Mineralogy and Genesis of the Kihabe Zn-Pb-V Prospect, Aha Hills, Northwest Botswana
Abstract
:1. Introduction
2. Geological Setting
2.1. Regional Geology
2.2. The Kihabe Prospect
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Analyte | KDD 143-16 | KDD 143-16 | KDD 143-16 | KDD 143-16 | KDD143-22 | KDD143-22 | KDD143-22 | KDD143-22 | KDD143-21 | KDD143-21 | KDD143-22 | KDD143-22 | KDD143-22 | KDD143-22 | KDD143-22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Willemite | Willemite | Willemite | Willemite | Willemite | Willemite | Willemite | Willemite | Smithsonite | Smithsonite | Smithsonite | Smithsonite | Smithsonite | Cerussite | Cerussite | |
| SiO2 | 25.55 | 26.17 | 25.77 | 26.11 | 26.91 | 26.35 | 26.90 | 26.04 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| ZnO | 70.91 | 70.68 | 68.65 | 72.38 | 71.88 | 71.46 | 69.93 | 71.31 | 62.72 | 61.83 | 62.88 | 60.07 | 63.34 | 2.70 | 1.82 |
| FeO | 0.12 | 0.22 | 0.88 | 0.04 | 0.18 | 0.78 | 0.06 | 0.36 | 0.23 | 0.34 | 0.04 | 2.77 | 0.22 | 0.16 | b.d. |
| MnO | 0.13 | 0.09 | 0.02 | b.d. | 0.16 | b.d. | 0.18 | b.d. | b.d. | 0.18 | b.d. | b.d. | 0.06 | 0.14 | 0.29 |
| MgO | b.d. | 0.15 | b.d. | b.d. | 0.11 | 0.07 | 0.19 | 0.28 | 0.28 | 0.33 | b.d. | 0.38 | 0.42 | 0.14 | 0.15 |
| CaO | 0.06 | 0.17 | 0.10 | 0.01 | b.d. | b.d. | 0.02 | 0.06 | 0.08 | b.d. | 0.06 | 0.06 | 0.24 | 0.55 | 0.41 |
| CdO | 0.14 | 0.42 | b.d. | b.d. | 0.08 | b.d. | 0.08 | b.d. | 0.25 | b.d. | 0.32 | 0.67 | 0.40 | b.d. | b.d. |
| PbO | b.d. | 0.03 | 0.16 | 0.18 | b.d. | 0.21 | 1.51 | 0.26 | 0.30 | 0.07 | 0.21 | 0.16 | 0.46 | 78.47 | 78.33 |
| BaO | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.24 | 0.15 |
| As2O5 | 2.87 | 2.18 | 2.43 | 1.91 | 1.35 | 1.90 | 0.52 | 2.02 | 0.58 | 0.78 | 0.72 | 0.13 | 0.29 | b.d. | b.d. |
| CO2 * | 34.85 | 34.50 | 34.57 | 34.97 | 35.42 | 17.78 | 17.14 | ||||||||
| Total | 99.78 | 100.12 | 98.00 | 100.64 | 100.66 | 100.76 | 99.40 | 100.33 | 99.29 | 98.04 | 98.80 | 99.22 | 100.83 | 100.19 | 98.28 |
| Analyte | Rule and Radke (1988) | KDD143-21 | KDD 143-22 | KDD143-21 | KDD 143-22 | KDD 143-22 | KDD143-24 | KDD143-24 | KDD 143-26 | KDD 143-26 | KDD 143-26 | KDD 143-26 | KDD 143-26 | KDD 143-26 | KDD143-26 | KDD143-26 | KDD143-26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 5 | Site 5 | Site 7 | Site 2 | Site 3 | Site 12 | Site 12 | Site 1 | Site 4 | Site 4 | Site 7 | Site 7 | Site 5 | Site 5 | Site 10 | Site 1 | ||
| Spectrum 3 | Spectrum 1 | Spectrum 3 | Spectrum 3 | Spectrum 2 | Spectrum 1 | Spectrum 2 | Spectrum 3 | Spectrum 8 | Spectrum 4 | Spectrum 1 | Spectrum 2 | Spectrum 2 | Spectrum 2 | Spectrum 4 | Spectrum 3 | ||
| SiO2 | 32.00 | 28.48 | 29.09 | 30.00 | 31.41 | 31.30 | 29.68 | 29.88 | 30.74 | 30.48 | 29.52 | 30.28 | 27.55 | 31.32 | 30.91 | 26.68 | 26.06 |
| TiO2 | 0.01 | 0.07 | 0.09 | 0.13 | − | 0.08 | 0.12 | − | 0.36 | 0.32 | 0.14 | − | − | − | − | ||
| Al2O3 | 12.40 | 10.97 | 11.55 | 10.27 | 12.91 | 12.89 | 11.35 | 11.66 | 15.31 | 15.15 | 14.14 | 16.05 | 13.84 | 14.19 | 15.42 | 13.57 | 14.24 |
| FeO | 12.90 | 0.51 | 0.65 | 0.15 | 0.31 | 1.10 | 1.29 | 1.60 | 1.62 | 1.01 | 0.87 | 1.15 | 0.79 | 1.36 | 1.76 | 1.64 | 0.79 |
| MnO | 0.15 | − | 0.11 | 0.04 | − | 0.17 | 0.25 | 0.14 | 0.12 | 0.04 | 0.18 | 0.13 | − | 0.03 | − | 0.06 | − |
| MgO | 4.60 | 1.84 | 1.95 | 1.69 | 2.09 | 1.68 | 2.14 | 2.32 | 1.96 | 1.90 | 1.70 | 2.44 | 3.57 | 1.76 | 2.09 | 2.19 | 1.52 |
| CaO | 1.00 | 0.35 | 0.42 | 0.64 | 0.57 | 0.64 | 0.65 | 0.59 | 0.45 | 0.51 | 0.55 | 0.42 | 0.38 | 0.58 | 0.31 | 0.36 | 0.27 |
| ZnO | 30.50 | 47.64 | 46.26 | 46.02 | 45.26 | 41.58 | 43.22 | 43.76 | 40.65 | 42.04 | 41.71 | 41.70 | 41.57 | 37.47 | 38.91 | 43.42 | 44.38 |
| CuO | − | − | − | − | − | − | 0.12 | − | 1.10 | 1.40 | 1.13 | 0.90 | 0.88 | 1.54 | 0.72 | 0.55 | 0.50 |
| Total | 93.55 | 89.80 | 90.03 | 88.88 | 92.64 | 89.49 | 88.70 | 90.02 | 92.06 | 92.53 | 90.16 | 93.40 | 88.72 | 88.25 | 90.12 | 88.46 | 87.76 |
| apfu | tetrahedral cations (Σ = 4) | ||||||||||||||||
| Si | 3.52 | 3.46 | 3.49 | 3.63 | 3.57 | 3.64 | 3.56 | 3.53 | 3.46 | 3.44 | 3.44 | 3.36 | 3.28 | 3.62 | 3.51 | 3.25 | 3.21 |
| AlIV | 0.48 | 0.54 | 0.51 | 0.37 | 0.43 | 0.36 | 0.44 | 0.47 | 0.54 | 0.56 | 0.56 | 0.64 | 0.72 | 0.38 | 0.49 | 0.75 | 0.79 |
| AlVI | 1.13 | 1.04 | 1.12 | 1.10 | 1.30 | 1.40 | 1.17 | 1.16 | 1.49 | 1.45 | 1.38 | 1.46 | 1.23 | 1.56 | 1.57 | 1.20 | 1.28 |
