Concentration of Rare Earth Elements (Sc, Y, La, Ce, Nd, Sm) in Bauxite Residue (Red Mud) Obtained by Water and Alkali Leaching of Bauxite Sintering Dust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solid Phase Characterization
2.2. Reagents
2.3. Experimentation
3. Results and Discussion
3.1. Water Leaching of ESP Dust
3.2. Kinetics of ESP Dust Leaching by Alkaline Aluminate Liquor
4. Conclusions
- The chemical and phase composition of the electrostatic precipitation dust and sinter obtained in the same technological process are different. The content of rare-earth elements in the dust of electrostatic precipitators is 70–80% higher than in the sinter, which may be explained by a large number of cycles of ESP dust return to the kiln due to the very small size of particles.
- Similar to the red mud of the Bayer process, scandium in ESP dust and in the solid residue after ESP dust leaching is associated to a greater extent with hematite, because of an insufficient transformation of latter into sodium ferrite in the sintering process and to a lower extent with disilication product (sodalite and cancrinite); the complete recovery of REE requires the destruction of the hematite at first.
- The following conditions allow to obtain red mud from ESP dust that contains three times more REE than traditional waste red mud of the Ural Alumina Refinery: pre-leaching with water at 90 °C followed by autoclave leaching with an alkaline-aluminate liquor at 240 °C for 90 min.
- The kinetic patterns of leaching alumina with the alkali liquor have been studied for the original electrostatic precipitation dust and water-leached dust. The change in the limiting stage of the process after water-treatment has been shown by an increase of the activation energy from 24.98 to 33.19 kJ/mol; changes in the form of the kinetic curves have also been demonstrated.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qi, D. Hydrometallurgy of Rare Earths: Extraction and Separation; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-813921-9. [Google Scholar]
- Savchenkov, S.A.; Bazhin, V.Y.; Brichkin, V.N.; Kosov, Y.I.; Ugolkov, V.L. Production Features of Magnesium-Neodymium Master Alloy Synthesis. Metallurgist 2019, 63, 394–402. [Google Scholar] [CrossRef]
- Wübbeke, J. Rare earth elements in China: Policies and narratives of reinventing an industry. Resour. Policy 2013, 38, 384–394. [Google Scholar] [CrossRef]
- Savchenkov, S.; Bazhin, V.; Brichkin, V.; Povarov, V.; Ugolkov, V.; Kasymova, D. Synthesis of magnesium-zinc-yttrium master alloy. Lett. Mater. 2019, 9, 339–343. [Google Scholar] [CrossRef]
- Røyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, C.Y. Separation and purification of scandium by solvent extraction and related technologies: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1237–1246. [Google Scholar] [CrossRef]
- Li, Z.; Din, J.; Xu, J.; Liao, C.; Yin, F.; Lǚ, T.; Cheng, L.; Li, J. Discovery of the REE minerals in the Wulong–Nanchuan bauxite deposits, Chongqing, China: Insights on conditions of formation and processes. J. Geochem. Explor. 2013, 133, 88–102. [Google Scholar] [CrossRef]
- Ou, Z.; Li, J.; Wang, Z. Application of mechanochemistry to metal recovery from second-hand resources: A technical overview. Environ. Sci. Process. Impacts 2015, 17, 1522–1530. [Google Scholar] [CrossRef]
- Rychkov, V.; Koukkari, P.; Kirillov, S.; Kirillov, E. Best Practices of Russia and Finland in Extracting REE from Fertilizer Waste. KnE Mater. Sci. 2017, 2, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Diallo, M.S.; Baier, G.; Moyer, B.A.; Hamelers, B. Critical Materials Recovery from Solutions and Wastes: Retrospective and Outlook. Environ. Sci. Technol. 2015, 49, 9387–9389. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Wang, J.; Wang, G.; Xue, Q. Innovative Methodology for Separating of Rare Earth and Iron from Bayan Obo Complex Iron Ore. ISIJ Int. 2012, 52, 1772–1777. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Ayora, C.; Macías, F.; Torres, E.; Lozano, A.; Carrero, S.; Nieto, J.-M.; Pérez-López, R.; Fernández-Martínez, A.; Castillo-Michel, H. Recovery of Rare Earth Elements and Yttrium from Passive-Remediation Systems of Acid Mine Drainage. Environ. Sci. Technol. 2016, 50, 8255–8262. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, D.L.; Puhakka, V.; Repo, E.; Sillanpää, M. Selective separation of scandium from iron, aluminium and gold rich wastewater using various amino and non-amino functionalized silica gels—A comparative study. J. Clean. Prod. 2018, 170, 890–901. [Google Scholar] [CrossRef]
- Smirnov, A.L.; Titova, S.M.; Rychkov, V.N.; Bunkov, G.M.; Semenishchev, V.S.; Kirillov, E.V.; Poponin, N.N.; Svirsky, I.A. Study of scandium and thorium sorption from uranium leach liquors. J. Radioanal. Nucl. Chem. 2017, 312, 277–283. [Google Scholar] [CrossRef]
- Rychkov, V.N.; Kirillov, E.V.; Kirillov, S.V.; Semenishchev, V.S.; Bunkov, G.M.; Botalov, M.S.; Smyshlyaev, D.V.; Malyshev, A.S. Recovery of rare earth elements from phosphogypsum. J. Clean. Prod. 2018, 196, 674–681. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Tareeva, O.A.; Elizarova, I.P. A study of the sulfuric acid leaching of rare-earth elements, phosphorus, and alkali metals from phosphodihydrate. Russ. J. Appl. Chem. 2010, 83, 958–964. [Google Scholar] [CrossRef]
