Hydrothermal Alteration of Etna Ash and Implications for Mars
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Ash and Mineral Phases
2.2. Hydrothermal Alteration of Ashes
3. Results
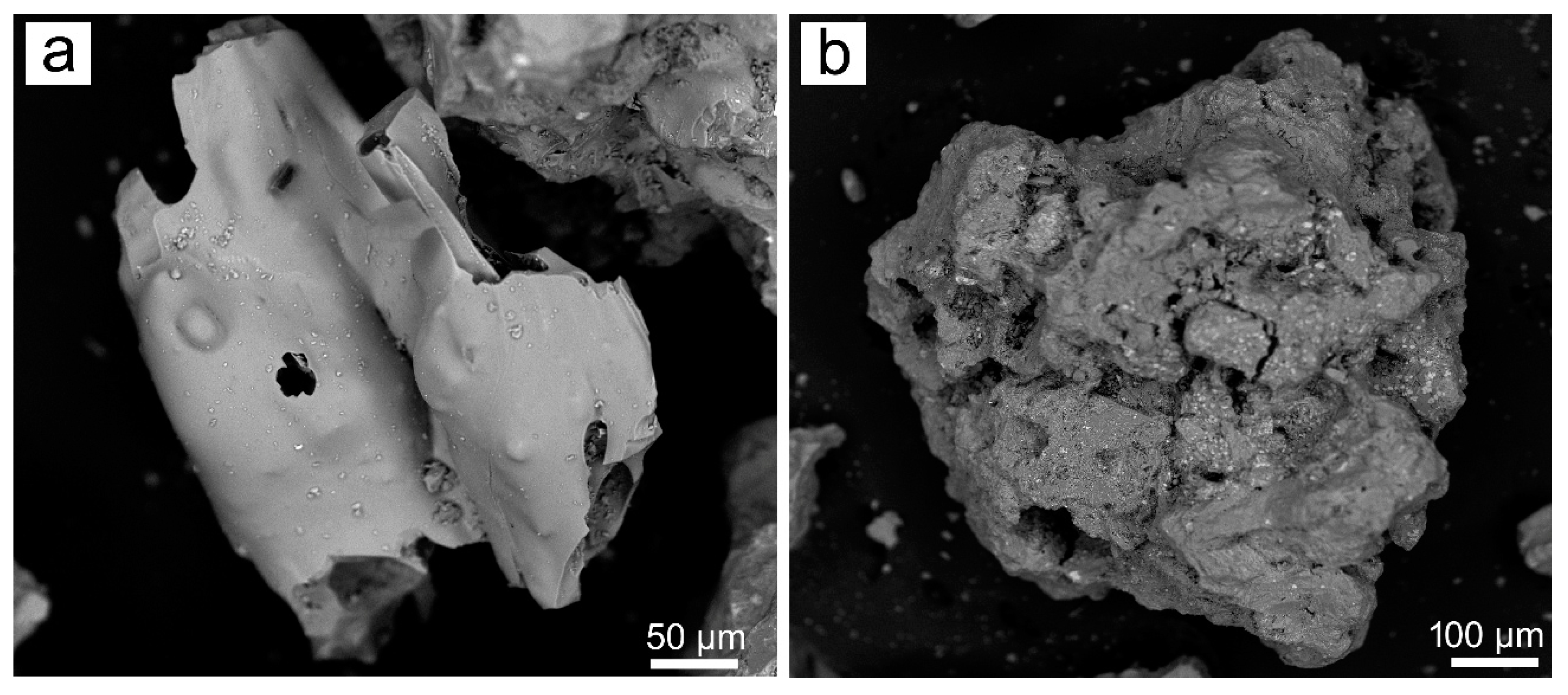
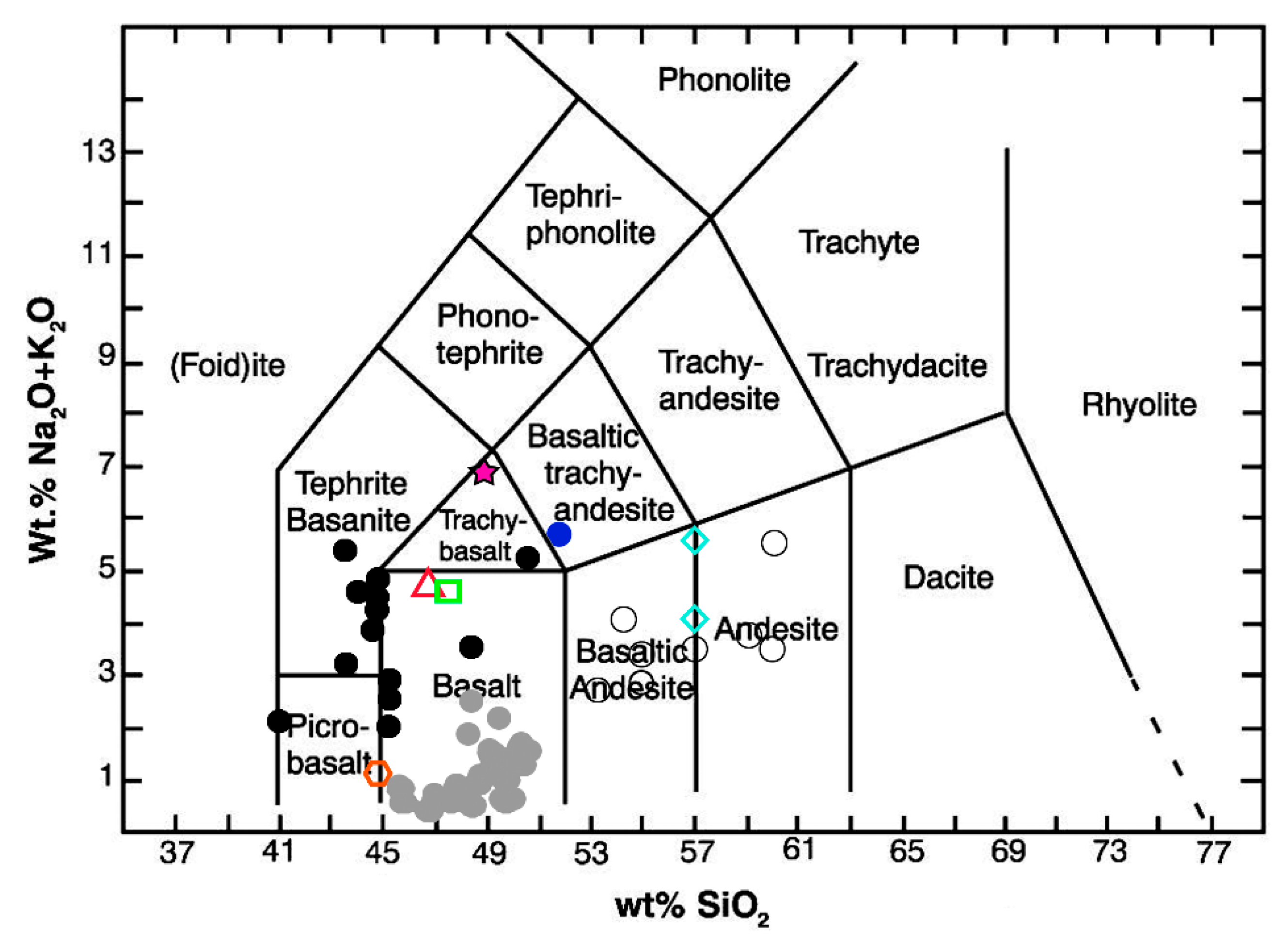
3.1. Ashes Characterization
3.2. Characterization of Run Products
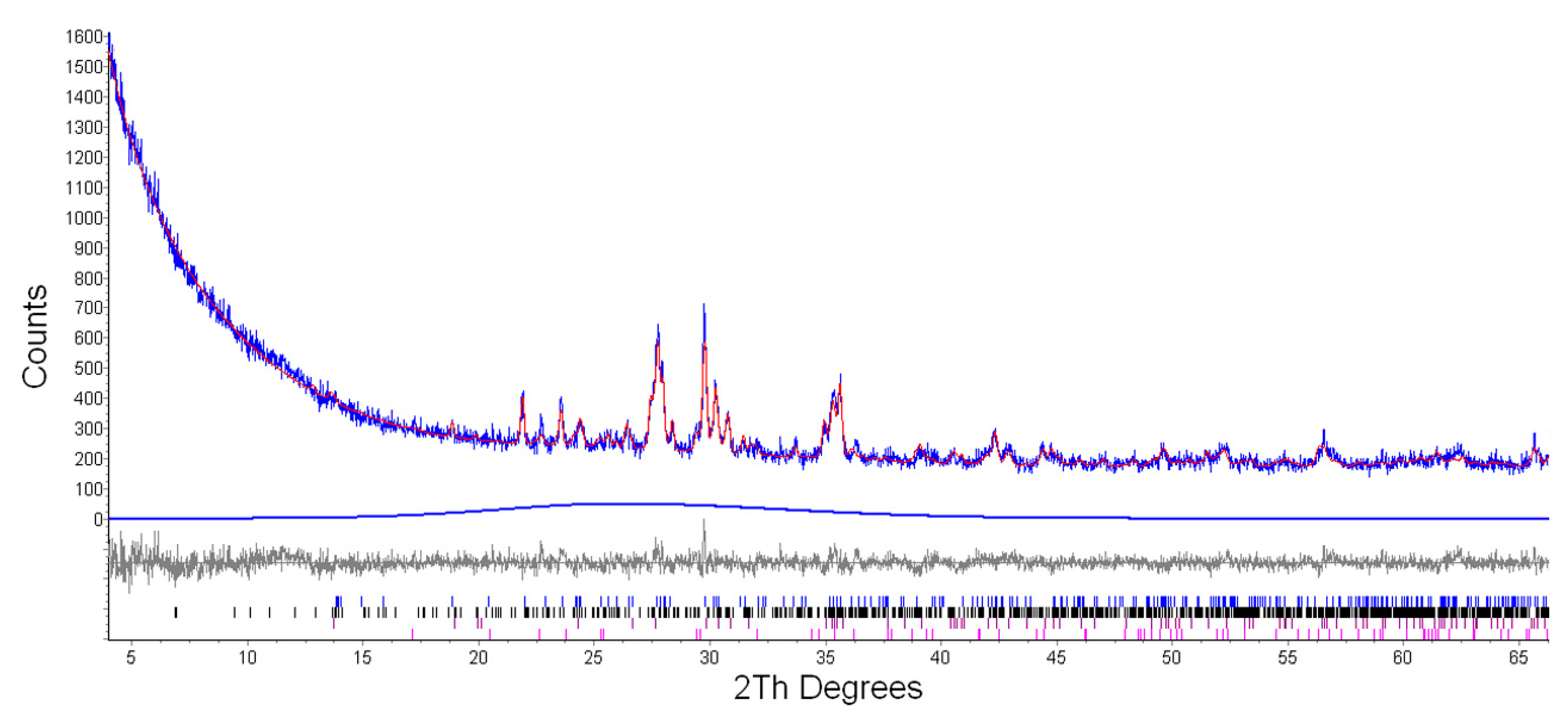
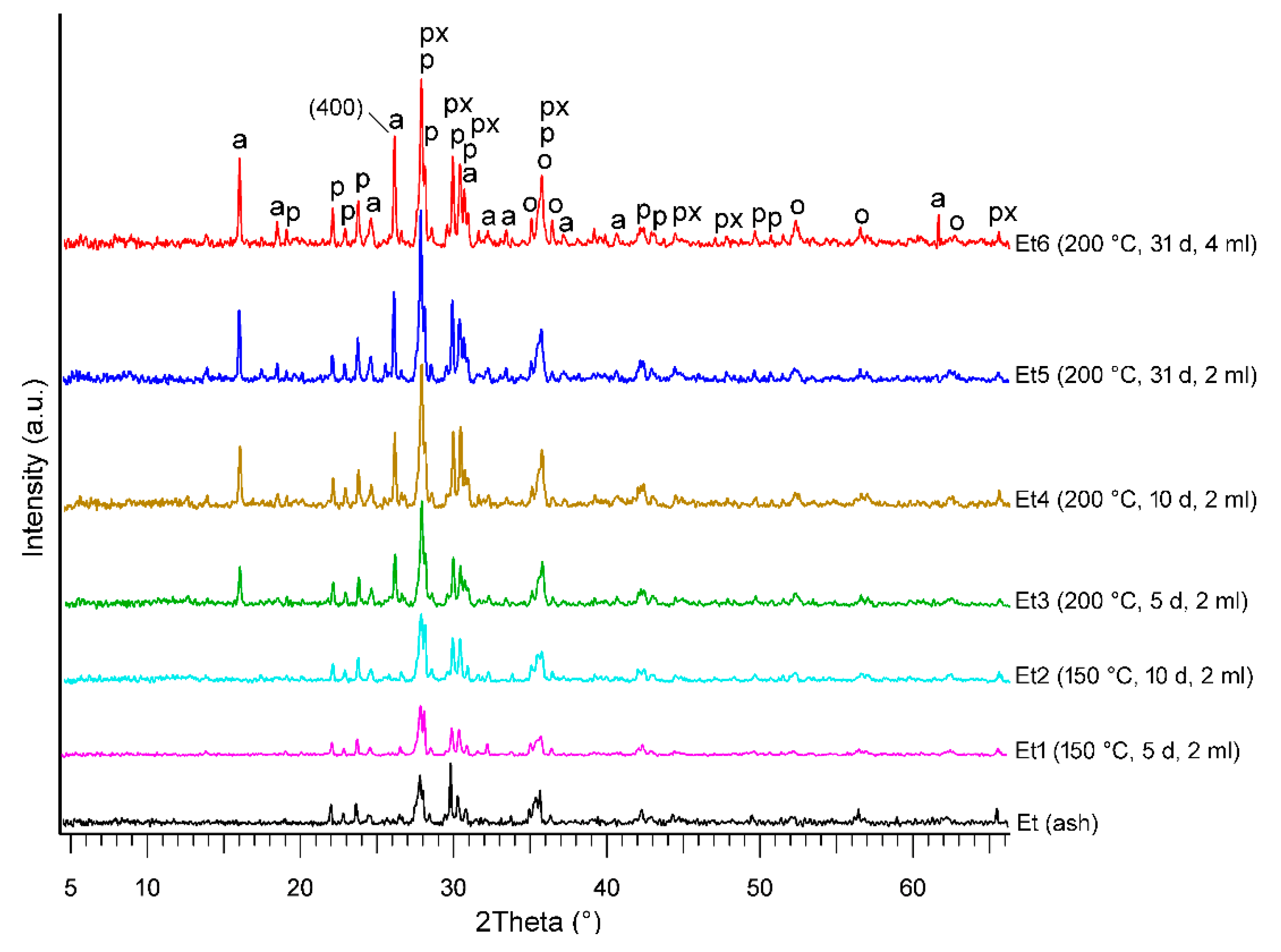
3.2.1. XRPD
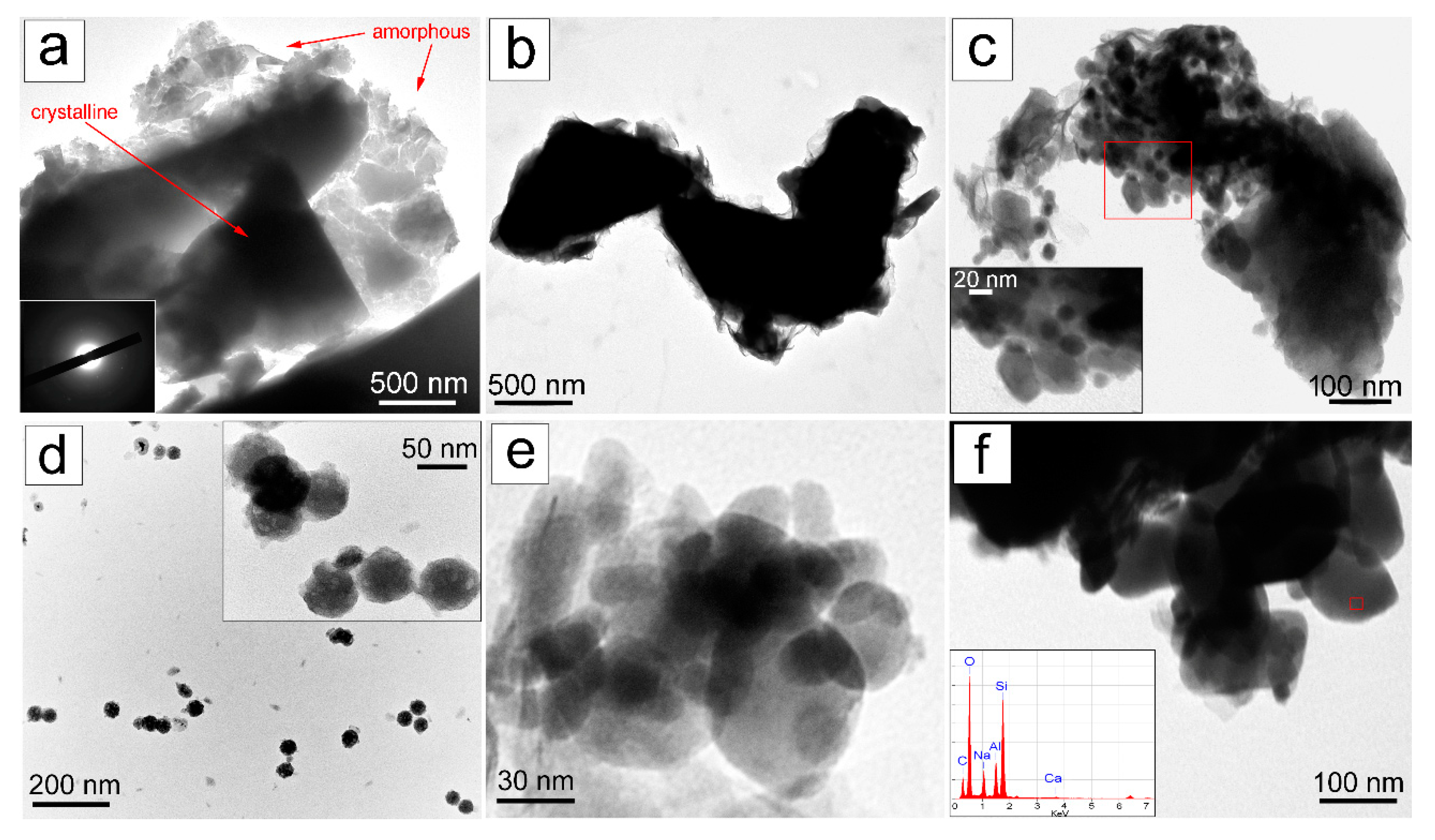
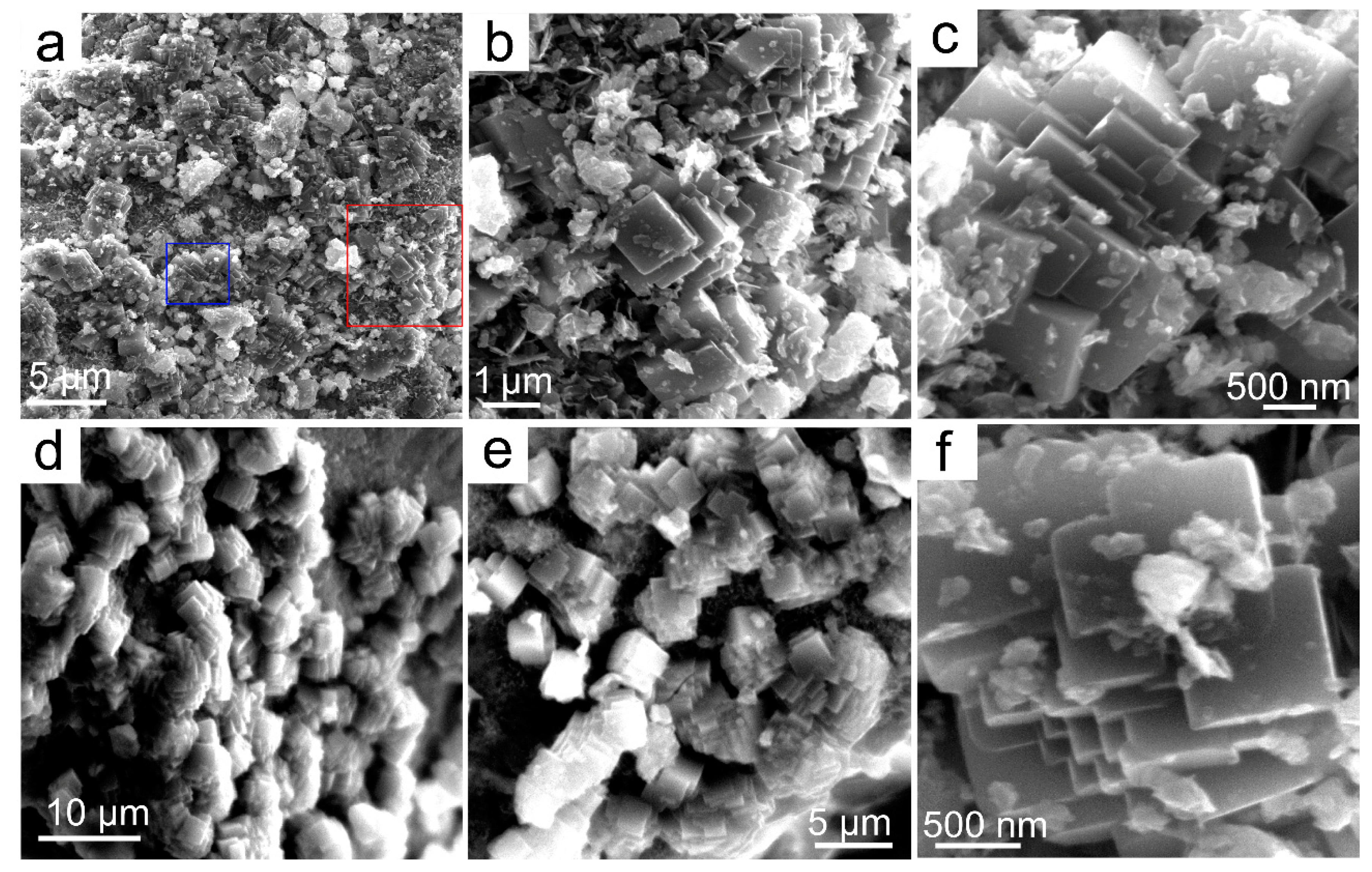
3.2.2. SEM/TEM/EDS
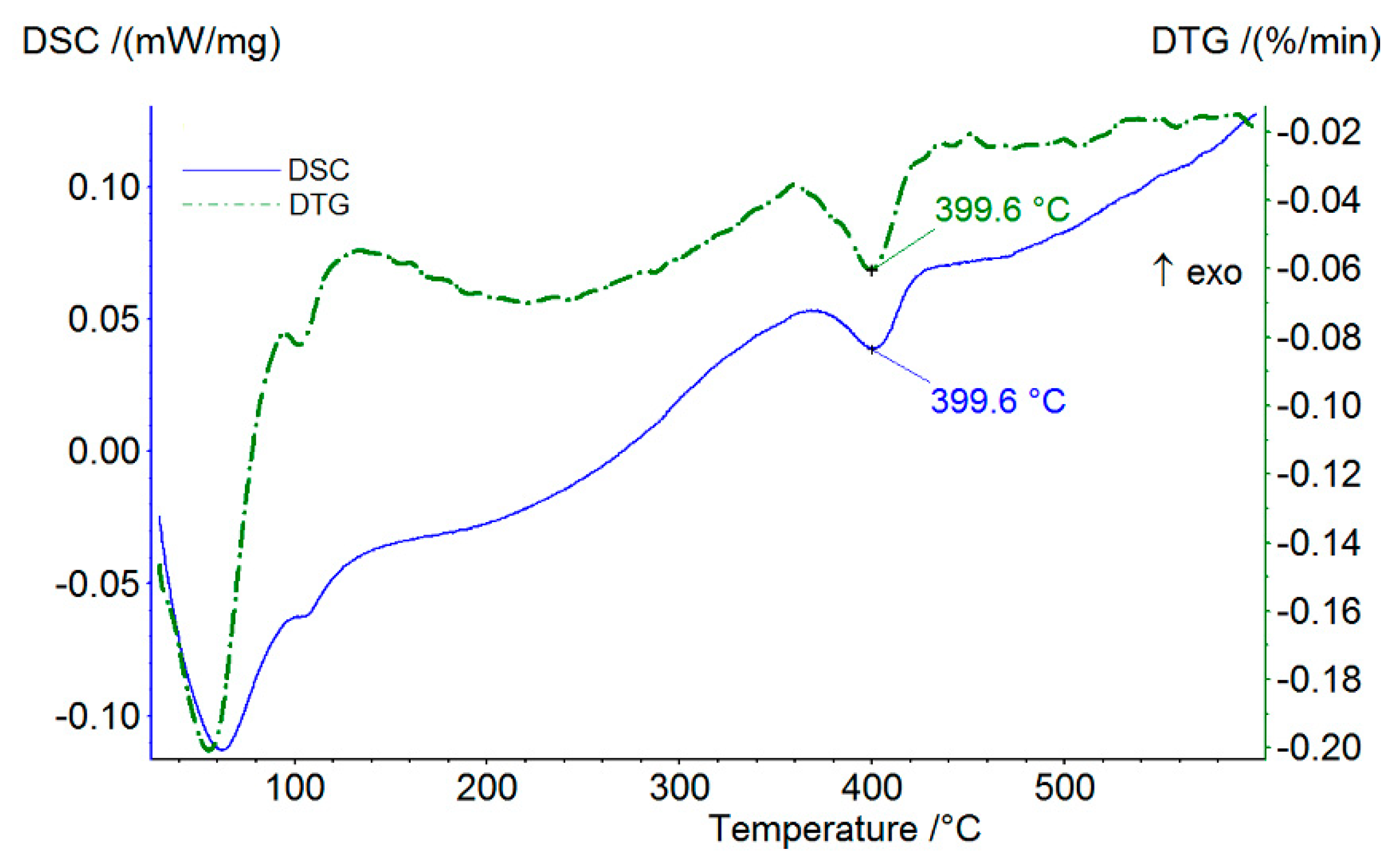
3.2.3. DSC/DTG Characterization
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poulet, F.; Bibring, J.-P.; Mustard, J.F.; Gendrin, A.; Mangold, N.; Langevin, Y.; Arvidson, R.E.; Gondet, B.; Gomez, C. Phyllosilicates on Mars and implications for early Martian climate. Nature 2005, 438, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Gendrin, A.; Mangold, N.; Bibring, J.-P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.; Arvidson, R.; et al. Sulfates in Martian layered terrains: The OMEGA/Mars Express view. Science 2005, 307, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Bibring, J.-P.; Langevin, Y.; Mustard, J.F.; Poulet, F.; Arvidson, R.; Gendrin, A.; Gondet, B.; Mangold, N.; Pinet, P.; Forget, F. Global mineralogical and aqueous Mars history derived from OMEGA/Mars Express data. Science 2006, 312, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.F.; Murchie, S.L.; Pelkey, S.M.; Ehlmann, B.L.; Milliken, R.E.; Grant, J.A.; Bibring, J.-P.; Poulet, F.; Bishop, J.; Dobrea, E.N.; et al. Hydrated silicate minerals on Mars observed by the CRISM instrument on MRO. Nature 2008, 454, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Ehlmann, B.L.; Berger, G.; Mangold, N.; Michalski, J.R.; Catling, D.C.; Ruff, S.W.; Chassefière, E.; Niles, P.B.; Chevrier, V.; Poulet, F. Geochemical Consequences of Widespread Clay Mineral Formation in Mars’ Ancient Crust. Space Sci. Rev. 2013, 174, 329–364. [Google Scholar] [CrossRef]
- Ming, D.W.; Gooding, J.L. Zeolites on Mars: Possible environmental indicators in soils and sediments. In Workshop on Mars Sample Return Science, Proceedings of A Lunar and Planetary Institute Workshop, Houston, TX, USA, 16–18 November 1987; Drake, M.J., Greeley, R., McKay, G.A., Blanchard, D.P., Carr, M.H., Gooding, J., McKay, C.P., Spudis, P.D., Squyres, S.W., Eds.; Lunar and Planetary Institute: Houston, TX, USA, 1988; pp. 124–125. [Google Scholar]
- Ehlmann, B.L.; Mustard, J.F.; Murchie, S.L.; Bibring, J.P.; Meunier, A.; Fraeman, A.A.; Langevin, Y. Subsurface water and clay mineral formation during the early history of Mars. Nature 2011, 479, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Viviano, C.E.; Moersch, J.E.; McSween, H.Y. Implications for early hydrothermal environments on Mars through the spectral evidence for carbonation and chloritization reactions in the Nili Fossae region. J. Geophys. Res. Planets 2013, 118, 1858–1872. [Google Scholar] [CrossRef]
