Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques
Abstract
1. Introduction
2. Materials and Methods
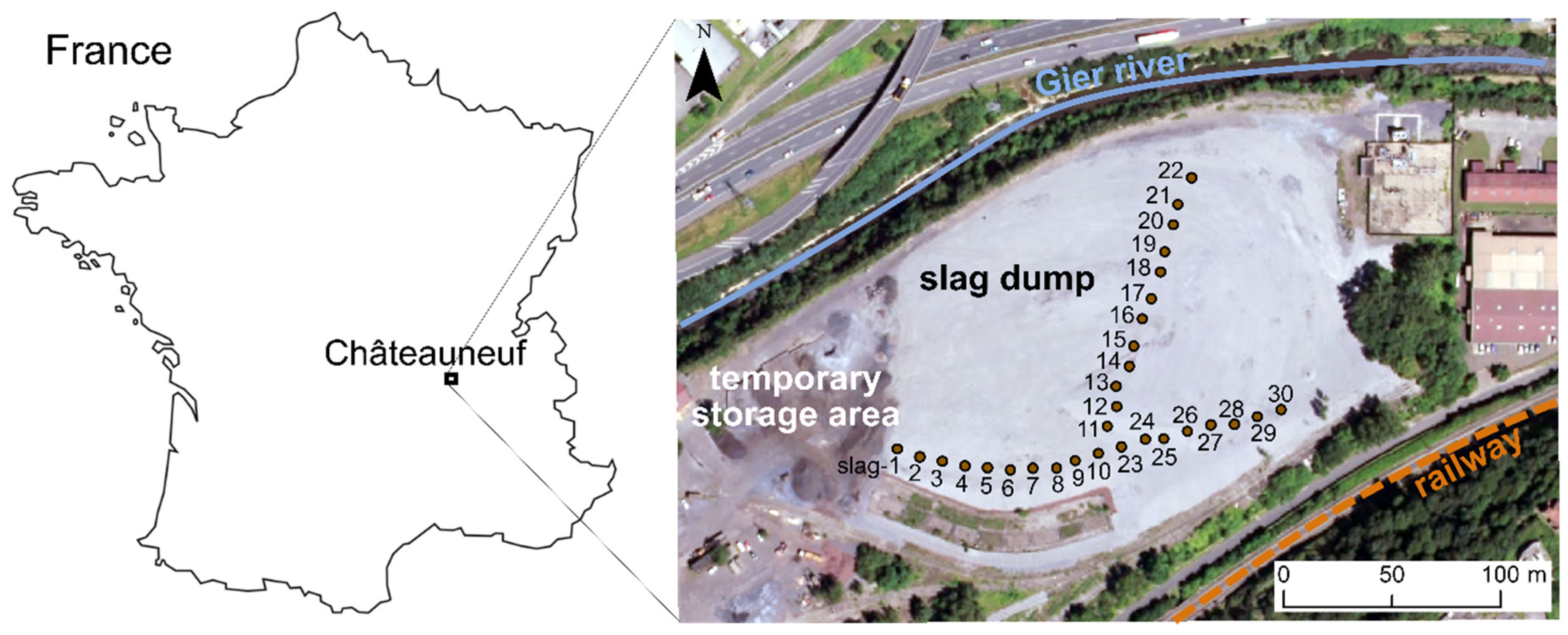
2.1. Sampling
- -
- Two sub-samples of 8 cm3 (approximately 15 g) were taken with a beaker from each of the 30 initial samples for magnetic and chemical analyses. This method allows to evaluate the variability within the same sample and provides a sufficient amount of data for a statistical analysis (see Section 2.2.4). These samples are hereafter referred to as “slag-1A”, “slag-1B”, “slag-2A”, “slag-2B” and so forth.
- -
- The sample slag-13, representative of the average chemical composition of the 30 EAF slag samples (Table 2), was selected to be sieved so as to explore the main properties of the slag as a function of grain size. Eight grain size fractions were examined: <0.25 mm, 0.25–0.5 mm, 0.5–1.0 mm, 1.0–1.6 mm, 1.6–2.5 mm, 2.5–5 mm, 5–10 mm and >10 mm.
- -
- A last sample called Mix 1 was prepared by mixing equal volumes from each of the 30 initial samples (16 cm3 taken with a beaker). It was then roughly crushed with a jaw crusher, to a size smaller than 4 mm, with the aim to get a representative set of grains the size of which is convenient for micro-analyses (EMPA and Raman spectroscopy) and thermo-magnetic measurements (0.5–1.0 mm).
