1. Introduction
The flotation of iron ores is performed in circuits that consist of mechanical cells, columns, or a combination of both types of machine. The feed consists of particles in the size range between 10 μm and 150 μm [
1]. The slimes (fraction < 10 μm) are removed in hydrocyclones and the top size is limited to 5% to 10% > 150 μm. This wide range of particles size impairs process selectivity due to the possible differences in behavior concerning properties, such as hydrophobicity, specific surface area, weight, etc. [
2]. Understanding the characteristics and behavior of the slimes is of fundamental importance on the iron ore flotation industry, as the operational conditions can be better adjusted to maximize Fe recovery and product quality [
3]
The harmful effects of iron slimes on the reverse flotation of quartz have been known since the beginning of its operation [
1]. The main effects refer to the increase of reagents consumption, the lower selectivity and the froth stability [
4]. The removal of iron ore slimes using hydrocyclones was introduced with the USBM process [
5]. Investigations that were carried out by Lima and coworkers for different iron ores from the Iron Quadrangle in the Southeast of Brazil indicated that the by-pass levels on the desliming hydrocyclones lower than 4% did not affect the flotation recoveries or the quality of the concentrates [
6]. The results of this study indicated that the specific surface area with different by pass levels can explain the flotation response on the presence of slimes.
Filippov and coworkers reported the effect of desliming in wet magnetic separation, applied to tailings from magnetite ores processing, at the Mikhailovsky plant in Russia [
7]. The authors demonstrated that desliming at 10 µm reveals higher mass pull and lower iron grades when desliming at 20 µm. Despite 10% higher mass pull at 10 µm, cutting at 20 µm have shown a dramatic increase on the flotation selectivity.
Table 1 shows the effect of the desliming size on the flotation response.
Studies that were conducted by Thella and coworkers with the iron slimes stored in slimes pond showed the possibility to obtain concentrates with 64.46% Fe, 2.66% Al
2O
3, and 2.05% SiO
2 with the combination of two stages of hydrocyclones of 50 mm of diameter with vortex finders of 14.3 mm and 11 mm of diameters to remove the particles with high content of Al
2O
3 and enhance the flotation selectivity while using amines and starch [
8]. Ma and coworkers observed that starch adsorption in kaolinite is pH dependent, with starch adsorption decreasing with increasing pH from 7 to 10.5 [
9] at pH 10.5, increasing ionic strength significantly enhances the adsorption density of starch on kaolinite.
Weissenborn and coworkers studied the optimization of selective flocculation of ultra-fine iron ore aiming the increase of Fe content in tailings samples where kaolinite was the main gangue mineral [
10]. The results indicated the possibility to increase the Fe content from 46.6% to 57%. Wheat starch was the most appropriate selective flocculant. The recovery of all minerals was very sensitive to changes in flocculation conditions, and careful control of the many process variables was required in order to achieve optimum recovery.
Xu and coworkers evaluated the floatability of kaolinite in different sizes and with different chain length collectors [
11]. The anomalous flotation behaviour of kaolinite have been explained based on crystal structure considerations. The results showed that fine kaolinite particles have more edges to adsorb fewer cationic collectors than that of coarse kaolinite particles, which is responsible for the poorer floatability of fine kaolinite.
Dey and coworkers evaluated the concentration of Indian iron ore slime using column flotation [
12]. A sample with 16% passing in 25 µm size fraction containing 58.7% Fe, 5.2% SiO
2, and 4.9% Al
2O
3 was used during the investigations in a column of 7.5 cm diameter and 3 m height. The tests of direct flotation were carried out using a pulp with 10% of solids (
w/
w), sodium silicate as depressant, MIBC as frother and sodium oleate as collector. A concentrate with 62% Fe and 59% yield was produced using 2.5 kg/t of collector at 0.5 cm/s of superficial air velocity.