- Rivera, R.M.; Ounoughene, G.; Malfliet, A.; Vind, J.; Panias, D.; Vassiliadou, V.; Binnemans, K.; Van Gerven, T. A Study of the Occurrence of Selected Rare-Earth Elements in Neutralized–Leached Bauxite Residue and Comparison with Untreated Bauxite Residue. J. Sustain. Metall. 2019, 5, 57–68. [Google Scholar] [CrossRef]
- Loginova, I.V.; Shoppert, A.A.; Chaikin, L.I. Extraction of Rare-Earth Metals During the Systematic Processing of Diaspore-Boehmite Bauxites. Metallurgist 2016, 60, 198–203. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Mordberg, L.E. Patterns of distribution and behaviour of trace elements in bauxites. Chem. Geol. 1993, 107, 241–244. [Google Scholar] [CrossRef]
- Anawati, J.; Azimi, G. Recovery of Strategic Materials from Canadian Bauxite Residue by Smelting Followed by Acid Baking–Water Leaching. In Rare Metal Technology 2020; Azimi, G., Forsberg, K., Ouchi, T., Kim, H., Alam, S., Baba, A.A., Eds.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2020; pp. 139–150. ISBN 978-3-030-36757-2. [Google Scholar]
- Sinha, S.; Sinha, M.K.; Pandey, B.D. Extraction of lanthanum and cerium from Indian red mud. Int. J. Miner. Process. 2014, 127, 70–73. [Google Scholar] [CrossRef]
- Anhaeusser, C.R.; Geological Society of South Africa (Eds.) Mineral Deposits of Southern Africa: In 2 Vol. 1; Geological Society of South Africa: Johannesburg, Southern Africa, 1986; ISBN 978-0-620-09438-2. [Google Scholar]
- Lokshin, E.P.; Tareeva, O.A. Production of high-quality gypsum raw materials from phosphogypsum. Russ. J. Appl. Chem. 2015, 88, 567–573. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Lokshin, E.P.; Tareeva, O.A. Activation of leaching of rare earth elements from phosphohemihydrate. Russ. J. Appl. Chem. 2013, 86, 1638–1642. [Google Scholar] [CrossRef]
- Kovács, T.; Horváth, M.; Csordás, A.; Bátor, G.; Tóth-Bodrogi, E. Tobacco plant as possible biomonitoring tool of red mud dust fallout and increased natural radioactivity. Heliyon 2020, 6, e03455. [Google Scholar] [CrossRef]
- Alam, S.; Das, B.K.; Das, S.K. Dispersion and Sedimentation Characteristics of Red Mud. J. Hazard. Toxic Radioact. Waste 2018, 22, 04018025. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Rivera, R.M.; Xakalashe, B.; Ounoughene, G.; Binnemans, K.; Friedrich, B.; Van Gerven, T. Selective rare earth element extraction using high-pressure acid leaching of slags arising from the smelting of bauxite residue. Hydrometallurgy 2019, 184, 162–174. [Google Scholar] [CrossRef]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Qu, Y.; Li, H.; Tian, W.; Wang, X.; Wang, X.; Jia, X.; Shi, B.; Song, G.; Tang, Y. Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Miner. Eng. 2015, 81, 1–4. [Google Scholar] [CrossRef]