2.2. Laboratory Methods and Procedures
2.2.1. Bulk Chemical Analyses
2.2.2. Mineralogical Characterisation
2.2.3. Magnetic Measurements
2.2.4. Principal Component Analysis
3. Results
3.1. Bulk Chemical Composition
3.2. Mineralogical Composition
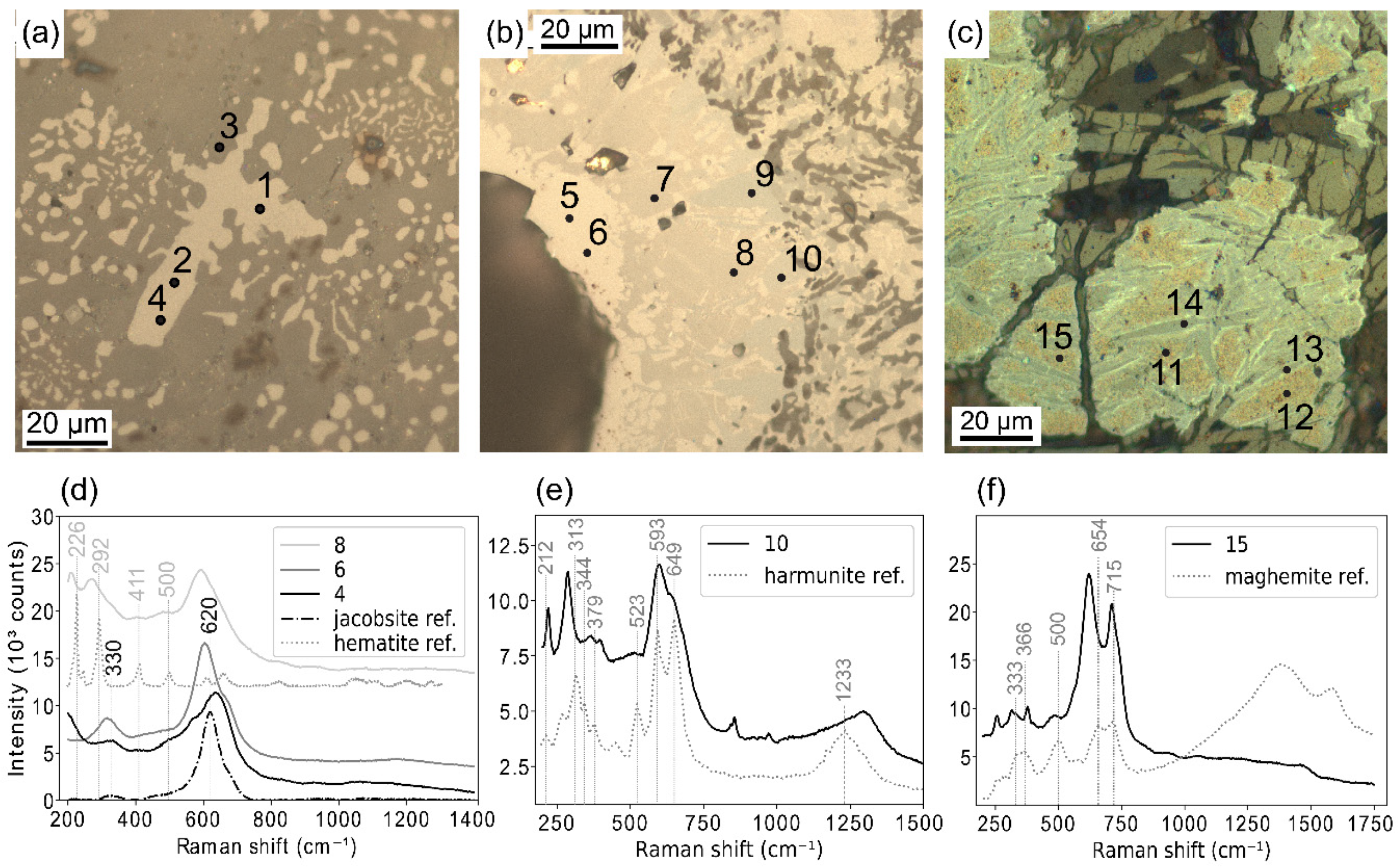
- Particles with patchy zonations. On Figure 4b, three different phases are actually distinguishable within the same particle: Fe rich jacobsite (points 5 and 6), jacobsite (points 7 and 8) and harmunite, CaFe2O4 (points 9 and 10) based on the work of Galuskina et al. [34]. Some hematite peaks visible on Raman spectra for point 8 suggest that the corresponding phase is partially oxidized (Figure 4d). However, this oxidation may be caused by Raman laser heating [35,36].