- Viviano-Beck, C.E.; Murchie, S.L.; Beck, A.W.; Dohm, J.M. Compositional and structural constraints on the geologic history of eastern Tharsis rise, Mars. Icarus 2017, 284, 43–58. [Google Scholar] [CrossRef]
- Wray, J.J.; Murchie, S.L.; Squyres, S.W.; Seelos, F.P.; Tornabene, L.L. Diverse aqueous environments on ancient Mars revealed in the southern highlands. Geology 2009, 37, 1043–1046. [Google Scholar] [CrossRef]
- Ansan, V.; Loizeau, D.; Mangold, N.; Le Mouélic, S.; Carter, J.; Poulet, F.; Dromart, G.; Lucas, A.; Bibring, P.; Gendrin, A.; et al. Stratigraphy, mineralogy, and origin of layered deposits inside Terby crater, Mars. Icarus 2011, 211, 273–304. [Google Scholar] [CrossRef]
- Carter, J.; Loizeau, D.; Mangold, N.; Poulet, F.; Bibring, J.-P. Widespread surface weathering on early Mars: A case for a warmer and wetter climate. Icarus 2015, 248, 373–382. [Google Scholar] [CrossRef]
- Carter, J.; Poulet, F.; Bibring, J.; Murchie, S.; Ansan, V.; Mangold, N. Mineralogy of layered deposits in Terby Crater, N. Hellas Planitia. In Proceedings of the 41st Lunar and Planetary Science Conference, The Woodlands, TX, USA, 1–5 March 2010. [Google Scholar]
- Carter, J.; Poulet, F.; Bibring, J.P.; Mangold, N.; Murchie, S. Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. J. Geophys. Res. Planets 2013, 118, 831–858. [Google Scholar] [CrossRef]
- Pajola, M.; Rossato, S.; Carter, J.; Baratti, E.; Pozzobon, R.; Erculiani, M.S.; Coradini, M.; McBride, K. Eridania Basin: An ancient paleolake floor as the next landing site for the Mars 2020 rover. Icarus 2016, 275, 163–182. [Google Scholar] [CrossRef]
- Singer, R.; McCord, T.; Clark, R.; Adams, J.; Huguenin, R. Mars surface composition from reflectance spectroscopy: a summary. J. Geophys. Res. 1979, 84, 8415–8426. [Google Scholar] [CrossRef]
- Bell, J.F.; McCord, T.B.; Owensby, P.D. Observational evidence of crystalline iron oxides on Mars. J. Geophys. Res. 1990, 95, 14447–14461. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Mustard, J.F.; Clark, R.N.; Swayze, G.A.; Murchie, S.L. Evidence for low-grade metamorphism, hydrothermal alteration, and diagnosis on Mars from phyllosilicate mineral assemblages. Clays Clay Miner. 2011, 59, 359–377. [Google Scholar] [CrossRef]
- Gooding, J.L. Soil mineralogy and chemistry on Mars: Possible clues from salts and clays in SNC meteorites. Icarus 1992, 99, 28–41. [Google Scholar] [CrossRef]
- Treiman, A.H.; Barrett, R.A.; Gooding, J.L. Preterrestrial aqueous alteration of the Lafayette (SNC) meteorite. Meteoritics 1993, 28, 86–97. [Google Scholar] [CrossRef]
- Sharp, Z.; Williams, J.; Shearer, C.; Agee, C.; McKeegan, K. The chlorine isotope composition of Martian meteorites 2. Implications for the early solar system and the formation of Mars. Meteorit. Planet. Sci. 2016, 51, 2111–2126. [Google Scholar] [CrossRef]
