The Important Role of Dissolved Oxygen Supply Regulated by the Hydraulic Shear Force during the Biosynthesis of Iron Hydroxysulfate Minerals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Modified 9K Liquid Medium
2.2. Preparation of the A. ferrooxidans Resting Cells
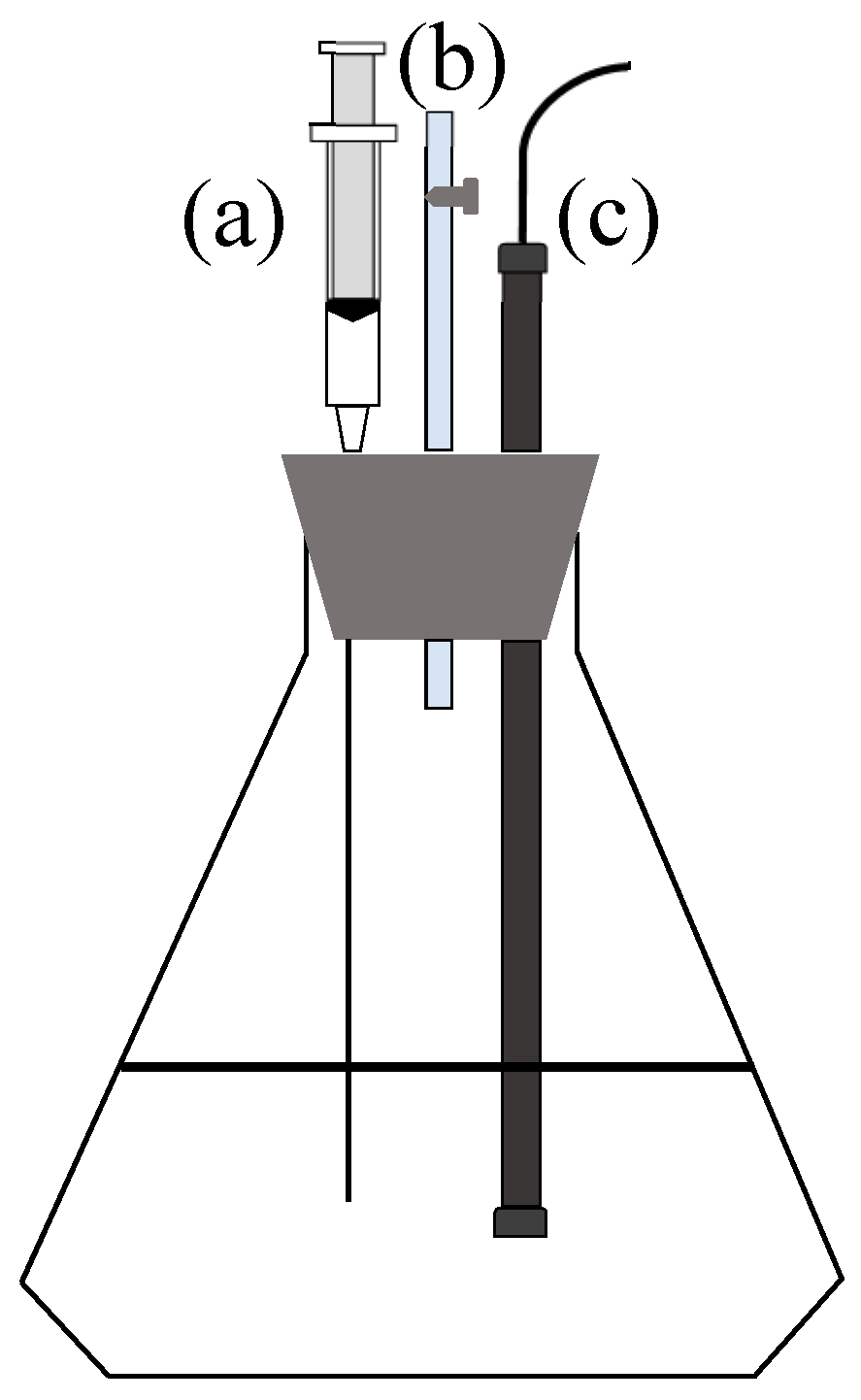
2.3. Effect of Hydraulic Shear Force on Fe2+ Bio-Oxidation and Fe3+ Hydrolysis by A. ferrooxidans
2.4. Effect of the O2 Supply Time on the A. ferrooxidans-Mediated Biomineralization
2.5. Determination Methods
3. Results and Discussion
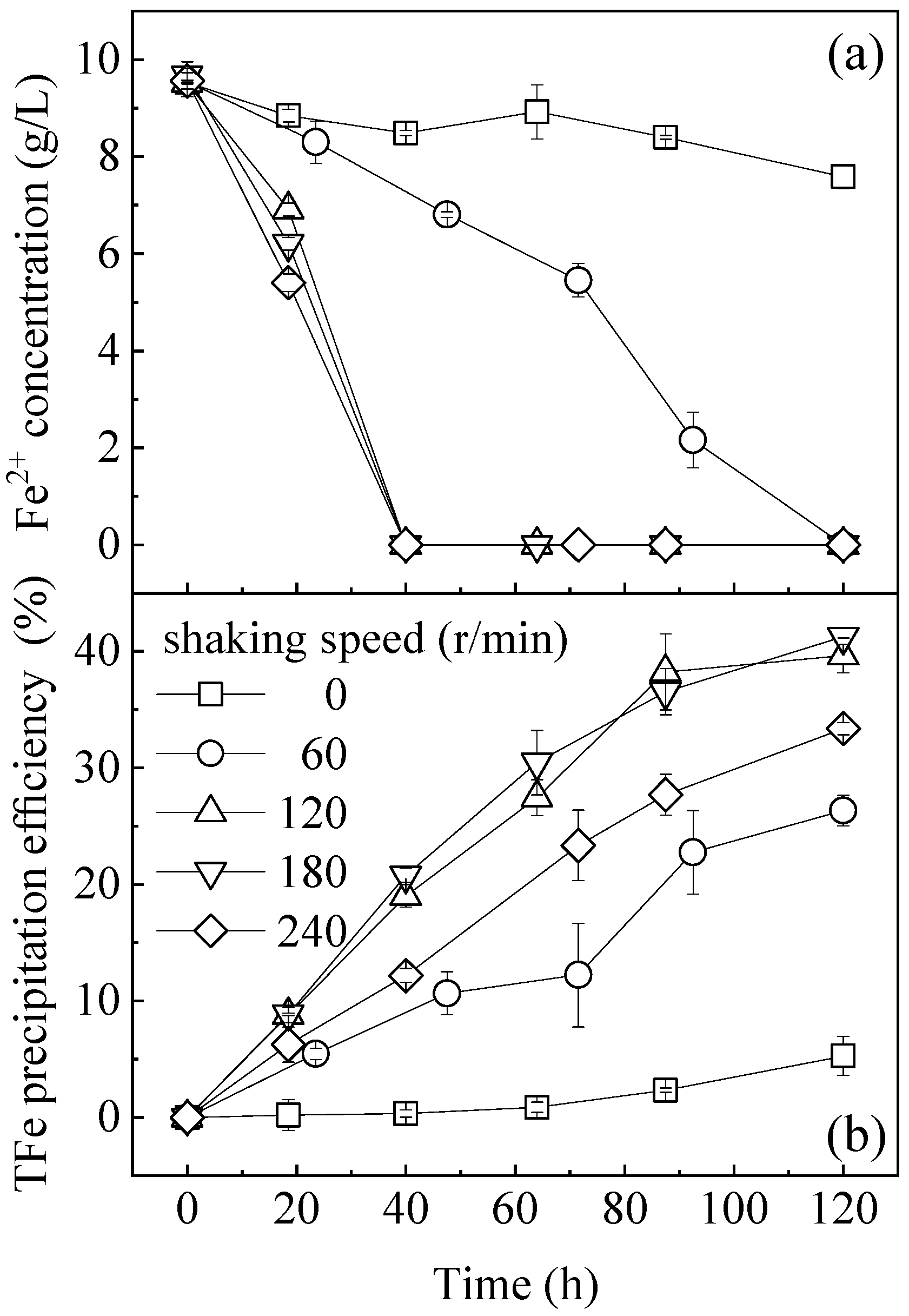
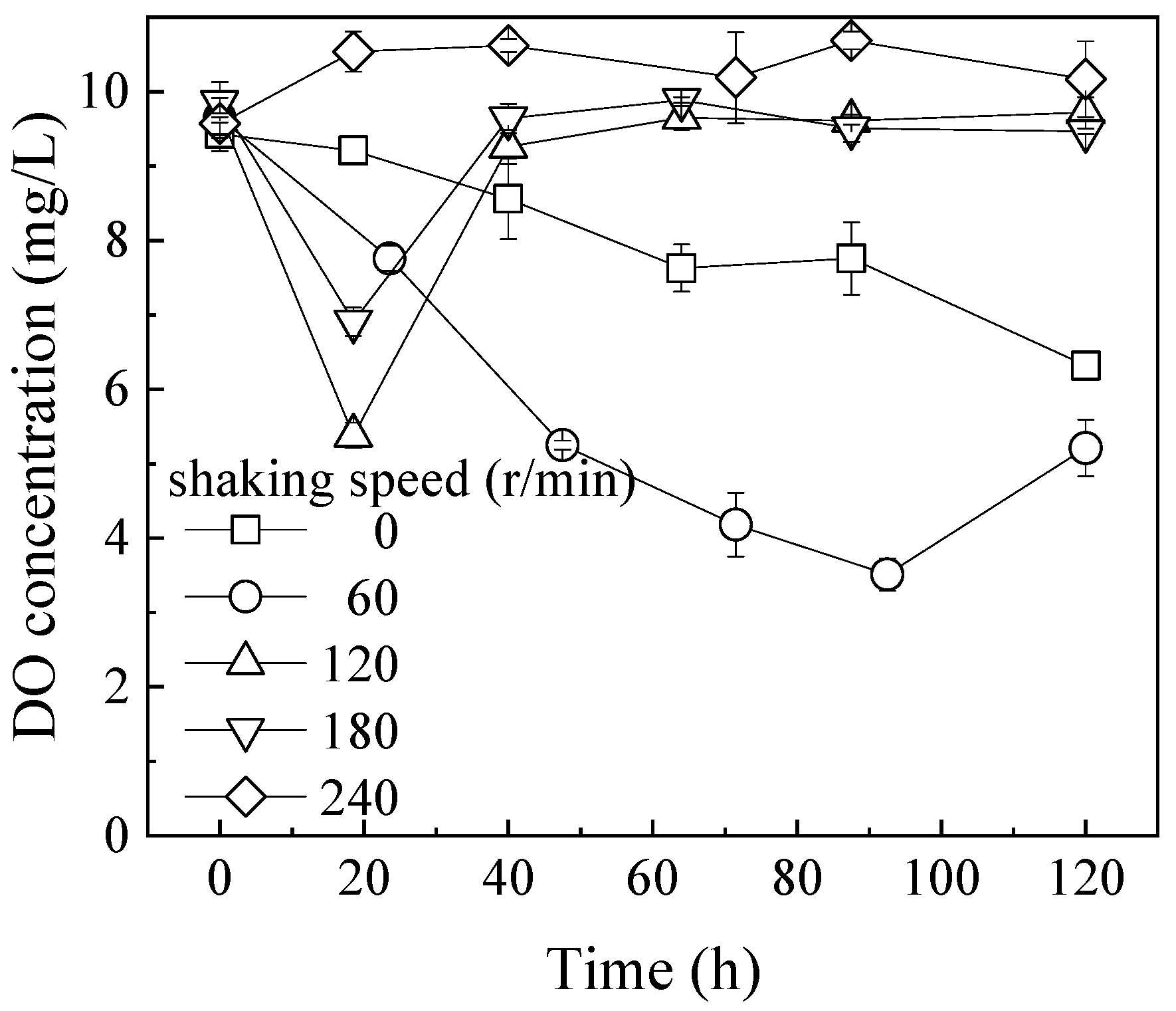
3.1. Effect of Hydraulic Shear Force on the Fe2+ Oxidation and TFe Precipitation Efficiency
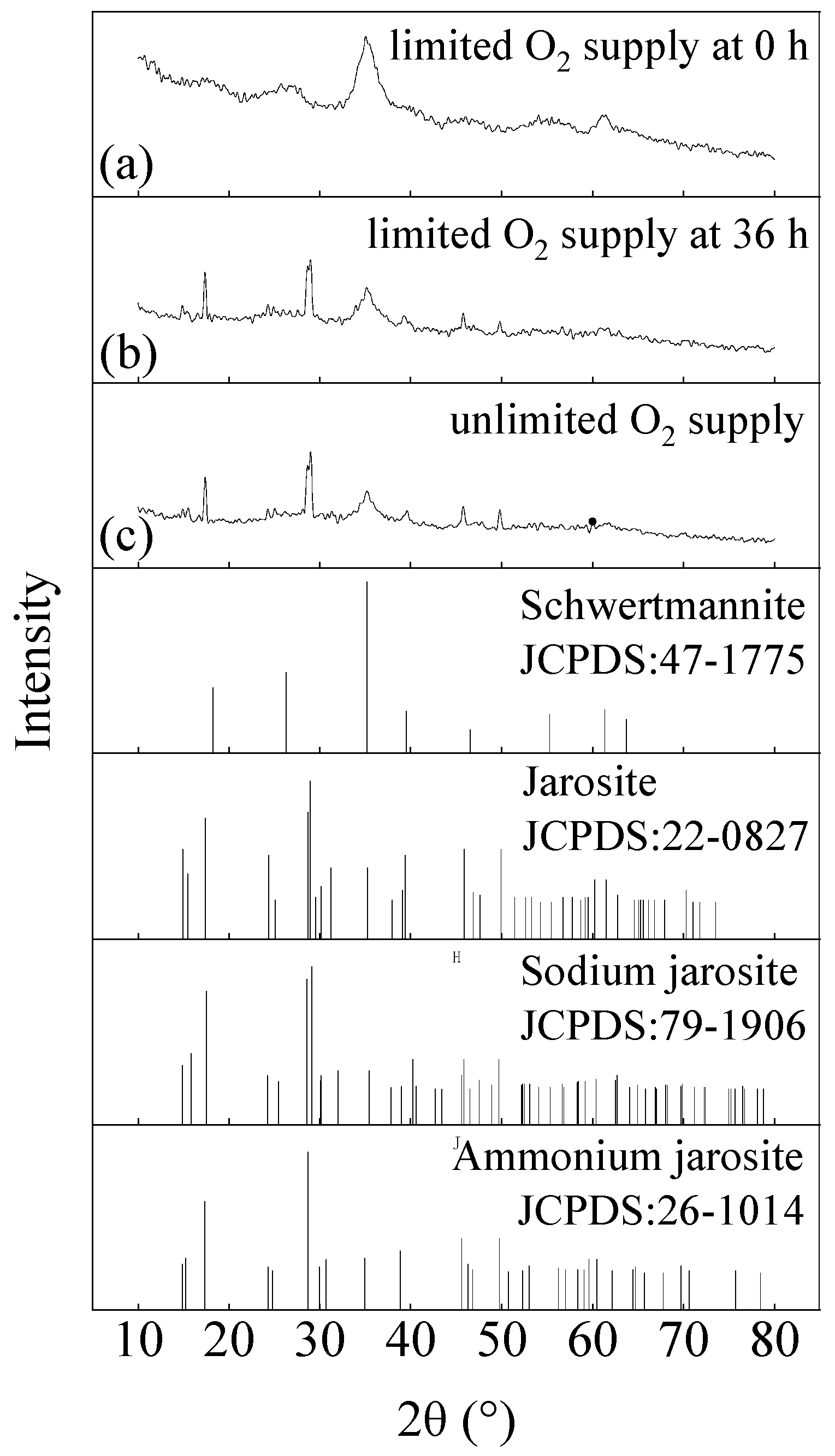
3.2. Effect of the O2 Supply Time on the Formation of Iron Hydroxysulfate Minerals
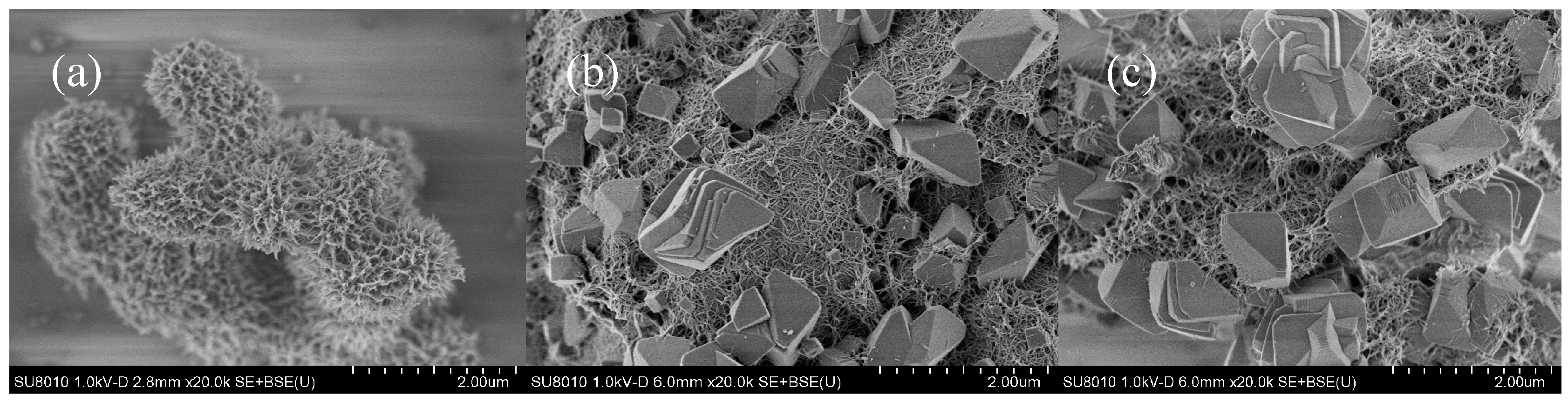
3.3. Characteristics of the Minerals Obtained from Different O2 Supply Conditions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balci, N.; Demirel, C. Prediction of Acid Mine Drainage (AMD) and Metal Release Sources at the Küre Copper Mine Site, Kastamonu, NW Turkey. Mine Water Environ. 2018, 37, 56–74. [Google Scholar] [CrossRef]
- Yucel, D.S.; Balci, N.; Baba, A. Generation of Acid Mine Lakes Associated with Abandoned Coal Mines in Northwest Turkey. Arch. Environ. Contam. Toxicol. 2016, 70, 757–782. [Google Scholar] [CrossRef] [Green Version]
- Balci, N.; Shanks, W.C., III; Mayer, B.; Mandernack, K.W. Oxygen and sulfur isotope systematics of sulfate produced by bacterial and abiotic oxidation of pyrite. Geochim. Cosmochim. Acta 2007, 71, 3796–3811. [Google Scholar] [CrossRef]