- Partially oxidized jacobsite (identified as Mg-Mn rich maghemite) with harmunite exsolutions. On Figure 4c, mottled areas correspond to the Mg-Mn rich maghemite (points 11, 12 and 15) according to Raman spectrum (Figure 4f) and EMPA point analyses (Table 5). Grey lamellae are identified as exsolved harmunite thanks to EMPA (Table 5), however it could not be confirmed with Raman analyses since the laser beam was to large. Thin light borders around the lamellae may be pure magnetite or wustite.
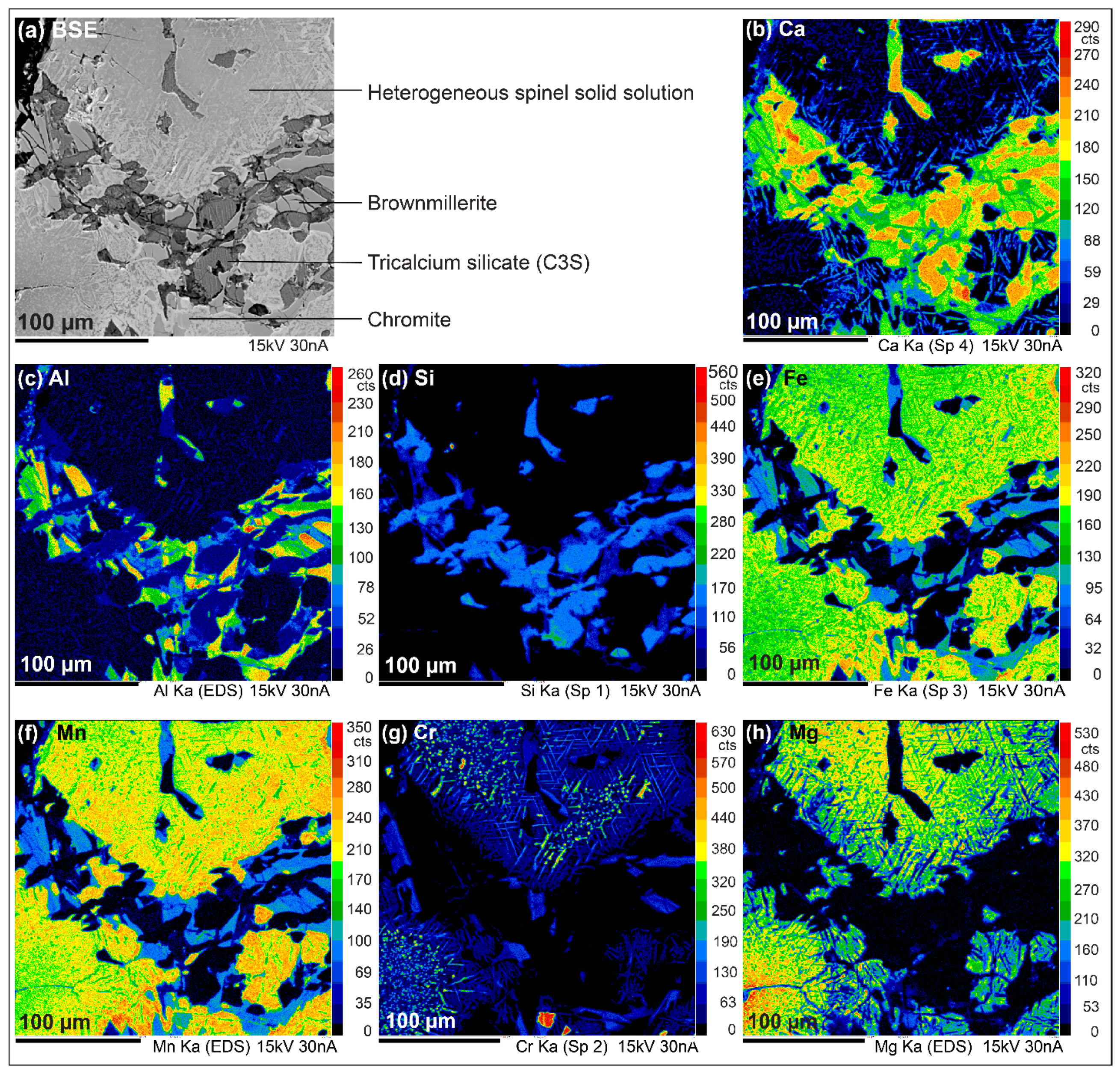
- Grains with Mg and Mn zonations. For example, in Figure 5, Mg content decreases gradually from the centre of the grain to the edge (Figure 5h), while Mn content follows the opposite trend (Figure 5f) Fe content is slightly higher at the extreme edge (Figure 5e). Some needle shaped minerals are included in the grains, composed of Ca and Cr at the centre of the grain (Figure 5b,g) and Ca, Fe and Mn at the edge (Figure 5b,e,f).