Slimes have higher surface area, which increases the reagent consumption and they can increase flotation residence time resulting in high froth volumes ([
13]. Lima and coworkers evaluated the effect of the by-pass on the reverse flotation of quartz for samples with different mineralogical compositions [
6]. The specular hematite sample was affected by the increase of by-pass level from 4% to 6%, on the contrary of the goethite/porous hematite sample, which was not affected when the by-pass level increased from 2% to 8%. This study showed the higher specific surface area of the specular hematite sample with 6% of by-pass can be the cause of the higher grade of SiO
2 on the concentrate, as reported in
Table 2.
Bandini and coworkers analyzed the effect of colloidal iron ore slime on the flotation of galena and quartz particles by coatings [
14]. These authors investigated the formation of iron ore slime coatings and methods for their removal, using quartz as a model for deslimed galena, since it shows similar surface electrical properties and slime particle adsorption characteristics. The use of pH control alone was ineffective in removing iron oxide slime coatings from galena or quartz. By increasing the pH to 10, both iron oxide and galena/quartz particles become negatively charged; however, there is still an energy barrier to overcome for slime detachment. Combining pH control with high shear mixing or sonication removed rapid and completely the iron ore slime coatings.
Rulyov and coworkers evaluated the microfotation of particles below 1 µm. The use of 40 µm bubbles and the coagulation or flocculation of particles to reach 7 µm were necessary to increase their recovery [
15].
The presence of slimes in the beneficiation plants of the Iron Ore Quadrangle in Brazil have increased even more, reaching almost 40 million of tons per annum. Understanding the harmful effect of these slimes on the iron ore flotation is a challenging and it can be useful to develop alternatives to increase the Fe recovery on the beneficiation plants.
2. Materials and Methods
2.1. Samples
This study was carried out using eight samples, as described in
Table 3.
Sample 1 was collected on the underflow of the thickener of an industrial beneficiation plant in the Iron Quadrangle, before the disposal on tailings dam. Samples 1A and 1B were obtained from sample 1 after three cleaning and three of scavenging stages, aiming to obtain the highest grades of Fe on the concentrate and silicates on the tailing.
Sample 1 was also submitted to settling in a 2000 mL beaker, aiming to obtain settling products (underflow) with different percentage of gangue minerals, especially aluminosilicates. The sedimentation tests were carried out on the following conditions. The percentage of solids in the feed was 5%. The pulp pH was 10.5 aiming the dispersion of the pulp. Settling time after dispersion of the pulp was put at rest for 5 min. Cut-off-size during desliming was at 10 µm and the corn starch dosages were 0, 100, 250, and 500 g/t. The obtained underflow samples were used for bench scale flotation tests with 100 g/t of collector (Ether amine Flotigam 7100 by Clariant, Muttenz, Switzerland), 500 g/t of corn starch as depressant at pH 10 and using Denver 1200 mL bench scale cell.
Samples 2A, 2B, and 2C were collected on the desliming hydrociclones feed of the same industrial beneficiation plant. The samples were collected in different days, when the% of SiO2 on the +74 µm were 20%, 23%, and 31%, respectively. Each of these three samples were deslimed at bench scale to obtain 1.5%, 3.5%, 5.5%, and 10.0% of slimes in the flotation feed composition. These tests were done aiming at evaluate the combined effect of slimes and the distribution of SiO2 on the +74 µm in the flotation feed. The flotation tests combining the% of SiO2 on the +74 µm fraction and the% of slimes were done with 100 g/t of collector (Ether amine Flotigam 7100 by Clariant), 500 g/t of corn starch, and pH 10.5 in a 1200 mL Denver cell.
