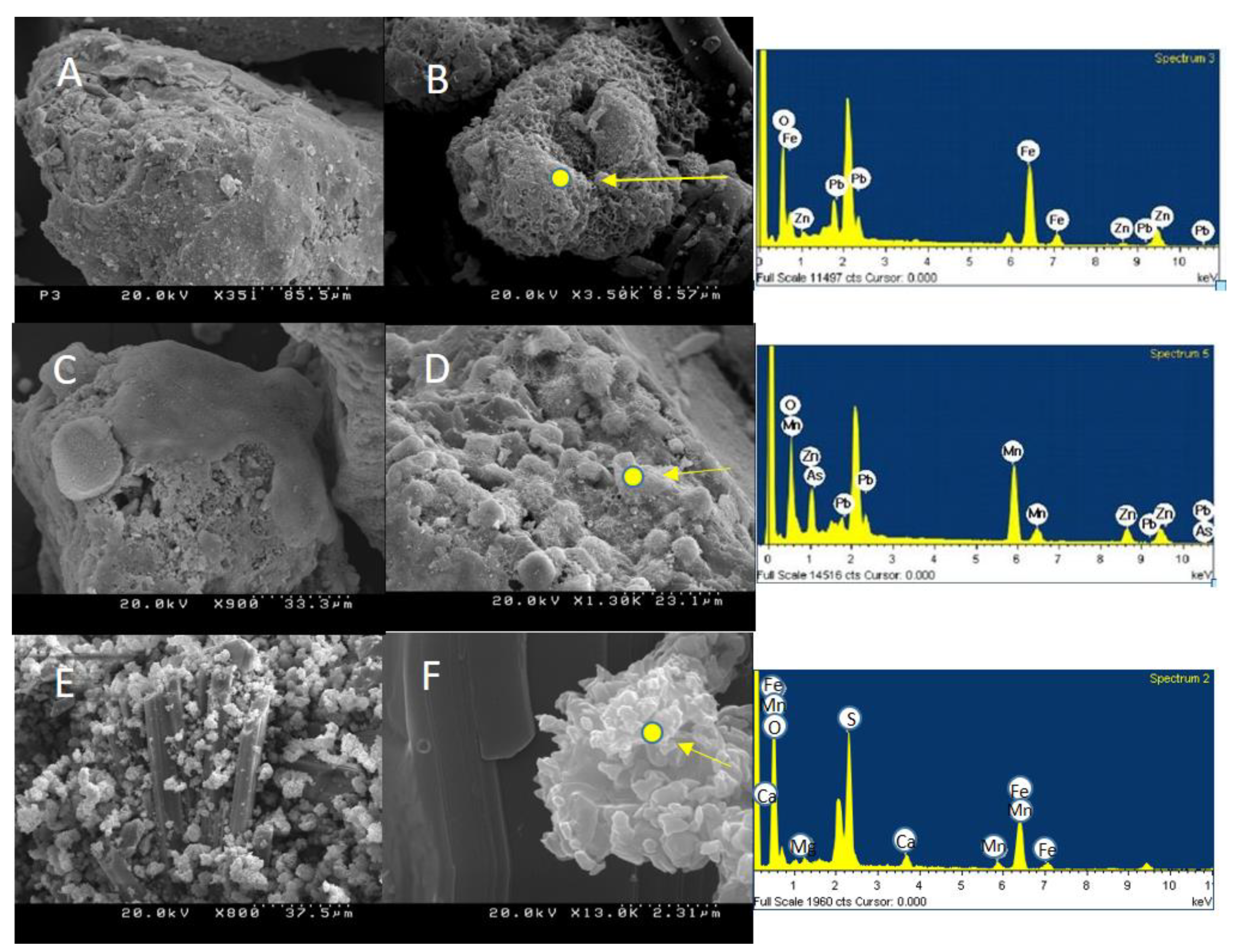
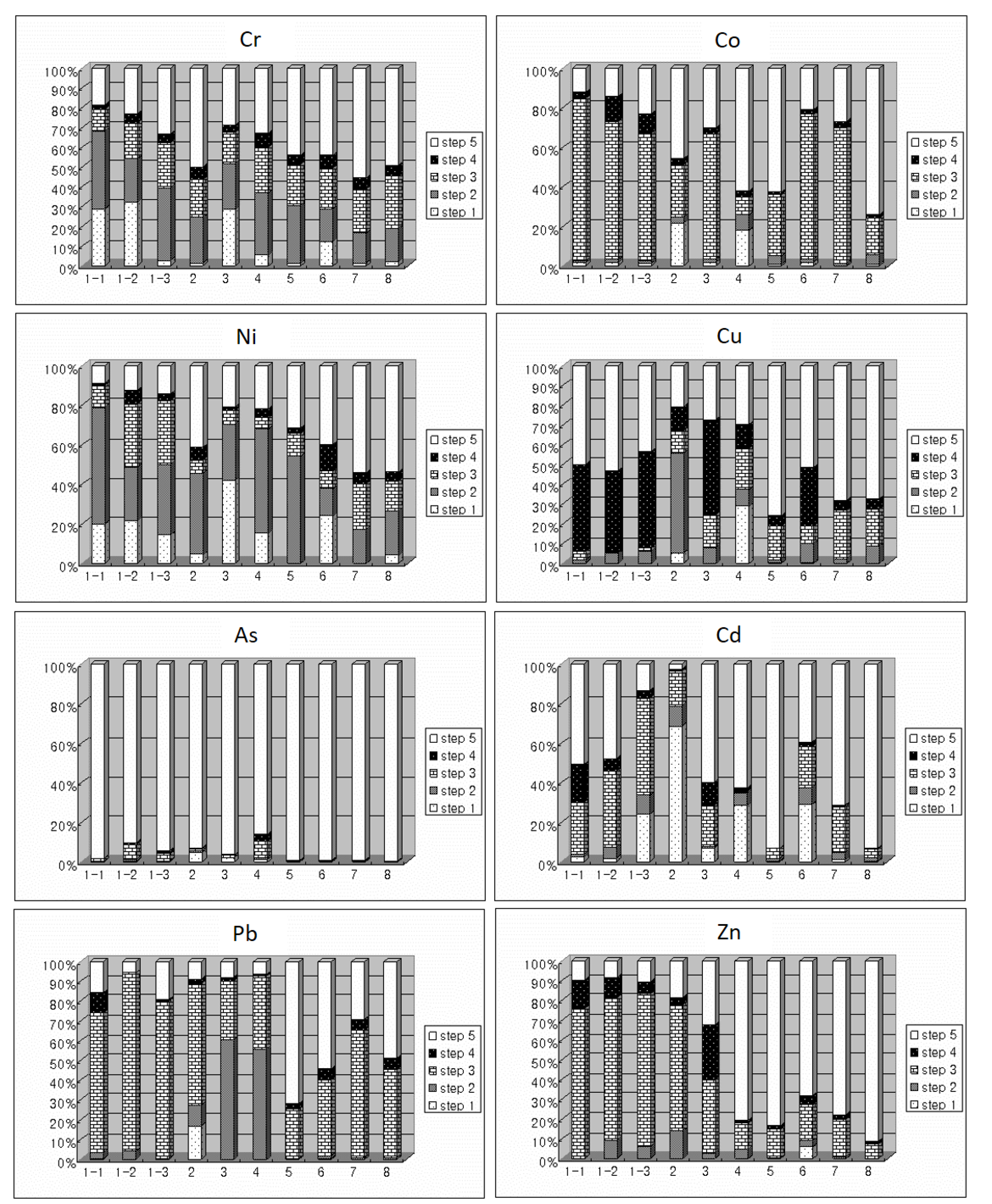
| Sampling Point | Number of Samples | Average Thickness | Color | pH (Average Value) |
|---|---|---|---|---|
| 1 | 11 | 55 cm | Varied, mostly black | 3.83–7.93 (6.68) |
| 2 | 3 | 25 cm | Brown, interlayered with gray | 3.22–4.05 (3.57) |
| 3 | 4 | 45 cm | Red, gray, and black | 4.15–4.87 (4.50) |
| 4 | 4 | 15 cm | Gray, brown, black, and yellow | 2.59–3.35 (3.10) |
| 5 | 2 | 3 cm | Red with a thin black layer | 6.08–6.26 (6.17) |
| 6 | 4 | 56 cm | Red and black | 4.07–5.82 (4.79) |
| 7 | 2 | 8 cm | Brown | 6.57–6.66 (6.62) |
| 8 | 3 | 28 cm | Brown, gray, and black | 6.62–6.68 (6.64) |
| B | D | E | |||
|---|---|---|---|---|---|
| Element | wt% | Element | wt% | Element | wt% |
| O | 39.29 | O | 36.67 | O | 56.80 |
| Fe | 49.72 | Mn | 36.04 | Mg | 1.10 |
| Zn | 2.82 | Zn | 17.21 | S | 16.11 |
| Pb | 8.18 | As | 1.82 | Ca | 1.95 |
| Pb | 8.25 | Mn | 2.82 | ||
| Fe | 22.22 | ||||
| Total | 100 | 100 | 100 | ||
| 1-1 | 1-2 | 1-3 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | 6.84 | 5.79 | 6.37 | 11.16 | 9.42 | 9.26 | 7.13 | 5.94 | 11.43 | 11.4 |
| CaO | 6.52 | 15.28 | 6.28 | 2.31 | 2.85 | 2.55 | 5.67 | 9.74 | 3.58 | 3.94 |
| Fe2O3 | 20.85 | 14.37 | 13.15 | 7.44 | 8.3 | 6.36 | 21.33 | 15.93 | 14.93 | 18.89 |
| K2O | 1.10 | 0.83 | 1.58 | 2.87 | 3.02 | 3.4 | 1.45 | 1.36 | 2.10 | 1.93 |