- Cravotta, C.A.; Trahan, M.K. Limestone drains to increase pH and remove dissolved metals from acidic mine drainage. Appl. Geochem. 1999, 14, 581–606. [Google Scholar] [CrossRef]
- Vhahangwele, M. A novel technology for neutralizing acidity and attenuating toxic chemical species from acid mine drainage using cryptocrystalline magnesite tailings. J. Water Process Eng. 2016, 10, 67–77. [Google Scholar]
- Wang, X.M.; Jiang, H.K.; Fang, D.; Liang, J.R.; Zhou, L.X. A novel approach to rapidly purify acid mine drainage through chemically forming schwertmannite followed by lime neutralization. Water Res. 2019, 151, 515–522. [Google Scholar] [CrossRef]
- Song, Y.W.; Wang, M.; Liang, J.R.; Zhou, L.X. High-rate precipitation of iron as jarosite by using a combination process of electrolytic reduction and biological oxidation. Hydrometallurgy 2014, 143, 23–27. [Google Scholar] [CrossRef]
- Meschke, K.; Herdegen, V.; Aubel, T.; Janneck, E.; Repke, J.U. Treatment of opencast lignite mining induced acid mine drainage (AMD) using a rotating microfiltration system. J. Environ. Chem. Eng. 2015, 4, 2848–2856. [Google Scholar] [CrossRef]
- Lee, W.C.; Lee, S.W.; Yun, S.T.; Lee, P.K.; Hwang, Y.S.; Kim, S.O. A novel method of utilizing permeable reactive kiddle (PRK) for the remediation of acid mine drainage. J. Hazard. Mater. 2016, 301, 332–341. [Google Scholar] [CrossRef]