- Chevrier, V.; Mathé, P.E. Mineralogy and evolution of the surface of Mars: a review. Planet. Space Sci. 2007, 55, 289–314. [Google Scholar] [CrossRef]
- Berger, G.; Toplis, M.J.; Treguier, E.; d’Uston, C.; Pinet, P. Evidence in favor of small amounts of ephemeral and transient water during alteration at Meridiani Planum, Mars. Am. Mineral. 2009, 94, 1279–1282. [Google Scholar] [CrossRef]
- Poulet, F.; Gomez, C.; Bibring, J.-P.; Langevin, Y.; Gondet, B.; Pinet, P.; Belluci, G.; Mustard, J. Martian surface mineralogy from Observatoire pour la Mine’ralogie, l’Eau, les Glaces et l’Activite’ on board the Mars Express spacecraft (OMEGA/MEx): Global mineral maps. J. Geophys. Res. 2007, 112, 08S02. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Mustard, J.F.; Swayze, G.A.; Clark, R.N.; Bishop, J.L.; Poulet, F.; Des Marais, D.J.; Roach, L.H.; Milliken, R.E.; Wray, J.J.; et al. Identification of hydrated silicate minerals on Mars using MRO-CRISM: geologic context near Nili Fossae and implications for aqueous alteration. J. Geophys. Res. 2009, 114, 1–33. [Google Scholar] [CrossRef]
- Yokomori, Y.; Idaka, S. The crystal structure of analcime. Microporous Mesoporous Mater. 1998, 21, 365–370. [Google Scholar] [CrossRef]
- Ferraris, G.; Jones, D.W.; Terkess, J. A neutron-diffraction study of the crystal structure of analcime, NaAlSi2O6·H2O. Z. Krist. Cryst. Mater. 1972, 135, 240–252. [Google Scholar] [CrossRef]
- Ballirano, P.; Pacella, A.; Bloise, A.; Giordani, M.; Mattioli, M. Thermal Stability of Woolly Erionite-K and Considerations about the Heat-Induced Behaviour of the Erionite Group. Minerals 2018, 8, 28. [Google Scholar] [CrossRef]
- Ballirano, P.; Bloise, A.; Gualtieri, A.F.; Lezzerini, M.; Pacella, A.; Perchiazzi, N.; Dogan, M.; Dogan, A.U. The crystal structure of mineral fibers. In Mineral Fibers: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; Volume 18, pp. 17–53. [Google Scholar]
- Bandfield, J.L.; Hamilton, V.E.; Christensen, P.R. A global view of martian surface compositions from MGS-TES. Science 2000, 287, 1626–1630. [Google Scholar] [CrossRef]
- Hoefen, T.M.; Clark, R.N.; Bandfield, J.L.; Smith, M.D.; Pearl, J.C.; Christensen, P.R. Discovery of olivine in the Nili Fossae region of Mars. Science 2003, 302, 627–630. [Google Scholar] [CrossRef]
- Mustard, J.F.; Poulet, F.; Gendrin, A.; Bibring, J.-P.; Langevin, Y.; Gondet, B.; Mangold, N.; Bellucci, G.; Altieri, F. Olivine and pyroxene diversity in the crust of Mars. Science 2005, 307, 1594–1597. [Google Scholar] [CrossRef]
- Hamilton, V.E.; Christensen, P.R. Evidence for extensive, olivine-rich bedrock on Mars. Geology 2005, 33, 433–436. [Google Scholar] [CrossRef]