| MgO | 1.49 | 1.55 | 1.33 | 1.14 | 0.95 | 0.84 | 1.45 | 1.08 | 1.45 | 1.20 |
| MnO | 4.78 | 4.07 | 5.27 | 0.80 | 1.63 | 0.74 | 2.28 | 2.72 | 1.61 | 1.61 |
| Na2O | 0.37 | 0.22 | 0.55 | 1.31 | 1.19 | 1.45 | 0.56 | 0.61 | 1.34 | 0.97 |
| P2O5 | 0.14 | 0.14 | 0.11 | 0.09 | 0.09 | 0.11 | 0.13 | 0.1 | 0.14 | 0.11 |
| SiO2 | 50.00 | 38.72 | 57.33 | 64.98 | 67.62 | 71.17 | 51.18 | 48.32 | 54.82 | 49.84 |
| TiO | 0.34 | 0.36 | 0.33 | 0.49 | 0.36 | 0.44 | 0.43 | 0.32 | 0.52 | 0.43 |
| LOI | 7.18 | 18.14 | 7.95 | 7.88 | 5.23 | 3.85 | 9.07 | 13.48 | 8.13 | 9.29 |
| Total | 99.61 | 99.47 | 100.25 | 100.47 | 100.66 | 100.17 | 100.68 | 99.60 | 100.05 | 99.61 |
| 1-1 | 1-2 | 1-3 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| As | 2178 | 1281 | 1109 | 117.2 | 132.9 | 499.9 | 4191 | 2303 | 1746 | 2510 |
| Cd | 15.43 | 18.65 | 71.29 | 11.93 | 7.077 | <5 | 6.759 | 19.75 | 5.52 | 5.454 |
| Co | 20.38 | 24.53 | 12.29 | 7.432 | 12.39 | <5 | 6.56 | 13.7 | 14.59 | 6.233 |
| Cr | 62.49 | 22.5 | 12.69 | 30.12 | 17.89 | 11 | 27.43 | 19.55 | 131.3 | 25.91 |
| Cu | 173.2 | 135.4 | 135.7 | 50.27 | 70.58 | 20.03 | 105.6 | 95.33 | 66.64 | 60.38 |
| Ni | 17.53 | 34.46 | 24.97 | 11.34 | 12.39 | 8.446 | 12.92 | 14.51 | 14.98 | 12.27 |
| Pb | 3270 | 2261 | 1904 | 208.3 | 836.1 | 82.49 | 1355 | 1481 | 1406 | 2047 |
| Zn | 5364 | 3201 | 10996 | 762.8 | 1075 | 246.3 | 1010 | 1567 | 889.8 | 833.1 |