| Ti | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.03 | 0.03 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe2+ | 1.19 | 0.05 | 0.07 | 0.02 | 0.03 | 0.11 | 0.13 | 0.16 | 0.15 | 0.10 | 0.08 | 0.11 | 0.08 | 0.13 | 0.17 | 0.17 | 0.08 |
| Mn | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.02 | 0.03 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| Mg | 0.75 | 0.33 | 0.35 | 0.30 | 0.35 | 0.29 | 0.38 | 0.41 | 0.33 | 0.32 | 0.29 | 0.40 | 0.63 | 0.30 | 0.35 | 0.40 | 0.28 |
| Ca | 0.12 | 0.05 | 0.05 | 0.08 | 0.07 | 0.08 | 0.08 | 0.08 | 0.05 | 0.06 | 0.07 | 0.05 | 0.05 | 0.07 | 0.04 | 0.05 | 0.04 |
| Zn | 2.48 | 4.28 | 4.10 | 4.11 | 3.80 | 3.57 | 3.83 | 3.82 | 3.38 | 3.50 | 3.59 | 3.42 | 3.66 | 3.20 | 3.26 | 3.91 | 4.04 |
| Cu | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.00 | 1.10 | 1.40 | 1.13 | 0.90 | 0.88 | 1.54 | 0.72 | 0.55 | 0.50 |
| Sum Oct. | 5.68 | 5.75 | 5.70 | 5.62 | 5.56 | 5.48 | 5.74 | 5.65 | 6.52 | 6.83 | 6.59 | 6.38 | 6.54 | 6.80 | 6.11 | 6.29 | 6.22 |
| R2+ | 4.55 | 4.71 | 4.58 | 4.51 | 4.25 | 4.07 | 4.57 | 4.48 | 5.02 | 5.38 | 5.18 | 4.89 | 5.30 | 5.24 | 4.54 | 5.09 | 4.94 |
| Analyte | KDD143-24 | KDD143-24 | KDD143-24 | KDD143-26 | KDD143-26 | KDD143-26 | KDD143-26 | KDD143-26 | KDD143-22 | KDD143-22 | KDD143-22 | KDD143-22 | KDD143-22 | KDD143-22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mimetite | Mimetite | Mimetite | Pyromorphite | Pyromorphite | Hinsdalite | Hinsdalite | Hinsdalite | Fe-Oxy- hydroxides | Fe-Oxy- hydroxides | Fe-Oxy- hydroxides | Fe-Oxy- hydroxides | Fe-Oxy- hydroxides | Fe-Oxy- hydroxides | |
| Al2O3 | n.a. | n.a. | n.a. | n.a. | n.a. | 16.45 | 19.78 | 16.59 | 0.52 | 1.04 | 1.50 | 1.24 | 0.20 | 0.65 |
| Fe2O3 | n.a. | n.a. | n.a. | n.a. | n.a. | 5.64 | 1.28 | 4.20 | 95.98 | 91.49 | 91.30 | 87.22 | 81.00 | 74.52 |
| ZnO | b.d. | 0.50 | b.d. | 0.35 | 0.76 | 2.18 | 2.75 | 1.73 | 1.78 | 1.15 | 1.96 | 2.89 | 1.99 | 4.98 |
| FeO | b.d. | b.d. | 2.85 | 0.06 | 0.31 | 5.13 | 1.17 | 3.82 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| MnO | 0.22 | b.d. | b.d. | 0.28 | 0.06 | b.d. | b.d. | b.d. | b.d. | 0.20 | b.d. | b.d. | b.d. | 0.01 |
| MgO | b.d. | b.d. | b.d. | 0.11 | b.d. | b.d. | 0.16 | b.d. | b.d. | 0.39 | 0.14 | b.d. | 0.05 | 0.11 |
| CaO | 0.03 | 0.35 | 0.31 | 2.08 | 1.15 | 0.63 | 0.22 | 0.13 | 0.05 | b.d. | 0.05 | b.d. | 0.25 | 0.19 |
| CdO | 0.32 | 0.24 | 0.03 | b.d. | 0.20 | b.d. | b.d. | 0.18 | b.d. | 0.29 | 0.13 | 0.86 | b.d. | 0.02 |
| PbO | 74.57 | 73.23 | 74.47 | 78.46 | 80.47 | 45.98 | 38.22 | 42.21 | 1.02 | 3.13 | 1.65 | 1.36 | 5.61 | 2.89 |
| SrO | b.d. | b.d. | b.d. | 0.92 | 0.81 | 0.35 | 0.38 | 0.28 | b.d. | b.d. | 0.40 | 0.08 | 0.28 | 0.20 |
| BaO | 0.11 | 0.04 | 0.41 | 0.46 | b.d. | 0.74 | 0.18 | b.d. | b.d. | 0.15 | 0.12 | 0.25 | b.d. | b.d. |
| As2O5 | 22.08 | 21.22 | 21.95 | b.d. | 0.26 | 1.40 | 1.50 | 1.97 | 0.57 | 0.60 | 0.01 | 1.93 | 2.00 | b.d. |
| P2O5 | 0.44 | 0.53 | 0.27 | 16.19 | 15.27 | 11.38 | 10.04 | 9.35 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Cl | 2.46 | 2.62 | 3.32 | 2.93 | 2.91 | 0.50 | 0.10 | 0.38 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| SO3 | n.a. | n.a. | n.a. | n.a. | n.a. | 9.42 | 9.78 | 8.86 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Total | 100.23 | 98.73 | 103.63 | 101.83 | 102.18 | 99.78 | 85.54 | 89.70 | 99.92 | 98.43 | 97.26 | 95.83 | 91.38 | 83.56 |
| Analyte | Sample # | KDD125-2 | KDD125-4 | KDD125-5 | KDD125-7 | KDD125-8 | KDD125-9 | KDD125-10 | KDD125-12 | KDD125-14 | KDD143-16 | KDD143-18 | KDD143-20 | KDD143-21 | KDD143-22 | KDD143-23 | KDD143-24 | KDD143-25 | KDD143-26 | KDD143-27 | KDD143-29 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | Det. Lim. | ||||||||||||||||||||
| SiO2 | 0.01 | 81.03 | 76.58 | 76.6 | 79.17 | 77.47 | 80.65 | 81.44 | 82.05 | 82.15 | 81.97 | 74.09 | 79.8 | 75.41 | 76.63 | 67.85 | 72.35 | 75.59 | 76.26 | 83.27 | 78.04 |
| Ti | 0.001 | 0.08 | 0.09 | 0.08 | 0.12 | 0.09 | 0.09 | 0.14 | 0.13 | 0.06 | 0.07 | 0.24 | 0.07 | 0.08 | 0.10 | 0.09 | 0.08 | 0.06 | 0.09 | 0.11 | 0.07 |
| Al | 0.01 | 4.26 | 3.57 | 4.38 | 4.64 | 3.51 | 3.54 | 3.75 | 4.70 | 4.03 | 3.30 | 3.85 | 3.92 | 3.71 | 3.20 | 3.27 | 3.26 | 3.43 | 3.18 | 3.95 | 3.60 |
| Fe | 0.01 | 0.76 | 1.07 | 2.20 | 0.62 | 0.84 | 0.72 | 1.38 | 1.11 | 0.51 | 0.61 | 0.92 | 0.59 | 0.69 | 0.76 | 0.82 | 0.96 | 0.66 | 0.64 | 0.57 | 0.47 |
| Mg | 0.01 | 0.10 | 0.09 | 0.10 | 0.12 | 0.10 | 0.09 | 0.10 | 0.13 | 0.11 | 0.19 | 0.27 | 0.19 | 0.21 | 0.15 | 0.22 | 0.18 | 0.24 | 0.26 | 0.17 | 0.14 |