- Hamilton, V.E.; Christensen, P.R.; McSween, H.Y., Jr.; Bandfield, J.L. Searching for the source regions of Martian meteorites using MGS TES: Integrating Martian meteorities into the global distribution of igneous materials on Mars, Meteorit. Planet. Sci. 2003, 38, 871–885. [Google Scholar]
- Allen, C.C.; Gooding, J.L.; Keil, K. Hydrothermally altered impact melt rock and breccia: Contributions to the soil of Mars. J. Geophys. Res. Solid Earth. 1982, 87, 10083–10101. [Google Scholar] [CrossRef]
- Naumov, M.V. Principal features of impact-generated hydrothermal circulation systems: Mineralogical and geochemical evidence. Geofluids 2005, 5, 165–184. [Google Scholar] [CrossRef]
- Weisenberger, T.; Selbekk, R.S. Multi-stage zeolite facies mineralization in the Hvalfjordur area, Iceland. Int. J. Earth Sci. 2008, 98, 985–999. [Google Scholar] [CrossRef]
- Robert, C.; Goffe, B. Zeolitisation of basalts in subaqueous freshwater settings: Field observations and experimental studies. Geochim. Cosmochim. Acta 1993, 57, 3597–3612. [Google Scholar] [CrossRef]
- Hay, R.L. Geologic occurrence of zeolites and some associated minerals. Pure Appl. Chem. 1986, 58, 1339–1342. [Google Scholar] [CrossRef]
- Eugster, H.P. Geochemistry of evaporitic lacustrine deposits. Annu. Rev. Earth Planet. Sci. 1980, 8, 35–63. [Google Scholar] [CrossRef]
- Sheppard, R.A.; Hay, R.L. Formation of zeolites in open hydrologic systems. Rev. Mineral. Geochem. 2001, 45, 261–275. [Google Scholar] [CrossRef]
- Line, C.M.B.; Putnis, A.; Putnis, C.; Giampaolo, C. The dehydration kinetics and microtexture of analcime from two parageneses. Am. Mineral. 1995, 80, 268–279. [Google Scholar] [CrossRef]
- Luhr, J.F.; Kyser, T.K. Primary igneous analcime: The Colima minettes. Am. Mineral. 1989, 74, 216–223. [Google Scholar]
- Sætre, C.; Hellevang, H.; Riu, L.; Dypvik, H.; Pilorget, C.; Poulet, F.; Werner, S.C. Experimental hydrothermal alteration of basaltic glass with relevance to Mars. Meteorit. Planet. Sci. 2019 54, 357–378. [CrossRef]
- Viennet, J.C.; Bultel, B.; Riu, L.; Werner, S.C. Dioctahedral phyllosilicates versus zeolites and carbonates versus zeolites competitions as constraints to understanding early Mars alteration conditions. J. Geophys. Res. Planets 2017, 122, 2328–2343. [Google Scholar] [CrossRef]
- Hellevang, H.; Dypvik, H.; Kalleson, E.; Pittarello, L.; Koeberl, C. Can alteration experiments on impact melts from El’gygytgyn and volcanic glasses shed new light on the formation of the Martian surface? Meteorit. Planet. Sci. 2013, 48, 1287–1295. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefánsson, A. CO2-water–basalt interaction. Low temperature experiments and implications for CO2 sequestration into basalts. Geochim. Cosmochim. Acta 2012, 81, 129–152. [Google Scholar] [CrossRef]
- De’Gennaro, M.; Langella, A.; Cappelletti, P.; Colella, C. Hydrothermal conversion of trachytic glass to zeolite. 3. Monocationic model glasses. Clays Clay Miner. 1999, 47, 348–357. [Google Scholar] [CrossRef]