- Shoppert, A.; Loginova, I.; Rogozhnikov, D.; Karimov, K.; Chaikin, L. Increased as Adsorption on Maghemite-Containing Red Mud Prepared by the Alkali Fusion-Leaching Method. Minerals 2019, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Sabirzyanov, N.A.; Yatsenko, S.P. Gidrokhimicheskie Sposoby Kompleksnoĭ Pererabotki Boksita; IKhTT UrO RAN: Ekaterinburg, Russia, 2006; ISBN 978-5-7691-1629-2. (In Russia) [Google Scholar]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Abhilash; Meshram, P. Overview on Extraction and Separation of Rare Earth Elements from Red Mud: Focus on Scandium. Miner. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of Bauxite Residue (Red Mud) in View of Iron and Selective Rare Earths Recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Petrakova, O.V.; Panov, A.V.; Gorbachev, S.N.; Klimentenok, G.N.; Perestoronin, A.V.; Vishnyakov, S.E.; Anashkin, V.S. Improved Efficiency of Red Mud Processing through Scandium Oxide Recovery. In Light Metals 2015; Hyland, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 91–96. ISBN 978-1-119-09343-5. [Google Scholar]
- Yatsenko, S.P.; Pyagai, I.N. Red mud pulp carbonization with scandium extraction during alumina production. Theor. Found. Chem. Eng. 2010, 44, 563–568. [Google Scholar] [CrossRef]
- Loginova, I.V.; Shoppert, A.A.; Chaikin, L.I. Effect of Adding Sintering Furnace Electrostatic Precipitator Dust on Combined Leaching of Bauxites and Cakes. Metallurgist 2015, 59, 698–704. [Google Scholar] [CrossRef]
- Diev, V.N.; Sabirzyanov, N.A.; Skryabneva, L.M.; Yatsenko, S.P.; Anashkin, V.S.; Aminov, S.N.; Zavadskij, K.F.; Sysoev, A.V.; Ustich, E.P. Method for Scandium Extraction from Bauxite Treatment for Alumina Production. Russia Patent 2,201,988, 10 April 2003. [Google Scholar]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.; Tkaczyk, A.; Vassiliadou, V.; Panias, D. Rare Earth Element Phases in Bauxite Residue. Minerals 2018, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Other Valuable Metals from Bauxite Residue (Red Mud): A Review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- M Tóth, T.; Schubert, F.; Raucsik, B.; Fintor, K. Mineralogical and Geochemical Constraints of the REE Accumulation in the Almásfüzitő Red Mud Depository in Northwest Hungary. Appl. Sci. 2019, 9, 3654. [Google Scholar] [CrossRef] [Green Version]