- Umita, T. Biological mine drainage treatment. Resour. Conserv. Recycl. 1996, 16, 179–188. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Valente, T.; Grande, J.A.; De, I.T.M.L.; Santisteban, M.; Cerón, J.C. Mineralogy and environmental relevance of AMD-precipitates from the Tharsis mines, Iberian Pyrite Belt (SW, Spain). Appl. Geochem. 2013, 39, 11–25. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Gan, M.; Zhang, D.; Hu, Y.H.; Chai, L.Y. The nature of schwertmannite and jarosite mediated by two strains of Acidithiobacillus ferrooxidans with different ferrous oxidation ability. Mater. Sci. Eng. C 2013, 33, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.H.; Shi, T.H.; Wang, S.Z.; Huang, X.F.; Zhang, T.; Tang, Y.T.; Zhang, X.Y.; Qiu, R.L. Silane-based coatings on the pyrite for remediation of acid mine drainage. Water Res. 2013, 47, 4391–4402. [Google Scholar] [CrossRef]
- Regenspurg, S.; Brand, A.; Peiffer, S. Formation and stability of schwertmannite in acid mining lakes. Geochim. Cosmochim. Acta 2004, 68, 1185–1197. [Google Scholar] [CrossRef]
- Loan, M.; Richmond, W.R.; Parkinson, G.M. On the crystal growth of nanoscale schwertmannite. J. Cryst. Growth 2005, 275, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Barham, R.J. Schwertmannite: A unique mineral, contains a replaceable ligand, transforms to jarosites, hematites, and/or basic iron sulfate. J. Mater. Res. 1997, 12, 2751–2758. [Google Scholar] [CrossRef]
- Min, G.; Sun, S.J.; Zheng, Z.H.; Tang, H.J.; Sheng, J.R.; Zhu, J.Y.; Liu, X.X. Adsorption of Cr(VI) and Cu(II) by AlPO4 modified biosynthetic schwertmannite. Appl. Surf. Sci. 2015, 356, 986–997. [Google Scholar]