| Ca | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | b.d. | 0.04 | 0.10 | 0.05 | 0.05 | 0.05 | 0.05 | 0.06 | 0.08 | 0.11 | 0.09 | 0.10 |
| Na | 0.01 | b.d. | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | b.d. | 0.03 | 0.03 | 0.02 | 0.02 | 0.06 | 0.08 | 0.07 | 0.08 | 0.06 |
| K | 0.01 | 2.30 | 2.07 | 2.37 | 2.54 | 2.03 | 2.14 | 2.13 | 2.67 | 2.55 | 1.86 | 2.33 | 2.29 | 2.15 | 1.83 | 1.81 | 1.75 | 2.09 | 1.61 | 2.54 | 1.86 |
| P | 0.01 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 |
| S | 0.05 | 0.44 | 2.42 | 1.71 | 0.07 | 1.45 | 1.78 | 1.80 | 0.06 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.19 |
| Zn | 0.0005 | 0.03 | 4.30 | 3.08 | 0.19 | 4.28 | 3.09 | 1.40 | 0.07 | 0.53 | 3.37 | 4.91 | 3.49 | 6.04 | 5.12 | 9.32 | 7.06 | 4.66 | 5.24 | 0.96 | 1.47 |
| Pb | 0.0005 | 2.61 | 1.94 | 0.96 | 3.09 | 1.38 | 1.05 | 0.48 | 0.20 | 1.80 | 0.33 | 1.02 | 0.57 | 0.26 | 2.04 | 2.02 | 1.30 | 2.56 | 1.82 | 0.54 | 4.82 |
| mg/kg | |||||||||||||||||||||
| Li | 0.5 | 6.8 | 5.8 | 5.7 | 9.9 | 5.8 | 6.7 | 5.9 | 8 | 6.8 | 15.4 | 10.9 | 7.5 | 7.9 | 9.5 | 10.2 | 11 | 7.2 | 6.9 | 9.7 | 8.9 |
| V | 10 | 21 | 19 | 24 | 31 | 23 | 15 | 20 | 29 | 16 | 16 | 51 | 18 | 58 | 262 | 23 | 27 | 19 | 20 | 28 | 20 |
| Cr | 1 | 60 | 14 | 91 | 13 | 65 | 10 | 87 | 12 | 51 | 12 | 84 | 10 | 72 | 18 | 66 | 9 | 54 | 14 | 63 | 7 |
| Mn | 5 | 39 | 58 | 45 | 42 | 51 | 44 | 40 | 34 | 33 | 45 | 45 | 42 | 42 | 46 | 47 | 52 | 39 | 35 | 38 | 40 |
| Co | 1 | b.d. | 2 | 2 | b.d. | 4 | 5 | 7 | b.d. | b.d. | 2 | 9 | 8 | 10 | 8 | 25 | 18 | 32 | 31 | 5 | 3 |
| Ni | 0.5 | 3 | 4.9 | 6.8 | 1.1 | 7.3 | 8.6 | 15.7 | b.d. | 2.8 | 3.8 | 21.1 | 10.9 | 13.3 | 8.9 | 20 | 11.3 | 12.6 | 12.3 | 7.5 | 2.1 |
| Cu | 0.5 | 49.9 | 20.7 | 15.2 | 33.8 | 103.8 | 9.1 | 6.7 | 4.2 | 62.4 | 66.1 | 204.5 | 176.6 | 424.1 | 794.6 | 1831.5 | 1394.2 | 973.7 | 1131.5 | 477.1 | 1365.7 |
| As | 5 | 294 | 256 | 182 | 406 | 419 | 29 | 41 | 64 | 134 | 275 | 76 | 72 | 81 | 117 | 3647 | 2007 | 172 | 119 | 39 | 42 |
| Rb | 0.5 | 81.5 | 75.5 | 85.9 | 94.9 | 74 | 75.1 | 80.1 | 105.3 | 97.3 | 72.5 | 92.1 | 85.7 | 81.3 | 70.3 | 67.7 | 69.3 | 73.3 | 54.5 | 91.7 | 76.8 |
| Sr | 5 | 46 | 10 | 7 | 8 | 7 | 5 | 6 | 7 | 20 | 5 | 8 | 10 | 7 | 13 | 7 | 6 | 31 | 28 | 13 | 28 |
| Y | 0.5 | 2.5 | 2.9 | 2.8 | 4.3 | 25 | 8.7 | 10.3 | 11.6 | 6.5 | 5.9 | 9.7 | 6.7 | 6.3 | 6.6 | 6.2 | 6.3 | 6.6 | 6.8 | 7.2 | 6.9 |
| Zr | 0.5 | 52.7 | 48.9 | 48.3 | 62.1 | 48.5 | 45.6 | 72.5 | 66.6. | 43.9 | 41.6 | 113.8 | 46.3 | 44.7 | 48.4 | 44.6 | 40 | 48.7 | 50.1 | 49.7 | 44.2 |
| Nb | 0.5 | 3.5 | 3.1 | 3.1 | 4.5 | 3.1 | 3.8 | 5.2 | 4.5 | 3 | 3.3 | 8.9 | 3 | 3.4 | 3.9 | 3.1 | 3 | 2.3 | 3.6 | 4.2 | 3.1 |
| Mo | 0.5 | 1.4 | 1.4 | 1.6 | 1.5 | 1.4 | 1.3 | 1.4 | 1.8 | 1.1 | 1.4 | 1 | 2.2 | 2.1 | 3.7 | 11.9 | 2.8 | 2 | 2.3 | 1.5 | 4.4 |
| Ag | 0.5 | 50.3 | 38.8 | 20.7 | 99.8 | 39.3 | 12.1 | 7.8 | 27.2 | 27.7 | 17.6 | 49.2 | 15.9 | 17.8 | 47.3 | 39.8 | 39 | 103.1 | 112 | 27.7 | 102.8 |
| Cd | 0.5 | 0.5 | 200.7 | 100.9 | 6.4 | 114.2 | 271.4 | 69.8 | 6.5 | 35 | 50.7 | 144.7 | 86.6 | 186.9 | 131.4 | 410.2 | 417.8 | 7.8 | 10.2 | 1.4 | 2177.5 |
| Sb | 0.5 | 1.2 | 1.2 | 1.6 | 1.2 | 0.9 | 0.8 | b.d. | 1.2 | 0.5 | 3.6 | 2.6 | 1.5 | 4.2 | 9.1 | 28.3 | 24.6 | 5.7 | 5.7 | 2.6 | 12.2 |
| Ba | 5 | 140 | 92 | 112 | 153 | 109 | 97 | 100 | 146 | 307 | 166 | 218 | 205 | 204 | 188 | 183 | 165 | 347 | 1232 | 253 | 957 |
| La | 0.5 | 21.9 | 19.8 | 20.6 | 29.7 | 24.8 | 24.8 | 27.4 | 28.1 | 24.2 | 12.9 | 27.7 | 23.9 | 22 | 15.7 | 14.7 | 15.3 | 17.7 | 13.8 | 19.5 | 18.1 |
| Ce | 5 | 43 | 39 | 41 | 61 | 49 | 48 | 56 | 53 | 47 | 27 | 54 | 46 | 42 | 29 | 29 | 29 | 36 | 28 | 37 | 35 |
| Hf | 0.5 | 1.6 | 1.5 | 1.4 | 1.4 | 1.5 | 1.4 | 2.2 | 1.8 | 1.3 | 1.3 | 3.2 | 1.4 | 1.4 | 1.3 | 1.1 | 1 | 1.2 | 1.5 | 1.1 | 1 |
| Th | 0.5 | 5.1 | 5.9 | 6.2 | 7.4 | 5.5 | 5.6 | 10.2 | 7.5 | 4.2 | 4.1 | 16.9 | 4.6 | 4.7 | 8.2 | 6.2 | 5.1 | 4.1 | 7 | 6.6 | 4.8 |
| U | 0.5 | 2 | 3.2 | 2.7 | 5.5 | 4.7 | 1.3 | 1.7 | 1.8 | 1.9 | 0.8 | 1.9 | 1 | 1.1 | 1.8 | 2.6 | 2.1 | 1.6 | 1.3 | 1.2 | 1.3 |
References
- Botswana Geoscience Institute. Botswana Mineral Projects and Prospects. Ministry of Mineral Resources, Green Technology and Energy Security. 2020, p. 28. Available online: http://www.bgi.org.bw/sites/default/files/Brochure%20on%20Botswana%20Mineral%20Projects%20and%20Prospects%202020%20Version.pdf (accessed on 1 July 2020).