Samples 3 and 4 are pure quartz and hematite minerals from Minas Gerais, Brazil. They were crushed using jaw and gyratory crusher and milled using ceramic ball mill to reach the size fraction approximately below 200 µm. The quartz sample was screened to obtain +74 µm for the flotation tests. The hematite sample was classified using a cyclosizer in +20 µm and –20 µm fractions. The flotability of quartz in the presence of hematite (+20 µm and –20 µm) was evaluated using 100 g/t of collector (Ether amine Flotigam 7100 by Clariant), 500 g/t of corn starch as depressant at pH 10, and using MineMet 300 mL bench scale. The proportion of coarse quartz and fine hematite was almost the same in typical desliming feed, as 80% and 20%, respectively.
2.2. Characterizations
Chemical assays were done by X-ray fluorescence (XRF) by Thermo Fisher Scientific Niton XL3t XRF Analyzer (Thermo Fisher Scientific, Waltham, MA, USA), to determine Fe, SiO2, Al2O3, P, Mn, CaO, MgO, and LOI. The error of chemical analysis was evaluated to 2%.
Size distribution was obtained by laser sizer instrument (Malvern® Mastersizer 2000, Malvern, Malvern, UK).
The mineralogical composition of sample 1 was determined using an X-ray diffraction technique and the automated scanning electron microscope (QEMSCAN® system) with a tungsten source from the FEI company (Hillsboro, OR, USA). The iDiscover software (everly Hills, CA, USA) was used for the measurements and the data processing. The PMA (Particle Mineral Analysis) analysis model was used. The QEMSCAN® was operated using a voltage acceleration of 15 kV and a current of approximately 5 nA. For the X-ray diffraction, the samples were pressed and analyzed in a PANalytical X-ray diffractometer (Empyrean model) while using a Co tube. The crystalline phases were identified and interpreted by the High Score Plus software (Malvern Panalytical, Worchestershire, UK) and the mineralogical composition was quantified by the Rietveld method.
3. Results and Discussion
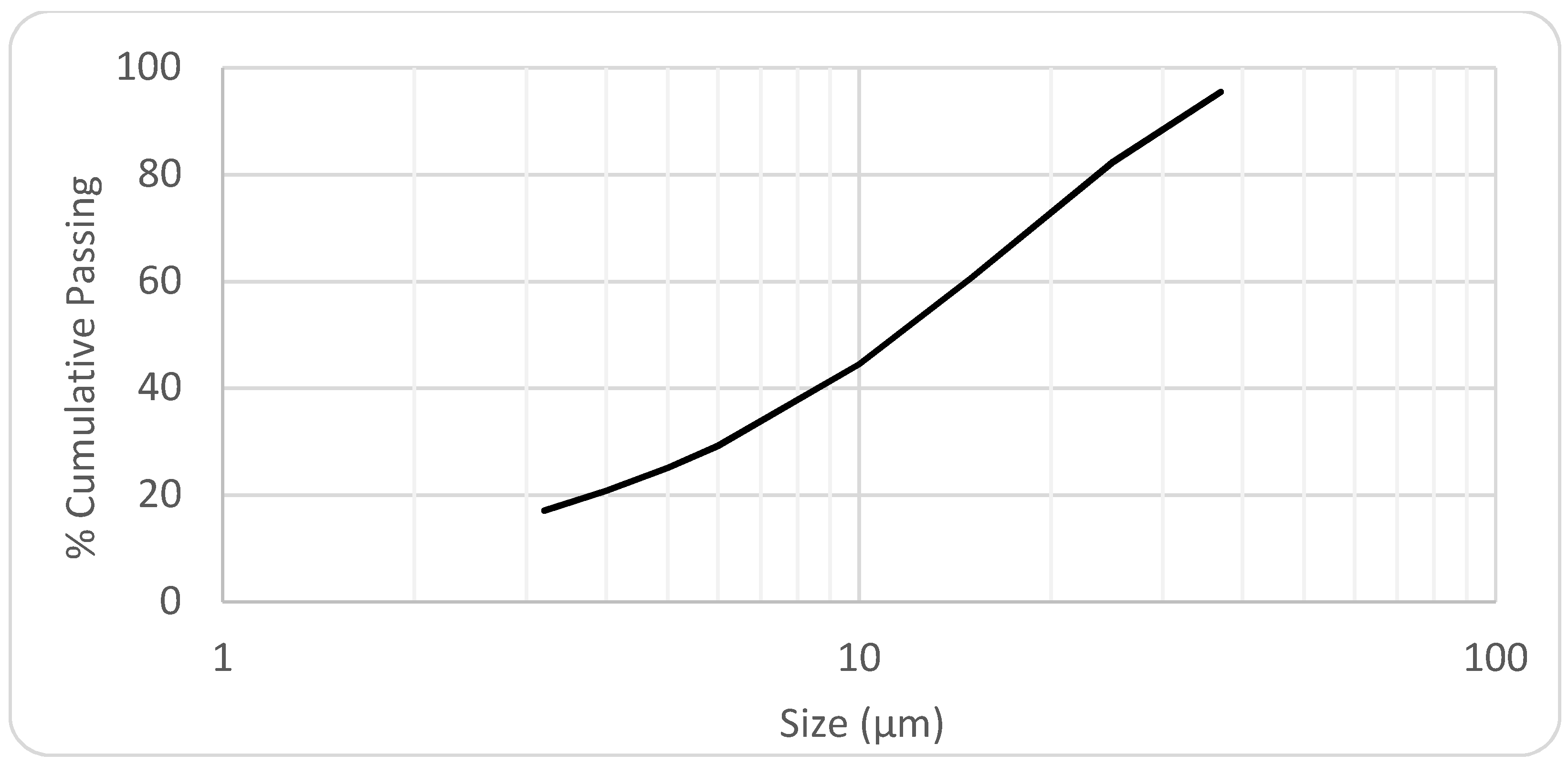
The chemical analyses and size distribution of the iron ore slime, sample 1 are shown in
Table 4 and