3.3. Potential Hazardous Metal Location
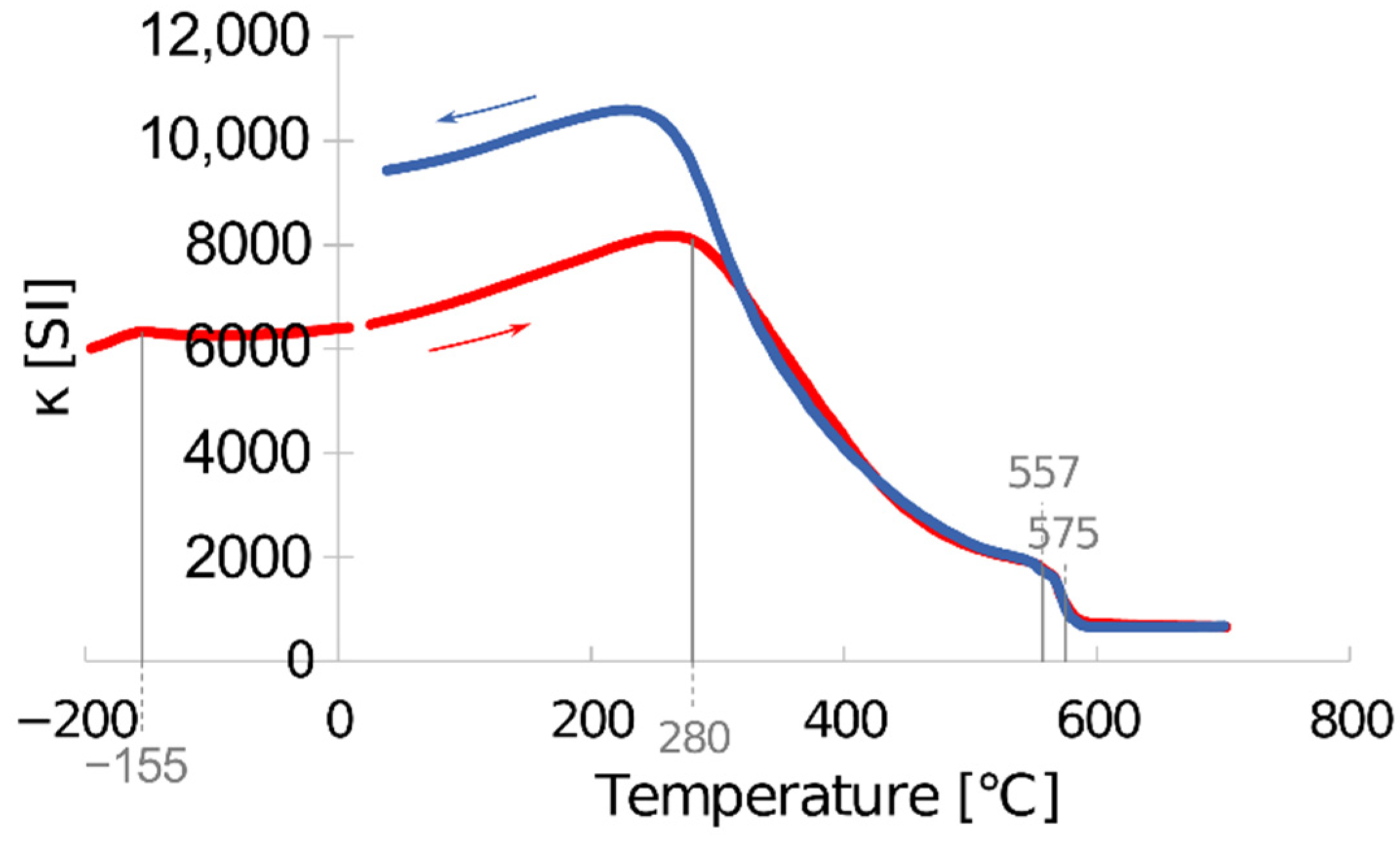
3.4. Focus on Magnetic Phases
3.5. Principal Component Analysis
4. Discussion
4.1. Comparison with Other EAF Slag
4.2. Hazardous Metals Location and Potential Mobility
4.3. Hazardous Metals and Magnetic Susceptibility
4.4. Magnetic Susceptibility as a Field Tool
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Steel Association Steel Industry Co-products. Available online: https://www.worldsteel.org/en/dam/jcr:1b916a6d-06fd-4e84-b35d-c1d911d18df4/Fact_By-products_2016.pdf (accessed on 13 January 2020).