- Cairncross, B. The Otavi Mountain Land Cu-Pb-Zn-V Deposits. Mineral. Rec. 1997, 28, 109–130. [Google Scholar]
- Melcher, F.; Oberthür, T.; Rammlmair, D. Geochemical and mineralogical distribution of germanium in the Khusib Springs Cu-Zn-Pb-Ag sulfide deposit, Otavi Mountain Land, Namibia. Ore Geol. Rev. 2006, 28, 32–56. [Google Scholar] [CrossRef]
- Pirajno, F.; Joubert, B.D. An overview of carbonate-hosted mineral deposits in the Otavi mountain land, Namibia; implications for ore genesis. J. Afr. Earth Sci. 1993, 16, 265–272. [Google Scholar] [CrossRef]
- Chetty, D.; Frimmel, H.E. The role of evaporites in the genesis of base metal sulphide mineralisation in the northern platform of the Pan-African Damara Belt, Namibia; geochemical and fluid inclusion evidence from carbonate wall rock alteration. Mineral. Depos. 2000, 35, 364–376. [Google Scholar] [CrossRef]
- Hughes, M.J. The Tsumeb Ore Body, Namibia, and Related Dolostone-Hosted Base Metal Ore Deposits of Central Africa. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 1987; p. 473. [Google Scholar]
- Hitzman, M.W.; Reynolds, N.A.; Sangster, D.F.; Allen, C.R.; Carman, C.E. Classification, genesis, and exploration guides for nonsulfide Zinc deposits. Econ. Geol. 2003, 98, 685–714. [Google Scholar] [CrossRef]
- Boni, M.; Mondillo, N. The “Calamines” and the “Others”: The great family of supergene nonsulfide zinc ores. Ore Geol. Rev. 2015, 67, 208–233. [Google Scholar] [CrossRef]
- Melcher, F. The Otavi Mountain Land in Namibia: Tsumeb, Germanium and Snowball Earth. Mitt. Österr. Miner. Ges. 2003, 148, 413–435. [Google Scholar]
- Mondillo, N.; Accardo, M.; Boni, M.; Boyce, A.; Herrington, R.; Rumsey, M.; Wilkinson, C. New insights into the genesis of willemite (Zn2SiO4) from zinc nonsulfide deposits, through trace elements and oxygen isotope geochemistry. Ore Geol. Rev. 2020, 118, 103307. [Google Scholar] [CrossRef]
- Mount Burgess Mining N.L. Available online: http://www.mountburgess.com (accessed on 1 July 2020).
- Carney, J.N.; Aldiss, D.T.; Lock, N.P. The geology of Botswana. Botsw. Geol. S. Bull. 1994, 37, 113. [Google Scholar]
- Key, R.; Ayers, N. The 1998 Edition of the Geological Map of Botswana. J. Afr. Earth Sci. 2000, 30, 427–451. [Google Scholar] [CrossRef]
- Lehmann, J.; Master, S.; Milani, L.; Kinnaird, J.A.; Naydenov, K.V.; Kumar, S.M. Regional aeromagnetic and stratigraphic correlations of the Kalahari Copperbelt in Namibia and Botswana. Ore Geol. Rev. 2015, 71, 169–190. [Google Scholar] [CrossRef]
- Mapeo, R.B.M.; Wendorff, M.; Ramokate, L.V.; Armstrong, R.A.; Mphinyane, T.; Koobokile, M. Zircon geochronology of basement granitoid gneisses and sedimentary rocks of the Tsodilo Hills Group in the Pan-African Damara Belt, western Botswana: Age constraints, provenance, and tectonic significance. J. Afr. Earth Sci. 2019, 159, 103576. [Google Scholar] [CrossRef]
- Meixner, H.M.; Peart, R.J. The Kalahari Drilling Project. Botsw. Geol. S. Bull. 1984, 27, 224. [Google Scholar]