- Zhang, S.L.; Jia, S.Y.; Yu, B.; Liu, Y.; Wu, S.H.; Han, X. Sulfidization of As(V)-containing schwertmannite and its impact on arsenic mobilization. Chem. Geol. 2016, 420, 270–279. [Google Scholar] [CrossRef]
- Song, Y.W.; Zhang, J.Y.; Wang, H.R. Initial pH and K+ concentrations jointly determine the types of biogenic ferric hydroxysulfate minerals and their effect on adsorption removal of Cr(VI) in simulated acid mine drainage. Water Sci. Technol. 2018, 78, 2183–2192. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Kaiman, S. Synthesis and properties of jarosite-type compounds. Can. Mineral. 1976, 14, 151–158. [Google Scholar]
- Dutrizac, J.E. The effectiveness of jarosite species for precipitating sodium jarosite. J. Mineral. Met. Mater. Soc. 1999, 51, 30–32. [Google Scholar] [CrossRef]
- Gramp, J.P.; Jones, F.S.; Bigham, J.M.; Tuovinen, O.H. Monovalent cation concentrations determine the types of Fe(III) hydroxysulfate precipitates formed in bioleach solutions. Hydrometallurgy 2008, 94, 29–33. [Google Scholar] [CrossRef]
- Song, Y.W.; Wang, H.R.; Yang, J.; Cao, Y.X. Influence of monovalent cations on the efficiency of ferrous ion oxidation, total iron precipitation, and adsorptive removal of Cr(VI) and As(III) in simulated acid mine drainage with inoculation of Acidithiobacillus ferrooxidans. Metals 2018, 8, 596. [Google Scholar] [CrossRef] [Green Version]
- Bigham, J.M.; Schwertmann, U.; Pfab, G. Influence of pH on mineral speciation in a bioreactor simulating acid mine drainage. Appl. Geochem. 1996, 11, 845–849. [Google Scholar] [CrossRef]