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- European Slag Association (EUROSLAG) Statistical Data. Available online: https://www.euroslag.com/research-library-downloads/downloads/ (accessed on 13 January 2020).
- Council of the European Union Council Directive 1999/31/EC 26 on the Landfill of Waste. 26 April 1999.
- Lekakh, S.N.; Rawlins, C.H.; Robertson, D.G.C.; Richards, V.L.; Peaslee, K.D. Kinetics of Aqueous Leaching and Carbonization of Steelmaking Slag. Metall. Mater. Trans. B 2008, 39, 125–134. [Google Scholar] [CrossRef]
- Albertsson, G.J.; Engström, F.; Teng, L. Effect of the Heat Treatment on the Chromium Partition in Cr-Containing Industrial and Synthetic Slags. Steel Res. Int. 2014, 85, 1418–1431. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Di Cecca, C.; Le Saout, G.; Garcia-Diaz, E. The effect of microstructure on the leaching behaviour of electric arc furnace (EAF) carbon steel slag. Process Saf. Environ. Prot. 2016, 102, 810–821. [Google Scholar] [CrossRef]
- Neuhold, S.; van Zomeren, A.; Dijkstra, J.J.; van der Sloot, H.A.; Drissen, P.; Algermissen, D.; Mudersbach, D.; Schüler, S.; Griessacher, T.; Raith, J.G.; et al. Investigation of Possible Leaching Control Mechanisms for Chromium and Vanadium in Electric Arc Furnace (EAF) Slags Using Combined Experimental and Modeling Approaches. Minerals 2019, 9, 525. [Google Scholar] [CrossRef]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Lidstrom Larsson, M.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef]
- Loncnar, M.; Mladenovic, A.; Zupancic, M.; Bukovec, P. Comparison of the mineralogy and microstructure of EAF stainless steel slags with reference to the cooling treatment. J. Min. Met. B Met. 2017, 53, 19–29. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical, and Morphological Properties of Steel Slag. Adv. Civ. Eng. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Neuhold, S.; Algermissen, D.; Drissen, P.; Adamczyk, B.; Presoly, P.; Sedlazeck, K.P.; Schenk, J.; Raith, J.G.; Pomberger, R.; Vollprecht, D. Tailoring the FeO/SiO2 Ratio in Electric Arc Furnace Slags to Minimize the Leaching of Vanadium and Chromium. Appl. Sci. 2020, 10, 2549. [Google Scholar] [CrossRef]
- Engström, F.; Adolfsson, D.; Yang, Q.; Samuelsson, C.; Björkman, B. Crystallization Behaviour of some Steelmaking Slags. Steel Res. Int. 2010, 81, 362–371. [Google Scholar] [CrossRef]
- Engström, F.; Larsson, M.L.; Samuelsson, C.; Sandström, Å.; Robinson, R.; Björkman, B. Leaching Behavior of Aged Steel Slags. Steel Res. Int. 2014, 85, 607–615. [Google Scholar] [CrossRef]
- Rosowiecka, O.; Nawrocki, J. Assessment of soils pollution extent in surroundings of ironworks based on magnetic analysis. Studia Geophys. Geod. 2010, 54, 185–194. [Google Scholar] [CrossRef]
- Cao, L.; Appel, E.; Hu, S.; Yin, G.; Lin, H.; Rösler, W. Magnetic response to air pollution recorded by soil and dust-loaded leaves in a changing industrial environment. Atmos. Environ. 2015, 119, 304–313. [Google Scholar] [CrossRef]
- Girault, F.; Perrier, F.; Poitou, C.; Isambert, A.; Théveniaut, H.; Laperche, V.; Clozel-Leloup, B.; Douay, F. Effective radium concentration in topsoils contaminated by lead and zinc smelters. Sci. Total Environ. 2016, 566–567, 865–876. [Google Scholar] [CrossRef]
- Attoucheik, L.; Jordanova, N.; Bayou, B.; Lagroix, F.; Jordanova, D.; Maouche, S.; Henry, B.; Boutaleb, A. Soil metal pollution from former Zn–Pb mining assessed by geochemical and magnetic investigations: case study of the Bou Caid area (Tissemsilt, Algeria). Environ. Earth Sci. 2017, 76. [Google Scholar] [CrossRef]