Figure 1, respectively.
The typical iron ore slime is about 95% passing in 37 µm, 45% passing 10 µm, and 17% passing 3.2 µm. By combining the sieving analysis with the XRF results, it was possible to estimate the distribution of Fe, Si, and Al in the different size fractions, as shown in
Figure 2. It was possible to observe that the majority of the iron (87%) is present in the fine particle size fractions (−20 μm), which are susceptible to report to the tailing by entrainment when performing reverse cationic flotation. At the same way, almost 91% of the Al (mainly kaolinite) is distributed in the size fraction below 20 μm, with low floatability behavior as reported by Xu and coworkers [
11]. On the contrary, 58% of Si is below 20 μm.
The mineralogical analyses are reported in
Figure 3, where hematite is the main iron mineral, and quartz and kaolinite are the main gangue minerals.
Figure 4 illustrates an image of the surface of the hematite, where it is possible to observe the presence of finely disseminated quartz inclusions that appear to be smaller than 2 μm, which can impact the selectivity of flotation.
Figure 5 shows the comparative of PSD between the iron ore slime (sample 1) and the products (samples 1A and 1B) obtained after several stages of magnetic concentration and
Table 5 show the comparative chemical analyses between them.
The hematite of the slime (sample 1A) is much finer than the silicates (1B), in accordance with the size distribution of
Figure 2. D50 of hematite is 9 µm, while d50 of the silicates is 20 µm.
From the results that are presented in
Figure 5, it was deduced that the Iron oxide minerals (hematite) present on the slime sample are much finer than the gangue minerals (silicates). Moreover, after three stages of cleaning with the magnetic separator, the tailing product contains 17.06% Fe, indicating some association between gangue minerals and Fe minerals.
The finer size distribution of the Fe minerals and their associations with the gangue minerals indicate the challenges and difficulties to concentrate the iron ore slimes and the maximum yielded recovery expected was evaluated as 55%.