- Smith, P. The processing of high silica bauxites—Review of existing and potential processes. Hydrometallurgy 2009, 98, 162–176. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Shirokova, A.G.; Koryakova, O.V.; Sabirzyanov, N.A.; Yatsenko, S.P. Complexing Properties of Scandium(III) in Alkaline Medium. Russ. J. Appl. Chem. 2004, 77, 1070–1073. [Google Scholar] [CrossRef]
- Anawati, J.; Azimi, G. Recovery of scandium from Canadian bauxite residue utilizing acid baking followed by water leaching. Waste Manag. 2019, 95, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Alex, T.C.; Kumar, R.; Roy, S.K.; Mehrotra, S.P. Towards ambient pressure leaching of boehmite through mechanical activation. Hydrometallurgy 2014, 144–145, 99–106. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: New York, NY, USA, 1999; ISBN 978-0-471-25424-9. [Google Scholar]
- Rogozhnikov, D.A.; Shoppert, A.A.; Dizer, O.A.; Karimov, K.A.; Rusalev, R.E. Leaching Kinetics of Sulfides from Refractory Gold Concentrates by Nitric Acid. Metals 2019, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, X.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z.; Zhou, K. Effects of Si-bearing minerals on the conversion of hematite into magnetite during reductive Bayer digestion. Hydrometallurgy 2019, 189, 105126. [Google Scholar] [CrossRef]


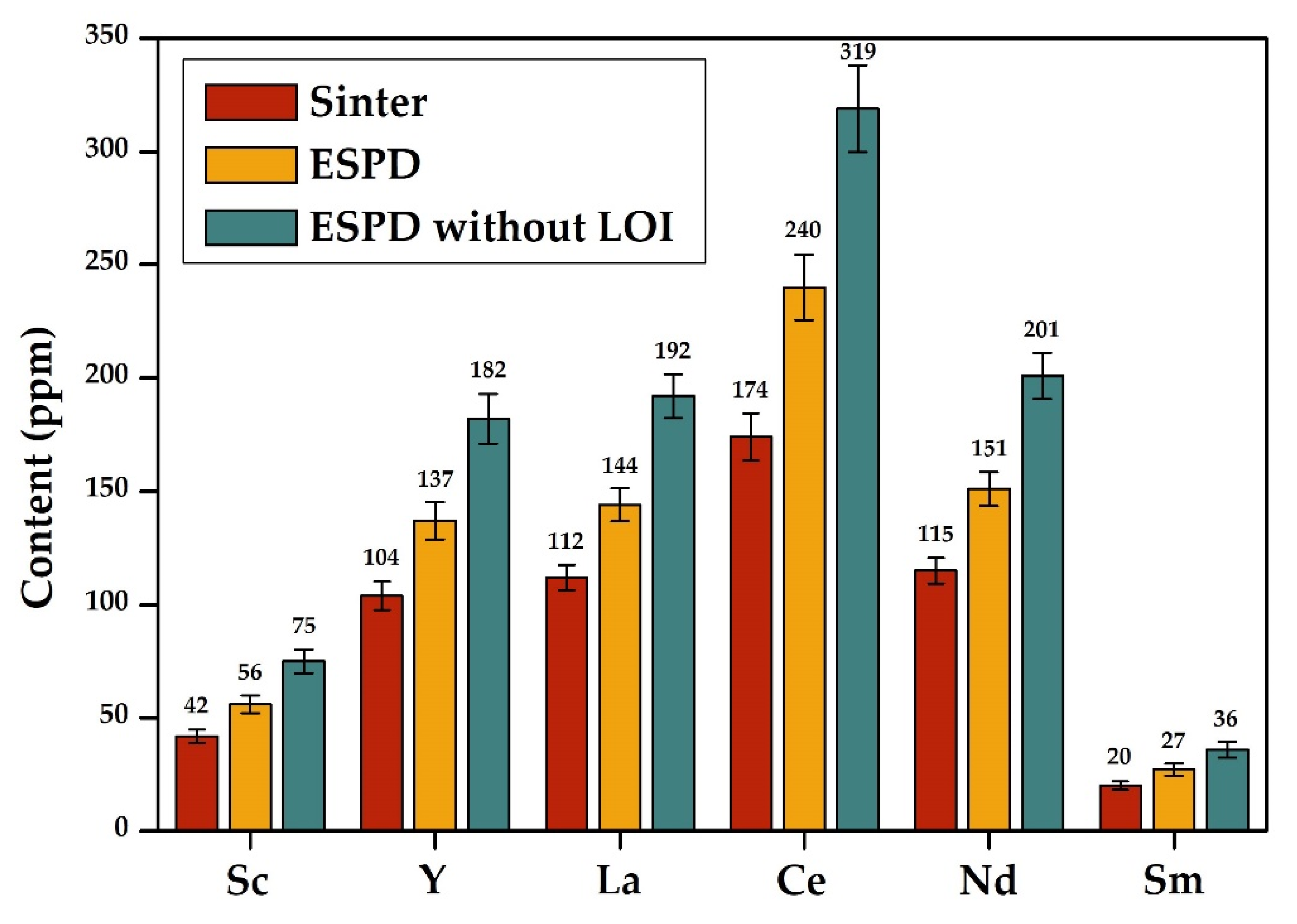





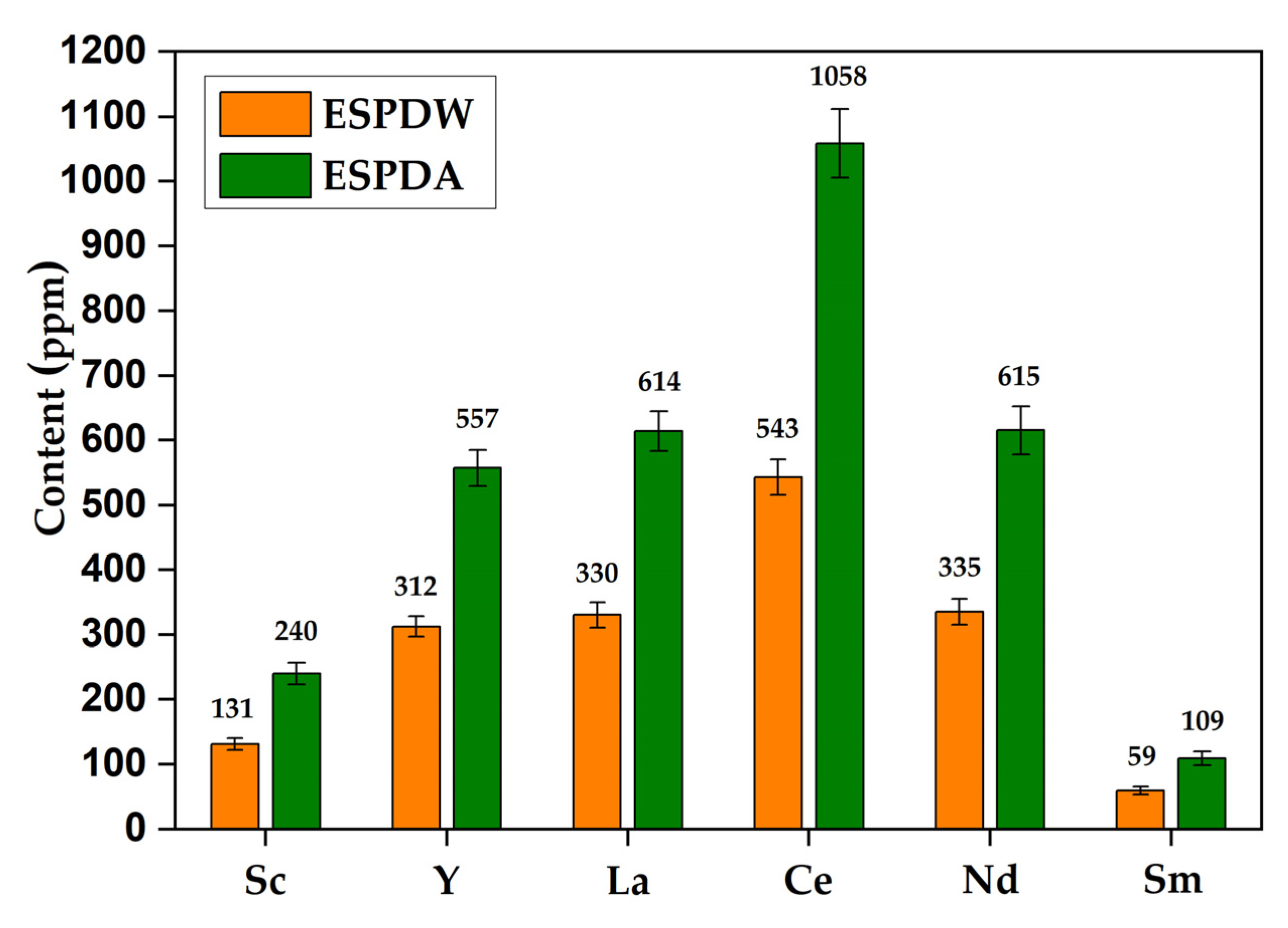

| Products | Al2O3 | SiO2 | Na2O | MgO | CaO | Fe2O3 | LOI 1 | μSi 2 | ΣREE |
|---|---|---|---|---|---|---|---|---|---|
| ESP dust | 25.5 | 2.5 | 28.3 | 0.4 | 2.5 | 12.2 | 24.8 | 10.2 | 755 |
| Bauxite sinter | 35.0 | 5.0 | 33.2 | 0.8 | 4.8 | 16.7 | 0.5 | 7.0 | 567 |
| Product | Al2O3 | SiO2 | Na2O | MgO | CaO | Fe2O3 | LOI | μSi |
|---|---|---|---|---|---|---|---|---|
| ESPDW | 39.8 | 3.1 | 1.7 | 1.0 | 6.2 | 30.5 | 18.3 | 12.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaikin, L.; Shoppert, A.; Valeev, D.; Loginova, I.; Napol’skikh, J. Concentration of Rare Earth Elements (Sc, Y, La, Ce, Nd, Sm) in Bauxite Residue (Red Mud) Obtained by Water and Alkali Leaching of Bauxite Sintering Dust. Minerals 2020, 10, 500. https://doi.org/10.3390/min10060500
Chaikin L, Shoppert A, Valeev D, Loginova I, Napol’skikh J. Concentration of Rare Earth Elements (Sc, Y, La, Ce, Nd, Sm) in Bauxite Residue (Red Mud) Obtained by Water and Alkali Leaching of Bauxite Sintering Dust. Minerals. 2020; 10(6):500. https://doi.org/10.3390/min10060500
Chicago/Turabian StyleChaikin, Leonid, Andrei Shoppert, Dmitry Valeev, Irina Loginova, and Julia Napol’skikh. 2020. "Concentration of Rare Earth Elements (Sc, Y, La, Ce, Nd, Sm) in Bauxite Residue (Red Mud) Obtained by Water and Alkali Leaching of Bauxite Sintering Dust" Minerals 10, no. 6: 500. https://doi.org/10.3390/min10060500