- Golden, N.; Zhang, C.; Potito, A.P.; Gibson, P.J.; Bargary, N.; Morrison, L. Impact of grass cover on the magnetic susceptibility measurements for assessing metal contamination in urban topsoil. Environ. Res. 2017, 155, 294–306. [Google Scholar] [CrossRef]
- Gołuchowska, B.; Strzyszcz, Z.; Kusza, G. Magnetic Susceptibility and Heavy Metal Content in Dust from the Lime Plant and the Cement Plant in Opole Voivodeship. Arch. Environ. Prot. 2012, 38, 71–80. [Google Scholar] [CrossRef]
- Jordanova, D.; Jordanova, N.; Hoffmann, V. Magnetic mineralogy and grain-size dependence of hysteresis parameters of single spherules from industrial waste products. Phys. Earth Planet. Inter. 2006, 154, 255–265. [Google Scholar] [CrossRef]
- Magiera, T.; Gołuchowska, B.; Jabłońska, M. Technogenic Magnetic Particles in Alkaline Dusts from Power and Cement Plants. Waterairsoil Pollut. 2013, 224. [Google Scholar] [CrossRef]
- Szuszkiewicz, M.; Magiera, T.; Kapička, A.; Petrovský, E.; Grison, H.; Gołuchowska, B. Magnetic characteristics of industrial dust from different sources of emission: A case study of Poland. J. Appl. Geophys. 2015, 116, 84–92. [Google Scholar] [CrossRef]
- Walach, G.; Scholger, R.; Cech, B. Geomagnetic and Geoelectric Prospection on a Roman Iron Production Facility in Hüttenberg, Austria (Ferrum Noricum): Geophysical Prospection in Hüttenberg, Austria. Archaeol. Prospect. 2011, 18, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Humphris, J.; Carey, C. New methods for investigating slag heaps: Integrating geoprospection, excavation and quantitative methods at Meroe, Sudan. J. Archaeol. Sci. 2016, 70, 132–144. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Occasional papers of the Geological Institute of Hungary; Geological Inst. of Hungary: Budapest, Hungary, 2011; ISBN 978-963-671-288-4. [Google Scholar]
- De Windt, L.; Chaurand, P.; Rose, J. Kinetics of steel slag leaching: Batch tests and modeling. Waste Manag. 2011, 31, 225–235. [Google Scholar] [CrossRef]
- Durinck, D.; Engström, F.; Arnout, S.; Heulens, J.; Jones, P.T.; Björkman, B.; Blanpain, B.; Wollants, P. Hot stage processing of metallurgical slags. Resour. Conserv. Recycl. 2008, 52, 1121–1131. [Google Scholar] [CrossRef]
- Waligora, J.; Bulteel, D.; Degrugilliers, P.; Damidot, D.; Potdevin, J.L.; Measson, M. Chemical and mineralogical characterizations of LD converter steel slags: A multi-analytical techniques approach. Mater. Charact. 2010, 61, 39–48. [Google Scholar] [CrossRef]
- Estrader, M.; López-Ortega, A.; Golosovsky, I.V.; Estradé, S.; Roca, A.G.; Salazar-Alvarez, G.; López-Conesa, L.; Tobia, D.; Winkler, E.; Ardisson, J.D.; et al. Origin of the large dispersion of magnetic properties in nanostructured oxides: FexO/Fe3O4 nanoparticles as a case study. Nanoscale 2015, 7, 3002–3015. [Google Scholar] [CrossRef]
- Hazen, R.M.; Jeanloz, R. Wüstite (Fe1−xO): A review of its defect structure and physical properties. Rev. Geophys. 1984, 22, 37. [Google Scholar] [CrossRef]
- Wang, Y.G.; Ping, D.H.; Guo, J.G. High-resolution transmission-electron-microscopy observation of the ultra-fine structure of natural magnetite. J. Appl. Cryst. 1994, 27, 96–102. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Vapnik, Y.; Lazic, B.; Armbruster, T.; Murashko, M.; Galuskin, E.V. Harmunite CaFe2O4: A new mineral from the Jabel Harmun, West Bank, Palestinian Autonomy, Israel. Am. Mineral. 2014, 99, 965–975. [Google Scholar] [CrossRef]