- Singletary, S.J.; Hanson, R.E.; Martin, M.W.; Crowley, J.L.; Bowring, S.A.; Key, R.M.; Ramokate, L.V.; Direng, B.B.; Krol, M.A. Geochronology of basement rocks in the Kalahari Desert, Botswana, and implications for regional Proterozoic tectonics. Precambrian Res. 2003, 121, 47–71. [Google Scholar] [CrossRef]
- Wendorff, M. Outline of lithostratigraphy sedimentation and tectonics of the Tsodilo Hills Group, Neoproterozoic Lower Paleozoic siliciclastic succession in NW Botswana. Ann. Soc. Geol. Pol. 2005, 75, 17–25. [Google Scholar]
- Killick, A.M. A preliminary account of the geology of the Kamtsas Formation of the Damara Sequence, eastern Gobabis District, South West Africa/Namibia. Trans. Geol. Soc. S. Afr. 1983, 86, 11–18. [Google Scholar]
- Hoffman, K.H. New aspects of lithostratigraphic subdivision and correlation of Late Proterozoic to Early Cambrian rocks of the southern Damara Belt and their correlation with the central and northern Damara Belt and the Gariep belt. Namib. Geol. Surv. Commun. 1989, 5, 59–67. [Google Scholar]
- Schwartz, M.O.; Kwok, Y.Y.; Davis, D.W.; Akangyang, P. Geology, geochronology and regional correlation of the Ghanzi Ridge, Botswana. S. Afr. J. Geol. 1996, 99, 245–250. [Google Scholar]
- Hanson, R.E. Proterozoic geochronology and tectonic evolution of southern Africa. Geol. Soc. Lond. Spec. Publ. 2003, 206, 427–463. [Google Scholar] [CrossRef]
- Reeves, C.V. Reconnaissance Aeromagnetic Survey of Botswana, 1975–1977; Final interpretation report; Terra Surveys Ltd., Botswana Geological Survey: Lobatse, Botswana, 1978. [Google Scholar]
- Chatupa, J.C.; Direng, B.B. Distribution of trace and major elements in the-180 + 75 µm and −75 µm fractions of the Sandveld regolith in northwest Ngamiland, Botswana. J. Afr. Earth Sci. 2000, 30, 515–534. [Google Scholar] [CrossRef]
- Loxton, R.F. A Photogeological Study of the Aha Hills, Northwest Botswana; Loxton, Hunting and Associates, Report for Billiton Botswana (Pty) Ltd.: Gaborone, Botswana, 1981; p. 27. [Google Scholar]
- Stalker, A.D. Aha Hills Prospecting Licence 39180; Final Report; Billiton Botswana (Pty) Limited: Gaborone, Botswana, 1983; p. 13. [Google Scholar]
- Mapeo, R.B. Geological and Structural Analysis of the Kihabe Base Metal Prospect in NW Botswana; Internal Report for Mount Burgess (Botswana) (Pty) Ltd.: Gaborone, Botswana, 2007; p. 41. [Google Scholar]
- Rule, A.C.; Radke, F. Baileychlore, the Zn end member of the trioctahedral chlorite series. Am. Mineral. 1988, 73, 135–139. [Google Scholar]
- Gray, D.R.; Foster, D.A.; Goscombe, B.; Passchier, C.W.; Trouw, R.A.J. 40Ar/39Ar thermochronology of the Pan-African Damara Orogen, Namibia, with implications for tectonothermal and geodynamic evolution. Prec. Res. 2006, 150, 49–72. [Google Scholar] [CrossRef]
- Schneider, J.; Boni, M.; Laukamp, C.; Bechstädt, T.; Petzel, V. Willemite (Zn2SiO4) as a possible Rb–Sr geochronometer for dating nonsulfide Zn–Pb mineralization: Examples from the Otavi Mountainland (Namibia). Ore Geol. Rev. 2008, 33, 152–167. [Google Scholar] [CrossRef]
- Boni, M.; Terracciano, R.; Evans, N.; Laukamp, C.; Schneider, J.; Bechstädt, T. Genesis of vanadium ore in Otavi Mountainland, Namibia. Econ. Geol. 2007, 102, 441–469. [Google Scholar] [CrossRef]