- Taddeucci, J.; Pompilio, M.; Scarlato, P. Conduit processes during the July–August 2001 explosive activity of Mt. Etna (Italy): inferences from glass chemistry and crystal size distribution of ash particles. J. Volcanol. Geotherm. Res. 2004, 137, 33–54. [Google Scholar] [CrossRef]
- Wang, X.; Boselli, A.; D’Avino, L.; Pisani, G.; Spinelli, N.; Amodeo, A.; Perrone, M.R. Volcanic dust characterization by EARLINET during Etna’s eruptions in 2001–2002. Atmos. Environ. 2008, 42, 893–905. [Google Scholar] [CrossRef]
- Witter, J.B.; Hamilton, V.E.; Houghton, B.F. Thermal infrared spectroscopy of explosively erupted terrestrial basalts: potential analogues for surface compositions on Mars. In Proceedings of the 36th Lunar and Planetary Science Conference, League City, TX, USA, 14–18 March 2005. [Google Scholar]
- Ákos, K.; Csorba, Á. Ásványok és kőzetek vizsgálata a Mars felszínén: vizsgálati, meghatározási lehetőségek. Földtani Közlöny 2010, 40/3, 293–302. [Google Scholar]
- McSween, H.Y.; Wyatt, M.B.; Gellert, R.; Bell, J.; Morris, R.V.; Herkenhoff, K.E.; Crumpler, L.S.; Milam, K.A.; Stockstill, K.R.; Tornabene, L.L. Characterization and petrologic interpretation of olivine-rich basalts at Gusev Crater, Mars. J. Geophys. Res. 2006, 111, 1–17. [Google Scholar] [CrossRef]
- Utada, M. Zeolites in hydrothermally altered rocks. Rev. Mineral. Geochem. 2001, 45, 30–322. [Google Scholar] [CrossRef]
- Hurowitz, J.A.; McLennan, S.L. A~3.5 Ga record of water-limited, acidic weathering conditions on Mars. Earth Planet. Sci. Lett. 2007, 260, 432–443. [Google Scholar] [CrossRef]
- Wang, A.; Korotev, R.L.; Jolliff, B.L.; Haskin, L.A.; Crumpler, L.; Farrand, W.H.; Herkenhoff, K.E.; de Souza Jr., P.; Kusack, A.G.; Hurowitz, J.A.; et al. Evidence of phyllosilicates in Wooly Patch, an altered rock encountered at West Spur, Columbia Hills, by the Spirit rover in Gusev crater, Mars. J. Geophys. Res. 2006, 111, 1–22. [Google Scholar] [CrossRef]
- Ayris, P.; Delmelle, P. Volcanic and atmospheric controls on ash iron solubility: A review. Phys. Chem. Earth 2012, 45, 103–112. [Google Scholar] [CrossRef]
- Farquhar, J.; Savarino, J.; Jackson, T.L.; Thiemens, M.H. Evidence of atmospheric sulphur in the martian regolith from sulphur isotopes in meteorites. Nature 2000, 404, 50–52. [Google Scholar] [CrossRef]
- Young, R.A. Introduction to the Rietveld method. In The Rietveld Method; Young, R.A., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 1–38. [Google Scholar]
- Gualtieri, A.F.; Gatta, G.D.; Arletti, R.; Artioli, G.; Ballirano, P.; Cruciani, G.; Guagliardi, A.; Malferrari, D.; Masciocchi, N.; Scardi, P. Quantitative phase analysis using the Rietveld method: towards a procedure for checking the reliability and quality of the results. Period. Mineral. 2019, 88, 147–151. [Google Scholar]