- Dariz, P.; Schmid, T. Ferruginous phases in 19th century lime and cement mortars: A Raman microspectroscopic study. Mater. Charact. 2017, 129, 9–17. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Dunlop, D.J.; Özdemir, Ö. Magnetizations in Rocks and Minerals. In Treatise on Geophysics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 255–308. ISBN 978-0-444-53803-1. [Google Scholar]
- Kropáček, V.; Krs, M. Distribution of the values of natural remanent magnetization and magnetic susceptibility of some minerals. Stud. Geophys. Geod. 1971, 15, 340–352. [Google Scholar] [CrossRef]
- Gautam, P.; Blaha, U.; Appel, E. Magnetic susceptibility of dust-loaded leaves as a proxy of traffic-related heavy metal pollution in Kathmandu city, Nepal. Atmos. Environ. 2005, 39, 2201–2211. [Google Scholar] [CrossRef]
- Lehndorff, E.; Urbat, M.; Schwark, L. Accumulation histories of magnetic particles on pine needles as function of air quality. Atmos. Environ. 2006, 40, 7082–7096. [Google Scholar] [CrossRef]
- Moreno, E.; Sagnotti, L.; Dinarès-Turell, J.; Winkler, A.; Cascella, A. Biomonitoring of traffic air pollution in Rome using magnetic properties of tree leaves. Atmos. Environ. 2003, 37, 2967–2977. [Google Scholar] [CrossRef]
- Gargiulo, J.D.; Kumar, R.S.; Chaparro, M.A.E.; Chaparro, M.A.E.; Natal, M.; Rajkumar, P. Magnetic properties of air suspended particles in thirty eight cities from south India. Atmos. Pollut. Res. 2016, 7, 626–637. [Google Scholar] [CrossRef]
- Jordanova, D.; Jordanova, N.; Petrov, P. Magnetic susceptibility of road deposited sediments at a national scale—Relation to population size and urban pollution. Environ. Pollut. 2014, 189, 239–251. [Google Scholar] [CrossRef]
- Hunt, C.P.; Moskowitz, B.M.; Banerjee, S.K. Magnetic Properties of Rocks and Minerals. In Rock Pysics and Phase Relations A Handbook of Physical Constants; American Geophysical Union: Washington, DC, USA, 1995; Volume 3, pp. 189–204. ISBN 0-87590-853-5. [Google Scholar]
- Hodel, F.; Macouin, M.; Triantafyllou, A.; Carlut, J.; Berger, J.; Rousse, S.; Ennih, N.; Trindade, R.I.F. Unusual massive magnetite veins and highly altered Cr-spinels as relics of a Cl-rich acidic hydrothermal event in Neoproterozoic serpentinites (Bou Azzer ophiolite, Anti-Atlas, Morocco). Precambrian Res. 2017, 300, 151–167. [Google Scholar] [CrossRef]
- Hanesch, M.; Scholger, R. Mapping of heavy metal loadings in soils by means of magnetic susceptibility measurements. Environ. Geol. 2002, 42, 857–870. [Google Scholar] [CrossRef]
- Jakšík, O.; Kodešová, R.; Kapička, A.; Klement, A.; Fér, M.; Nikodem, A. Using magnetic susceptibility mapping for assessing soil degradation due to water erosion. Soil Water Res. 2016, 11, 105–113. [Google Scholar] [CrossRef]
- Jordanova, N.; Jordanova, D.; Petrov, P. Soil magnetic properties in Bulgaria at a national scale—Challenges and benefits. Glob. Planet. Chang. 2016, 137, 107–122. [Google Scholar] [CrossRef]
- Martin, A.P.; Ohneiser, C.; Turnbull, R.E.; Strong, D.T.; Demler, S. Soil magnetic susceptibility mapping as a pollution and provenance tool: an example from southern New Zealand. Geophys. J. Int. 2018, 212, 1225–1236. [Google Scholar] [CrossRef]