- Liao, Y.H.; Zhou, L.X.; Liang, J.R.; Xiong, H.X. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans cell suspensions under different pH condition. Mater. Sci. Eng. C 2009, 29, 211–215. [Google Scholar] [CrossRef]
- Jensen, A.B.; Webb, C. Ferrous sulphate oxidation using Thiobacillus ferrooxidans: A review. Process Biochem. 1995, 30, 225–236. [Google Scholar] [CrossRef]
- Daoud, J.; Karamanev, D. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans. Mineral. Eng. 2006, 19, 960–967. [Google Scholar] [CrossRef]
- Ohmura, N.; Sasaki, K.; Matsumoto, N.; Saiki, H. Anaerobic respiration using Fe3+, S0, and H2 in the chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans. J. Bacteriol. 2002, 184, 2081–2087. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.E.; Babij, T.; Ritchie, A.I.M. Leaching of a sulphide ore by Thiobacillus ferrooxidans under anaerobic conditions. In Biohydrometallurgy; Rossi, G., Torma, A.E., Eds.; Associazionen Mineraria Sarde, Iglesias: Iglesias, Italy, 1983; pp. 361–376. [Google Scholar]
- Magdalena, G.; Roger, B.H.J.; Paul, C.F.K. Pyrite oxidation by Acidithiobacillus ferrooxidans at various concentrations of dissolved oxygen. Chem. Geol. 2006, 225, 16–19. [Google Scholar]
- Wang, Z.; Che, J.; Ye, C. Application of ferric chloride both as oxidant and complexant to enhance the dissolution of metallic copper. Hydrometallurgy 2010, 105, 69–74. [Google Scholar] [CrossRef]
- Sun, L.X.; Zhang, X.; Tan, W.S.; Zhu, M.L. Effect of agitation intensity on the biooxidation process of refractory gold ores by Acidithiobacillus ferrooxidans. Hydrometallurgy 2012, 127–128, 99–103. [Google Scholar] [CrossRef]