- Brugger, J.; McPhail, D.C.; Wallace, M.; Waters, J. Formation of Willemite in Hydrothermal Environments. Econ. Geol. 2003, 98, 819–835. [Google Scholar] [CrossRef]
- Kamona, A.F.; Friedrich, G.H. Geology, mineralogy and stable isotope geochemistry of the Kabwe carbonate-hosted Pb–Zn deposit, Central Zambia. Ore Geol. Rev. 2007, 30, 217–243. [Google Scholar] [CrossRef]
- Mondillo, N.; Herrington, R.; Boyce, A.J.; Wilkinson, C.; Santoro, L.; Rumsey, M. Critical elements in non-sulfide Zn deposits: A reanalysis of the Kabwe Zn-Pb ores (central Zambia). Mineral. Mag. 2018, 82, S89–S114. [Google Scholar] [CrossRef] [Green Version]
- White, A.J.R.; Pearce, M.A.; Meadows, H.R. Distinguishing regional- and local-scale metasomatic systems at the Prairie Downs Zn–Pb deposit. Lithos 2016, 262, 247–265. [Google Scholar] [CrossRef]
- Galley, A.G.; Hannington, M.D.; Jonasson, I.R. Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods. In Geological Association of Canada, Mineral Deposits Division, Special Publication No. 5; Goodfellow, W.D., Ed.; Geological Association of Canada, Mineral Deposits Division: St. John’s, NL, Canada, 2007; pp. 141–161. [Google Scholar]
- Spry, P.G.; Peter, J.M.; Slack, J.F. Meta-exhalites as exploration guides to ore. In Metamorphosed and Metamorphogenic Ore Deposits; Reviews in Economic Geology; Spry, P.G., Marshall, B., Vokes, F.M., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2000; Volume 11, pp. 163–201. [Google Scholar]
- Beaufort, D.; Rigault, C.; Billon, S.; Billault, V.; Inoue, A.; Inoue, S.; Patrier, P. Chlorite and chloritization processes through mixed-layer mineral series in lowtemperature geological systems—A review. Clay Miner. 2015, 50, 497–523. [Google Scholar] [CrossRef]
- Abad, I.; Jiménez-Millán, J.; Sánchez-Roa, C.; Nieto, F.; Velilla, N. Neocrystallization of clay minerals in the Alhama de Murcia Fault (southeast Spain): Implications for fault mechanics. Clay Miner. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Do Campo, M.; Bauluz, B.; Nieto, F.; Papa, C.; Hongn, F. SEM and TEM evidence of mixed-layer illite-smectite formed by dissolution crystallization processes in continental Paleogene sequences in northwestern Argentina. Clay Miner. 2016, 51, 723–740. [Google Scholar] [CrossRef] [Green Version]
- Bourdelle, F.; Cathelineau, M. Low-temperature chlorite geothermometry: A graphical representation based on a T–R2+–Si diagram. Eur. J. Mineral. 2015, 27, 617–626. [Google Scholar] [CrossRef]
- Pacey, A.; Wilkinson, J.J.; Cooke, D.R. Chlorite and epidote mineral chemistry in porphyry ore systems: A case study of the Northparkes District, New South Wales, Australia. Econ. Geol. 2020, 115, 701–727. [Google Scholar] [CrossRef]
- Balassone, G.; Nieto, F.; Arfè, G.; Boni, M.; Mondillo, N. Zn-clay minerals in the Skorpion Zn nonsulfide deposit (Namibia): Identification and genetic clues revealed by HRTEM and AEM study. Appl. Clay Sci. 2017, 150, 309–322. [Google Scholar] [CrossRef]
- Mondillo, N.; Nieto, F.; Balassone, G. Micro and nano-characterization of Zn-clays in nonsulfide supergene ores of southern Peru. Am. Mineral. 2015, 100, 2484–2496. [Google Scholar] [CrossRef] [Green Version]