Lima and coworkers calculated the degree of entrainment for Fe in different size fractions using an empirical correlation with the water recovery for the rougher, cleaner, and recleaner industrial cells [
16]. The results showed the highest entrainment degree for the particles were lower than 20 µm. This result suggests the iron oxide minerals present on the slimes can be easily lost by entrainment, as almost 80% are lower than 20 µm, as shown on the
Figure 2.
Table 6 shows the results of the sedimentation tests with the iron ore slime (sample 1), indicating that the corn starch promotes the concentration of Al
2O
3 to the underflow.
When considering that kaolinite is the main mineral of Al2O3 in the iron ore slime, the increase of the Al2O3 content to the underflow in the presence of corn starch, can indicate its adsorption on the kaolinite surface. This behavior indicates that the presence of slimes can affect the flotation of quartz by the depression of kaolinite with the corn starch damaging the hematite depression. As the main mechanism of interaction in cationic reverse flotation of quartz is electrostatic, the lack of depression can lead the hematite to be floated by the collector.
The underflows obtained in the sedimentation tests, with different Al
2O
3 contents were submitted to standard bench scale flotation tests. The results of the tests can be seen in the
Table 7, where higher percentage of Al
2O
3 on the feed (underflow) negatively affected the quality of the flotation concentrate.
The Al sites of the kaolinite seems to interact with the starch while the Si sites can be free to interact with the collector. This behavior can reduce the floatability of quartz particles, as shown in the results of
Table 7.
Figure 6 shows the combined effect of the distribution of SiO
2 in the +74 µm fraction and the presence of slimes on the flotation response, regarding the % of SiO
2 in the concentrate. These tests were realized with samples 2A, 2B, and 2C (desliming feeds).
The flotation response is drastically impacted with the combination of higher SiO
2 distribution in the +74 µm fraction, even for small quantities of slimes, as indicated on the
Figure 6. An increase from 1.0% to 3.0% of slimes can lead to a drop of almost 10% in the iron grade in the concentrate. The presence of slimes can adsorb part of the available collector, resulting in insufficient hydrophobicity of the coarse quartz, causing a dropback phenomenon. This behavior is in accordance with the studies conducted by Fugen and cowerkers, where the presence of ultrafine particles (−10 µm) caused the high consumption of reagents, suggesting splint the flotation circuit [
17]. Another hypothesis is slime coating of small kaolinite particles on the coarse quartz surface inhibiting the collector adsorption or/and the interaction between quartz particles and bubbles. The mechanism of coatings can be controlled by the electrostatic interactions with the kaolinite particles because of the face-edge structure kaolinite [
9]. While the negatively charged hematite particles adsorption on the coarse quartz is difficult to occur. Additionally, the coating may be enhanced by the bridging effect of the Ca2+ and other metallic cations [
18].
The original size distributions of samples 4 and 5 (pure hematite and pure quartz) are shown in
Figure 7. The flotation tests were realized with the mixtures: 80% +74 µm quartz/20% +20 µm hematite and 80% +74 µm quartz/20% −20 µm hematite.
The harmful effect of slimes on the reverse flotation of quartz can also be observed on the results of
Figure 8, where adding 20% of the –20 µm hematite reduced the floatability of the coarse quartz particles (+74 µm) from 98% to 62%. On the other hand, the same amount of +20 µm hematite particles did not affect the floatability of these quartz particles. According to the results of
Figure 4, 87% of the hematite in the slime is below 20 µm, which enhances its effect on the floatability of quartz. These ultrafine particles of hematite can adsorb a large amount of the collector, causing less hydrophobicity of the quartz particles. The other hypothesis to explain the harmful effect of the fine hematite (−20 µm) on the floatability of the coarse quartz is the slime coating, as in the study of Bandini and coworkers [
14]. At pH 10.5, both particles are negatively charged, however there is still an energy barrier to overcome for slime detachment. When considering that the 2 hematite fractions showed almost the same recovery to the froth, but only the finer (−20 µm) decreased the recovery of quartz to the froth, slime coating seems to be the responsible for this harmful effect.