- Silverman, M.P.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [CrossRef] [Green Version]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Water Works Assn: Washington, DC, USA, 2012. [Google Scholar]
- Wang, S.M.; Zhou, L.X. A renovated approach for increasing colony count efficiency of Thiobacillus ferrooxidans and Thiobacillus thiooxidans: Double-layer plates. Acta Sci. Circumstantiae 2005, 25, 1418–1420. [Google Scholar]
- Liu, F.W.; Gao, S.Y.; Wang, M.; Yu, H.Y.; Cui, C.H.; Zhou, L.X. Effect of KOH on the formation of biogenic secondary iron minerals in iron- and sulfate-rich acidic environment. Acta Sci. Circumstantiae 2015, 35, 476–483. [Google Scholar]
- Manna, B.; Ghosh, U.C. Adsorption of arsenic from aqueous solution on synthetic hydrous stannic oxide. J. Hazard. Mater. 2007, 144, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Bigham, J.M.; Murad, E. The first occurrence of schwertmannite in a natural stream environment. Eur. J. Mineral. 1995, 7, 547–552. [Google Scholar] [CrossRef]
- Bai, S.Y.; Liang, J.R.; Zhou, L.X. Effects of monovalent cation and dissolved organic matter on the formation of biogenic secondary iron minerals in bioleaching system. Acta Mineral. Sin. 2011, 31, 118–125. [Google Scholar]
- Thomas, C.R. Problems of shear in biotechnology. In Chemical Engineering Problems in Biotechnology; Elsevier: Oxford, UK, 1990; pp. 23–93. [Google Scholar]