- Feng, S.H.; Li, G.H. Hydrothermal and solvothermal syntheses. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–104. [Google Scholar]
- Jing, Z.; Cai, K.; Li, Y.; Fan, J.; Zhang, Y.; Miao, J.; Jin, F. Hydrothermal synthesis of pollucite, analcime and their solid solutions and analysis of their properties. J. Nucl. Mater. 2017, 488, 63–69. [Google Scholar] [CrossRef]
- Walton, R.I. Subcritical solvothermal synthesis of condensed inorganic materials. Chem. Soc. Rev. 2002, 31, 230–238. [Google Scholar] [CrossRef]
- Krammer, P.; Vogel, H. Hydrolysis of esters in subcritical and supercritical water. J. Supercrit. Fluids 2000, 16, 189–206. [Google Scholar] [CrossRef]
- LeBas, M.J.; Lemaitre, R.W.; Streckeisen, A.; Zanettin, B. A chemical classification of volcanic rocks based on total alkali silica diagram. J. Petrol. 1986, 27, 745–750. [Google Scholar]
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.; Bonin, B.; Bateman, P. Igneous Rocks: A Classification and Glossary of Terms: Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar]
- Treiman, A.H.; Bish, D.L.; Vaniman, D.T.; Chipera, S.J.; Blake, D.F.; Ming, D.W.; Morris, R.V.; Bristow, T.F.; Morrison, S.M.; Baker, M.B.; et al. Mineralogy, provenance, and diagenesis of a potassic basaltic sandstone on Mars: CheMin X-ray diffraction of the Windjana sample (Kimberley area, Gale Crater). J. Geophys. Res. Planets. 2016, 121, 75–106. [Google Scholar] [CrossRef]
- Filiberto, J.; Chin, E.; Day, J.M.D.; Franchi, I.A.; Greenwood, R.C.; Gross, J.; Penniston-Dorland, S.C.; Schwenzer, S.P.; Treiman, A.H. Geochemistry of intermediate olivine-phyric shergottite Northwest Africa 6234, with similarities to basaltic shergottite Northwest Africa 480 and olivine-phyric shergottite Northwest Africa 2990. Meteorit. Planet. Sci. 2012, 47, 1256–1273. [Google Scholar] [CrossRef]
- Clark, B.C.; Baird, A.K.; Weldon, R.J.; Tsusaki, D.M.; Schnabel, L.; Candelaria, M.P. Chemical composition of Martian fines. J. Geophys. Res. 1982, 87, 10059–10067. [Google Scholar] [CrossRef]
- Christensen, P.R.; Bandfield, J.L.; Smith, M.D.; Hamilton, V.E.; Clark, R.N. Identification of a basaltic component on the martian surface from Thermal Emission Spectrometer data. J. Geophys. Res. 2000, 105, 9609–9621. [Google Scholar] [CrossRef]
- Wyatt, M.B.; McSween, H.Y., Jr. Spectral evidence for weathered basalt as an alternative to andesite in the northern lowlands of Mars. Nature 2002, 417, 263–266. [Google Scholar] [CrossRef]
- Coombs, D.S.; Whetten, T. Composition of analcime from sedimentary and burial metamorphic rocks. Geol. Soc. Am. Bull. 1967, 78, 269–282. [Google Scholar] [CrossRef]
- Sugano, N.; Kyono, A. An experimental study of symmetry lowering of analcime. Phys. Chem. Miner. 2018, 45, 381–390. [Google Scholar] [CrossRef]
- Mumpton, F.A.; Ormsby, W.C. Morphology of zeolites in sedimentary rocks by scanning electron microscopy. Clays Clay Miner. 1976, 24, 1–23. [Google Scholar] [CrossRef]