- Choulet, F.; Charles, N.; Barbanson, L.; Branquet, Y.; Sizaret, S.; Ennaciri, A.; Badra, L.; Chen, Y. Non-sulfide zinc deposits of the moroccan high atlas: Multi-scale characterization and origin. Ore Geol. Rev. 2014, 56, 115–140. [Google Scholar] [CrossRef] [Green Version]
- Choulet, F.; Richard, J.; Boiron, M.-C.; Dekoninck, A.; Yans, J. Distribution of trace elements in willemite from the Belgium non-sulphide deposits. Eur. J. Mineral. 2019, 31, 983–997. [Google Scholar] [CrossRef]
- Coppola, V.; Boni, M.; Gilg, H.A.; Balassone, G.; Dejonghe, L. The “calamine” nonsulfide Zn–Pb deposits of Belgium: Petrographical, mineralogical and geochemical characterization. Ore Geol. Rev. 2008, 33, 187–210. [Google Scholar] [CrossRef]
- Monteiro, L.V.S.; Bettencourt, J.S.; Juliani, C.; de Oliveira, T.F. Geology, petrography, and mineral chemistry of the Vazante non-sulfide and Ambrósia and Fagundes sulfide-rich carbonate-hosted Zn-(Pb) deposits, Minas Gerais. Brazil. Ore Geol. Rev. 2006, 28, 201–234. [Google Scholar] [CrossRef]
- Gilg, H.A.; Boni, M.; Hochleitner, R.; Struck, U. Stable isotope geochemistry of carbonate minerals in supergene oxidation zones of Zn-Pb deposits. Ore Geol. Rev. 2008, 33, 117–133. [Google Scholar] [CrossRef]
- Markl, G.; Marks, M.A.W.; Holzäpfel, J.; Wenzel, T. Major, minor, and trace element composition of pyromorphite-group minerals as recorder of supergene weathering processes from the Schwarzwald mining district, SW Germany. Am. Mineral. 2014, 99, 1133–1146. [Google Scholar] [CrossRef]
- Partridge, T.C.; Maud, R.R. Geomorphic evolution of southern Africa since the Mesozoic. S. Afr. J. Geol. 1987, 90, 179–208. [Google Scholar]
- Sillitoe, R.H. Supergene silver enrichment reassessed. In Supergene environments, processes, and products. Soc. Econ. Geol. Spec. Publ. 2009, 14, 15–32. [Google Scholar]
- Boyle, D.R. Iodargyrite as an indicator of arid climatic conditions and its association with gold-bearing glacial tills of the Chibougamau-Chapais area, Quebec. Can. Mineral. 1997, 35, 23–34. [Google Scholar]
- Gammons, C.H.; Yu, Y. The stability of aqueous silver bromide and iodine complexes at 25–300 8C. Experiments, theory and geologic applications. Chem. Geol. 1997, 137, 155–173. [Google Scholar] [CrossRef]
- Golebiowska, B.; Pieczka, A.; Rzepa, G.; Matyszkiewicz, J.; Krajewski, M. Iodargyrite from Zalas (Cracow area, Poland) as an indicator of Oligocene–Miocene aridity in Central Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 296, 130–137. [Google Scholar] [CrossRef]
- Van der Wateren, F.M.; Dunai, T.G. Late Neogene passive margin denudation history—Cosmogenic isotope measurements from the central Namib desert. Glob. Planet. Chang. 2001, 30, 271–307. [Google Scholar] [CrossRef]



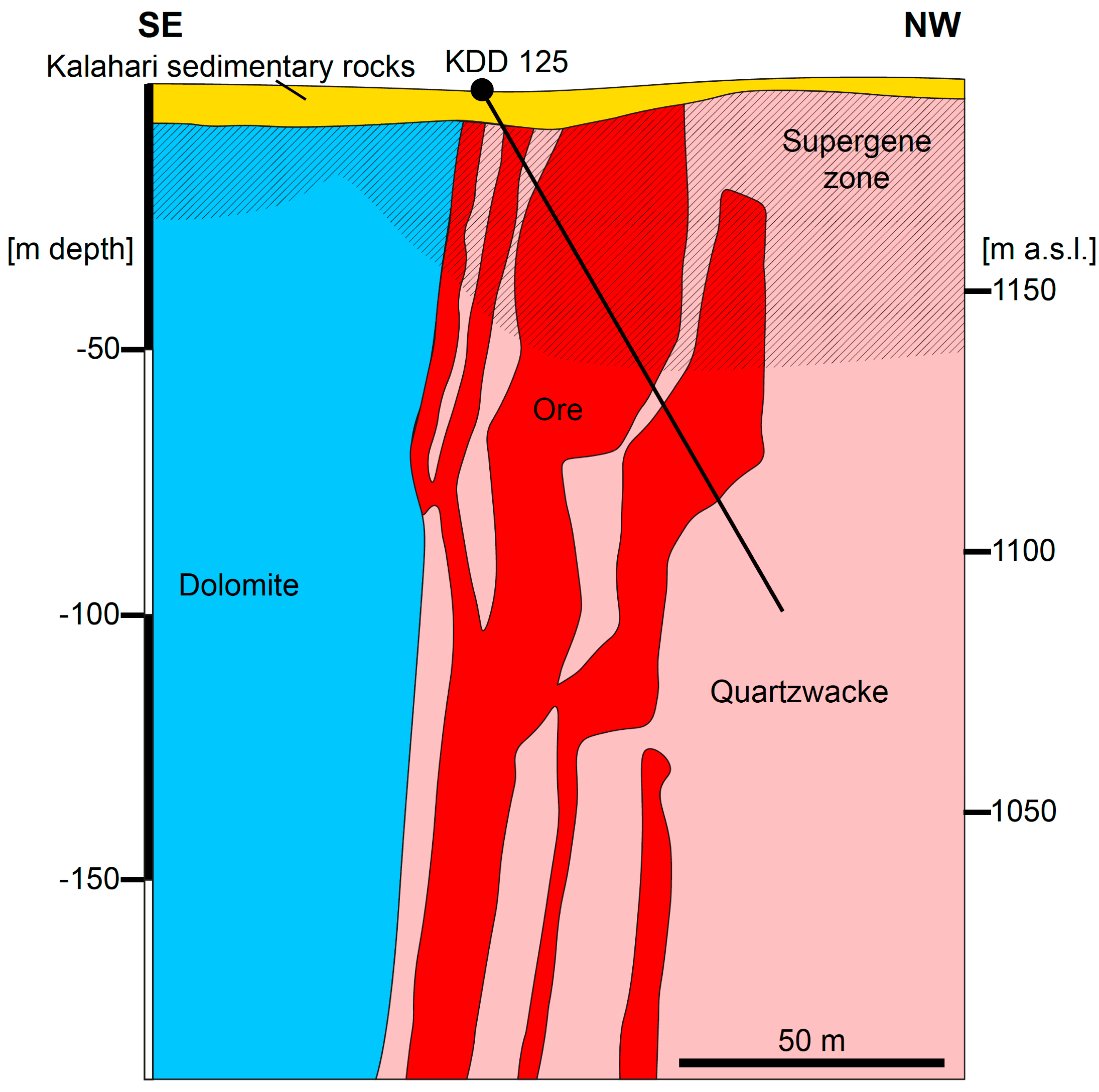

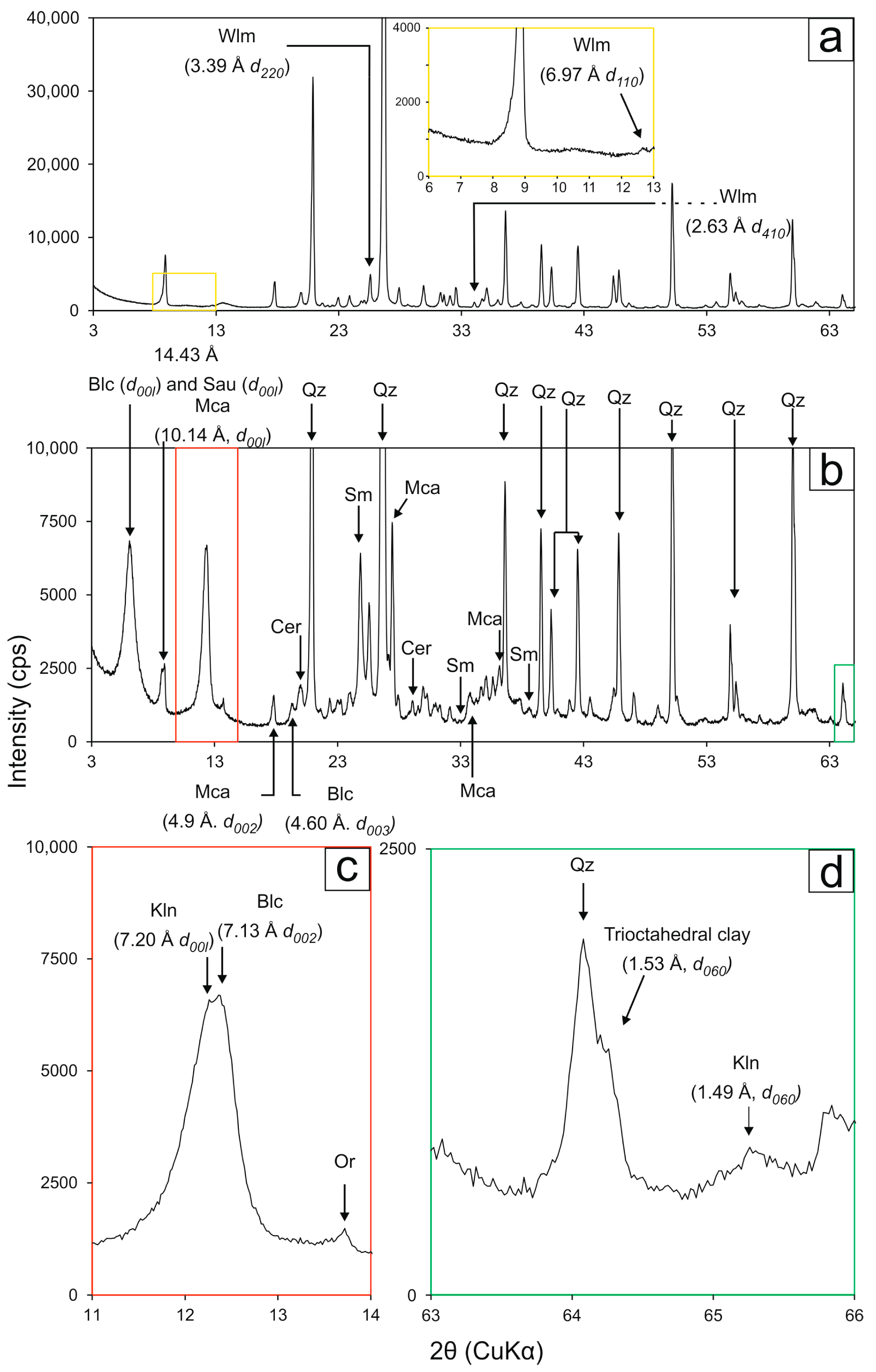
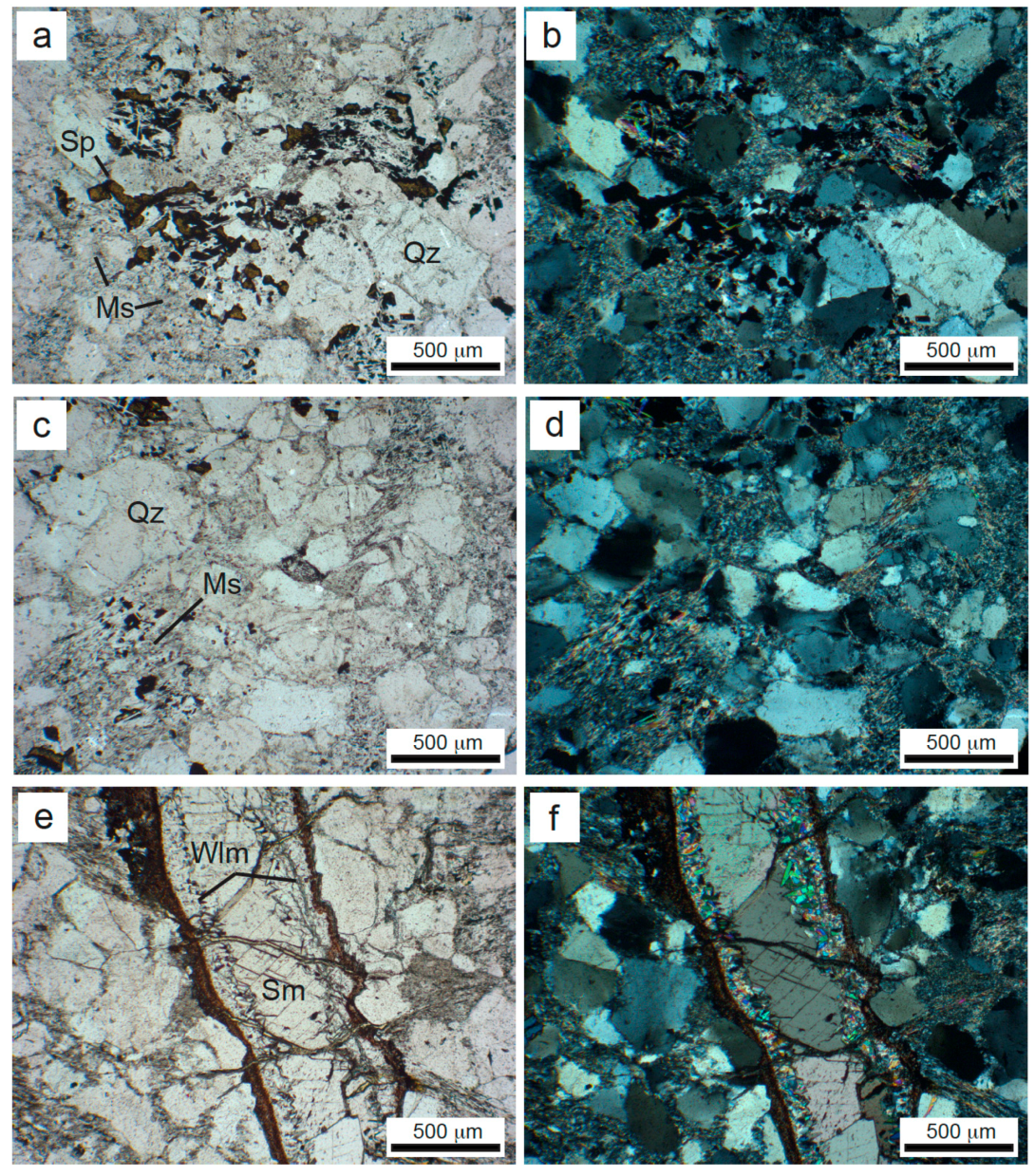



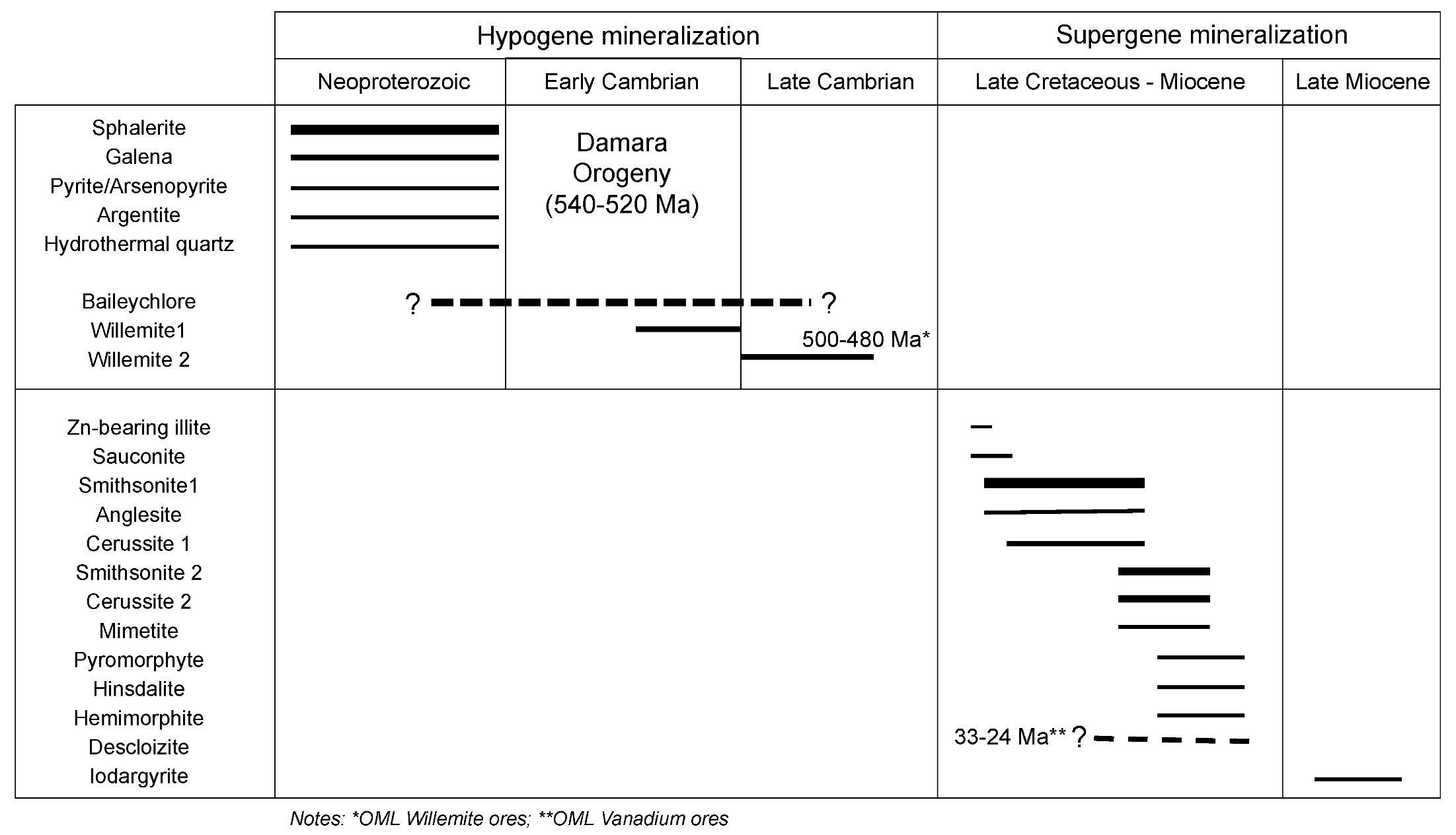

| Drillcore | Sampled Interval | Sample Label | Mineral Assemblage |
|---|---|---|---|