- Doran, M.P. Bioprocess Engineering Principles; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Liu, J.S.; Zhang, Y.H.; Li, B.M. The study kinetic for growth of Acidithiobacillus ferrooxidans. J. Microbiol. 2006, 2, 9–13. [Google Scholar]
- Majzlan, J.; Navrotsky, A. Thermodynamics of iron oxides: Part III. Enthalpies of formation and stability of ferrihydrite (−Fe(OH)3), schwertmannite (−FeO(OH)3/4(SO4)1/8), and ε-Fe2O3. Geochim. Cosmochim. Acta 2004, 68, 1049–1059. [Google Scholar] [CrossRef]
- Amouric, A.; Brochier, A.C.; Johnson, D.B.; Bonnefoy, V.; Hallberg, K.B. Phylogenetic and genetic variation among Fe(II)-oxidizing acidithiobacilli supports the view that these comprise multiple species with different ferrous iron oxidation pathways. Microbiology 2011, 157, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.M.; Bigham, J.M.; Tuovinen, O.H. Formation of schwertmannite and its transformation to jarosite in the presence of acidophilic iron-oxidizing microorganisms. Mater. Sci. Eng. C 2006, 26, 588–592. [Google Scholar] [CrossRef]
- Eskandarpour, A.; Onyango, M.S.; Ochieng, A.; Asai, S. Removal of fluoride ions from aqueous solution at low pH using schwertmannite. J. Hazard. Mater. 2008, 152, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Powder Diffraction Standards (JCPDS). Mineral Powder Diffraction Files; International Center for Diffraction Data: Newtown Square, PA, USA, 2002; pp. 856–975. [Google Scholar]
- Bai, S.Y.; Xu, Z.H.; Wang, M.; Liao, Y.H.; Liang, J.R.; Zheng, C.C.; Zhou, L.X. Both initial concentrations of Fe(II) and monovalent cations jointly determine the formation of biogenic iron hydroxysulfate precipitates in acidic sulfate-rich environments. Mater. Sci. Eng. C 2012, 32, 2323–2329. [Google Scholar] [CrossRef]