- Cruciani, G.; Gualtieri, A. Dehydration dynamics of analcime by in situ synchrotron powder diffraction. Am. Mineral. 1999, 84, 112–119. [Google Scholar] [CrossRef]
- Koizumi, M. The differential thermal analysis curves and the dehydration curves of zeolites. Mineral. J. 1953, 1, 36–47. [Google Scholar] [CrossRef]
- Bloise, A.; Kusiorowski, R.; Lassinantti Gualtieri, M.; Gualtieri, A.F. Thermal behaviour of mineral fibers. In Mineral Fibers: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtier, A.F., Ed.; European Mineralogical Union: London, UK, 2017; Volume 18, pp. 215–252. [Google Scholar]
- Cannata, C.B.; De Rosa, R.; Donato, P.; Taddeucci, J. Ash Features from Ordinary Activity at Stromboli Volcano. Int. J. Geosci. 2014, 5, 1361–1382. [Google Scholar] [CrossRef]
- Bloise, A. Synthesis and characterization of gillespite. Appl. Phys. A 2018, 124, 330. [Google Scholar] [CrossRef]
- Berger, G.; Cathala, A.; Fabre, S.; Borisova, A.Y.; Pages, A.; Aigouy, T.; Esvan, J.; Pinet, P. Experimental exploration of volcanic rocks-atmosphere interaction under Venus surface conditions. Icarus 2019, 329, 8–23. [Google Scholar] [CrossRef]
- Berger, G.; Claparols, C.; Guy, C.; Daux, V. Dissolution rate of a basalt glass in silica-rich solutions: Implications for long-term alteration. Geochim. Cosmochim. Acta 1994, 58, 4875–4886. [Google Scholar] [CrossRef]
- Crovisier, J.; Advocat, T.; Dussossoy, J. Nature and role of natural alteration gels formed on the surface of ancient volcanic glasses (Natural analogs of waste containment glasses). J. Nucl. Mater. 2003, 321, 91–109. [Google Scholar] [CrossRef]
- Hellmann, R.; Cotte, S.; Cadel, E.; Malladi, S.; Karlsson, L.S.; Lozano-Perez, S.; Cabie, M.; Seyeux, A. Nanometre- scale evidence for interfacial dissolution-reprecipitation control of silicate glass corrosion. Nat. Mater. 2015, 14, 307–311. [Google Scholar] [CrossRef]
- Michalski, J.; Poulet, F.; Bibring, J.P.; Mangold, N. Analysis of phyllosilicate deposits in the Nili Fossae region of Mars: Comparison of TES and OMEGA data. Icarus 2010, 206, 269–289. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Navarre-Sitchler, A.K.; Sak, P.B.; Steefel, C.I.; Brantley, S.L. Basalt weathering rates on Earth and the duration of liquid water on the plains of Gusev Crater, Mars. Geology 2008, 36, 67–70. [Google Scholar] [CrossRef]
- Carrozzo, F.G.; Di Achille, G.; Salese, F.; Altieri, F.; Bellucci, G. Geology and mineralogy of the Auki Crater, Tyrrhena Terra, Mars: A possible post impact induced hydrothermal system. Icarus 2017, 281, 228–239. [Google Scholar] [CrossRef]
- Rathbun, J.A.; Squyres, S.W. Hydrothermal systems associated with Martian impact craters. Icarus 2002, 157, 362–372. [Google Scholar] [CrossRef]
- Abramov, O.; Kring, D.A. Impact-induced hydrothermal activity on early Mars. J. Geophys. Res. Planets 2005, 110, E12S09. [Google Scholar] [CrossRef]