| KDD125 | 55.00–55.34 | KDD 125-1 | Qz, Ms, Cer |
| 55.34–55.76 | KDD 125-2 | Qz, Ms, Ang | |
| 55.76–56.05 | KDD 125-3 | Qz, Ms, Ang, Sp, Hem | |
| 56.05–56.50 | KDD 125-4 | Qz, Ms, Sp, Gn, Py | |
| 56.60–57.00 | KDD 125-5 | Qz, Ms, Sp, Gn, Py, Hem | |
| 57.00–57.30 | KDD 125-6 | Qz, Ms, Sp, Gn, Sm, Py, Hem | |
| 57.30–58.07 | KDD 125-7 | Qz, Ms, Or, Cer, Gn, Sm | |
| 57.08–58.57 | KDD 125-8 | Qz, Ms, Sm, Sp, Gn, Hem | |
| 58.57–58.89 | KDD 125-9 | Qz, Ms, Sp, Gn, Hem | |
| 58.89–59.10 | KDD 125-10 | Qz, Ms, Sp, Gn, Py | |
| 59.10–59.96 | KDD 125-11 | Qz, Ms, Sp, Gn, Py, Hem | |
| 59.96–60.10 | KDD 125-12 | Qz, Ms, Hem | |
| 60.10–60.32 | KDD 125-13 | Qz, Ms, Sp, Gn, Sm, Hem | |
| 60.32–60.63 | KDD 125-14 | Qz, Ms, Or, Kln, Gn, Cer | |
| 60.63–60.90 | KDD 125-15 | Qz, Ms, Or, Gn, Cer | |
| KDD143 | 50.00–50.04 | KDD 143-16 | Qz, Ms, Sm, Wlm |
| 50.04–50.40 | KDD 143-17 | Qz, Ms, Sm, Wlm, Hem | |
| 50.40–50.72 | KDD 143-18 | Qz, Ms, Or, Sm, Blc, Cer | |
| 50.72–50.92 | KDD 143-19 | Qz, Ms, Or, Sm, Blc | |
| 50.92–51.24 | KDD 143-20 | Qz, Ms, Or, Sm, Blc, Hem | |
| 51.24–51.60 | KDD 143-21 | Qz, Sm, Ms, Blc, Ill, Or | |
| 51.60–51.86 | KDD 143-22 | Qz, Ms, Or, Sm, Blc, Ill | |
| 51.86–52.20 | KDD 143-23 | Qz, Sm, Ms, Or, Blc, Wlm, Mim, Cer | |
| 52.20–52.55 | KDD 143-24 | Qz, Sm, Ms, Blc, Mim, Cer, Or | |
| 52.55–52.72 | KDD 143-25 | Qz, Blc, Or, Ms, Cer, Sau, Ill | |
| 52.72–53.00 | KDD 143-26 | Qz, Blc, Or, Ms, Cer, Sau, Ill, Wil, Sm | |
| 53.00–53.93 | KDD 143-27 | Qz, Ms, Or, Blc, Cer | |
| 53.93–54.26 | KDD 143-28 | Qz, Ms, Blc, Sm | |
| 54.26–54.65 | KDD 143-29 | Qz, Ms, Cer, Gn, Sm | |
| 54.65–54.93 | KDD 143-30 | Qz, Ms, Cer, Gn, Sm, Wlm |
| Mineral | Formula |
|---|---|
| Anglesite | PbSO4 |
| Argentite | Ag2S |
| Baileychlore | (Zn,Fe2+,Al,Mg)6(Si,Al)4O10(OH)8 |
| Cerussite | PbCO3 |
| Galena | PbS |
| Hemimorphite | Zn4(Si2O7)(OH)2·H2O |
| Hinsdalite | PbAl3(SO4)(PO4)(OH)6 |
| Iodargyrite | AgI |
| Mimetite | Pb5(AsO4)3Cl |
| Pyromorphite | Pb5(PO4)3Cl |
| Sauconite | Na0.3Zn3(Si,Al)4O10(OH)2·4H2O |
| Smithsonite | ZnCO3 |
| Sphalerite | ZnS |
| Willemite | Zn2SiO4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondillo, N.; Boni, M.; Balassone, G.; Forrester, N.; Putzolu, F.; Santoro, L. Mineralogy and Genesis of the Kihabe Zn-Pb-V Prospect, Aha Hills, Northwest Botswana. Minerals 2020, 10, 685. https://doi.org/10.3390/min10080685
Mondillo N, Boni M, Balassone G, Forrester N, Putzolu F, Santoro L. Mineralogy and Genesis of the Kihabe Zn-Pb-V Prospect, Aha Hills, Northwest Botswana. Minerals. 2020; 10(8):685. https://doi.org/10.3390/min10080685
Chicago/Turabian StyleMondillo, Nicola, Maria Boni, Giuseppina Balassone, Nigel Forrester, Francesco Putzolu, and Licia Santoro. 2020. "Mineralogy and Genesis of the Kihabe Zn-Pb-V Prospect, Aha Hills, Northwest Botswana" Minerals 10, no. 8: 685. https://doi.org/10.3390/min10080685
APA StyleMondillo, N., Boni, M., Balassone, G., Forrester, N., Putzolu, F., & Santoro, L. (2020). Mineralogy and Genesis of the Kihabe Zn-Pb-V Prospect, Aha Hills, Northwest Botswana. Minerals, 10(8), 685. https://doi.org/10.3390/min10080685