- Liu, F.W.; Bu, Y.S.; Tian, G.J.; Cui, C.H.; Zhou, L.X. Influence of temperature and pH on dissolution behavior of biogenic Schwertmannite in acidic environment and the adsorption of Cu2+. Acta Sci. Circumstantiae 2013, 33, 2445–2451. [Google Scholar]
- Sasaki, K.; Konno, H. Morphology of jarosite-group compounds precipitated from biologically and chemically oxidized Fe ions. Can. Mineral. 2000, 38, 45–56. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, R.; Wang, H.; Song, Y. The Important Role of Dissolved Oxygen Supply Regulated by the Hydraulic Shear Force during the Biosynthesis of Iron Hydroxysulfate Minerals. Minerals 2020, 10, 518. https://doi.org/10.3390/min10060518
Yang J, Wang R, Wang H, Song Y. The Important Role of Dissolved Oxygen Supply Regulated by the Hydraulic Shear Force during the Biosynthesis of Iron Hydroxysulfate Minerals. Minerals. 2020; 10(6):518. https://doi.org/10.3390/min10060518
Chicago/Turabian StyleYang, Jun, Rui Wang, Heru Wang, and Yongwei Song. 2020. "The Important Role of Dissolved Oxygen Supply Regulated by the Hydraulic Shear Force during the Biosynthesis of Iron Hydroxysulfate Minerals" Minerals 10, no. 6: 518. https://doi.org/10.3390/min10060518
APA StyleYang, J., Wang, R., Wang, H., & Song, Y. (2020). The Important Role of Dissolved Oxygen Supply Regulated by the Hydraulic Shear Force during the Biosynthesis of Iron Hydroxysulfate Minerals. Minerals, 10(6), 518. https://doi.org/10.3390/min10060518