- Schwenzer, S.P.; Kring D., A. Impact-generated hydrothermal systems capable of forming phyllosilicates on Noachian Mars. Geology 2009, 37, 1091–1094. [Google Scholar] [CrossRef]
- Wang, A.; Haskin, L.A.; Squyres, S.W.; Jolliff, B.L.; Crumpler, L.; Gellert, R.; Schröder, C.; Herkenhoff, K.; Hurowitz, J.; Tosca, N.J.; et al. Sulfate deposition in subsurface regolith in Gusev crater, Mars. J. Geophys. Res. Planets 2006, 111, 1–19. [Google Scholar] [CrossRef]
- Peretyazhko, T.S.; Sutter, B.; Morris, R.V.; Agresti, D.G.; Le, L.; Ming, D.W. Fe/Mg smectite formation under acidic conditions on early Mars. Geochim. Cosmochim. Acta 2016, 173, 37–49. [Google Scholar] [CrossRef]
- Shock, E.L. Hydrothermal systems as environments for the emergence of life. Ciba Found Symp. 1996, 202, 40–60. [Google Scholar]
- Newsom, H.E.; Hagerty, J.J.; Goff, F. Mixed hydrothermal fluids and the origin of the Martian soil. J. Geophys. Res. Planets. 1999, 104, 8717–8728. [Google Scholar] [CrossRef]
- Newsom, H.E.; Hagerty, J.J.; Thorsos, I.E. Location and sampling of aqueous and hydrothermal deposits in Martian impact craters. Astrobiology 2001, 1, 71–88. [Google Scholar] [CrossRef]

| Oxides | SiO2 | Na2O | K2O | Fe2O3 | FeO | MnO | Al2O3 | MgO | CaO | P2O5 | TiO2 | L.O.I. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt.% | 46.71 | 2.57 | 2.09 | 7.20 | 3.35 | 0.19 | 15.85 | 6.00 | 13.64 | 0.39 | 1.91 | 0.10 |
| Runs | T (°C) | t (Days) | W (mL) | W/R (mL/g) | Mineral Abundance | Newly Formed Mineral | Analcime Peak Intensity (400) (cps) | Analcime FWHM (400) (Δ°2θ) |
|---|---|---|---|---|---|---|---|---|
| Et (ash) | - | - | - | - | P > Py > O | - | - | - |
| Et1 | 150 | 5 | 2 | 2.8 | P > Py > O | - | - | - |
| Et2 | 150 | 10 | 2 | 2.8 | P > Py > O | - | - | - |
| Et3 | 200 | 5 | 2 | 2.8 | P > Py > O > An | An | 353 | 0.154 |
| Et4 | 200 | 10 | 2 | 2.8 | P > Py > O > An | An | 396 | 0.147 |
| Et5 | 200 | 31 | 2 | 2.8 | P > Py > O > An | An | 459 | 0.147 |
| Et6 | 200 | 31 | 4 | 5.7 | P > Py > An > O | An | 480 | 0.142 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloise, A.; Cannata, C.B.; Rosa, R.D. Hydrothermal Alteration of Etna Ash and Implications for Mars. Minerals 2020, 10, 450. https://doi.org/10.3390/min10050450
Bloise A, Cannata CB, Rosa RD. Hydrothermal Alteration of Etna Ash and Implications for Mars. Minerals. 2020; 10(5):450. https://doi.org/10.3390/min10050450
Chicago/Turabian StyleBloise, Andrea, Chiara Benedetta Cannata, and Rosanna De Rosa. 2020. "Hydrothermal Alteration of Etna Ash and Implications for Mars" Minerals 10, no. 5: 450. https://doi.org/10.3390/min10050450
APA StyleBloise, A., Cannata, C. B., & Rosa, R. D. (2020). Hydrothermal Alteration of Etna Ash and Implications for Mars. Minerals, 10(5), 450. https://doi.org/10.3390/min10050450