| As | Cd | Co | Cr | Cu | Ni | Pb | Zn | pH | |
|---|---|---|---|---|---|---|---|---|---|
| As | 1 | ||||||||
| Cd | 0.130 | 1 | |||||||
| Co | 0.044 | 0.195 | 1 | ||||||
| Cr | 0.157 | −0.268 | 0.269 | 1 | |||||
| Cu | 0.358 | 0.492 | 0.734 * | 0.046 | 1 | ||||
| Ni | 0.017 | 0.537 | 0.787 ** | −0.057 | 0.686 * | 1 | |||
| Pb | 0.470 | 0.270 | 0.676 * | 0.220 | 0.860 ** | 0.568 | 1 | ||
| Zn | −0.050 | 0.934 ** | 0.366 | −0.136 | 0.695 * | 0.592 | 0.518 | 1 | |
| pH | 0.460 | 0.344 | 0.555 | 0.345 | 0.720 * | 0.670 * | 0.853 ** | 0.546 | 1 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y. Geochemical Behavior of Potentially Toxic Elements in Riverbank-Deposited Weathered Tailings and Their Environmental Effects: Weathering of Pyrite and Manganese Pyroxene. Minerals 2020, 10, 413. https://doi.org/10.3390/min10050413
Kim Y. Geochemical Behavior of Potentially Toxic Elements in Riverbank-Deposited Weathered Tailings and Their Environmental Effects: Weathering of Pyrite and Manganese Pyroxene. Minerals. 2020; 10(5):413. https://doi.org/10.3390/min10050413
Chicago/Turabian StyleKim, Yeongkyoo. 2020. "Geochemical Behavior of Potentially Toxic Elements in Riverbank-Deposited Weathered Tailings and Their Environmental Effects: Weathering of Pyrite and Manganese Pyroxene" Minerals 10, no. 5: 413. https://doi.org/10.3390/min10050413