- Panaiotu, C.G.; Necula, C.; Panaiotu, C.E.; Axente, V. A magnetic investigation of heavy metals pollution in Bucharest. In Sustainability for Humanity & Environment in the Extended Connection Field Science-Economy-Policy, Scientific Reunion of the Special Program of Alexander von Humbold Foundation Concerning the Reconstruction of the South Eastern Europe; Editura Politehnica: Timisoara, Romania, 2005; pp. 83–86. ISBN 973.625-204-3. [Google Scholar]
- Dearing, J.A. Environmental Magnetic Susceptibility: Using the Bartington MS2 System; Chi Publishing: Kenilworth, UK, 1994. [Google Scholar]
- Thompson, R.; Oldfield, F. Environmental Magnetism; Allen & Unwin: Sydney, Australia, 1986; ISBN 978-94-011-8038-2. [Google Scholar]
- Coomarasamy, A.; Walzak, T.L. Effects of Moisture on Surface chemistry of Steel Slags and Steel Slag-Asphalt Paving Mixes. Transp. Res. Rec. 1995. [Google Scholar]
- Fällman, A.-M. Leaching of chromium and barium from steel slag in laboratory and field tests—A solubility controlled process? Waste Manag. 2000, 20, 149–154. [Google Scholar] [CrossRef]
- López, F.; López-Delgado, A.; Balcazar, N. Physico-chemical and mineralogical properties of EAF and AOD Slags. Afinidad 1996, 53, 39–46. [Google Scholar]
- Pillay, K.; von Blottnitz, H.; Petersen, J. Ageing of chromium(III)-bearing slag and its relation to the atmospheric oxidation of solid chromium(III)-oxide in the presence of calcium oxide. Chemosphere 2003, 52, 1771–1779. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E.; Nordström, U. Physicochemical and mineralogical properties of stainless steel slags oriented to metal recovery. Resour. Conserv. Recycl. 2004, 40, 245–271. [Google Scholar] [CrossRef]
- Suer, P.; Lindqvist, J.-E.; Arm, M.; Frogner-Kockum, P. Reproducing ten years of road ageing—Accelerated carbonation and leaching of EAF steel slag. Sci. Total Environ. 2009, 407, 5110–5118. [Google Scholar] [CrossRef]
- Cabrera-Real, H.; Romero-Serrano, A.; Zeifert, B.; Hernandez-Ramirez, A.; Hallen-Lopez, M.; Cruz-Ramirez, A. Effect of MgO and CaO/SiO2 on the immobilization of chromium in synthetic slags. J Mater Cycles Waste Manag. 2012, 14, 317–324. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J. Carbonation of Steel Slag for CO2 Sequestration: Leaching of Products and Reaction Mechanisms. Environ. Sci. Technol. 2006, 40, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.A.; Salem, H. The toxicology of chromium with respect to its chemical speciation: A review. J. Appl. Toxicol. 1993, 13, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.P. Vanadium geochemistry in the biogeosphere—Speciation, solid-solution interactions, and ecotoxicity. Appl. Geochem. 2019, 102, 1–25. [Google Scholar] [CrossRef]
- Gupta, U.C. Symptoms of Molybdenum Deficiency and Toxicity in Crops. In Molybdenum in Agriculture; Gupta, U.C., Ed.; Cambridge University Press: Cambridge, UK, 1997; pp. 160–170. ISBN 978-0-521-57121-0. [Google Scholar]
- Kubota, J.; Lazar, V.A.; Simonson, G.H.; Hill, W.W. The Relationship of Soils to Molybdenum Toxicity in Grazing Animals in Oregon. Soil Sci. Soc. Am. J. 1967, 31, 667–671. [Google Scholar] [CrossRef]
- Matern, K.; Rennert, T.; Mansfeldt, T. Molybdate adsorption from steel slag eluates by subsoils. Chemosphere 2013, 93, 2108–2115. [Google Scholar] [CrossRef]
- Lee, Y.; Nassaralla, C.L. Formation of hexavalent chromium by reaction between slag and magnesite-chrome refractory. Met. Mater. Trans B 1998, 29, 405–410. [Google Scholar] [CrossRef]
- Ziemniak, S.E.; Castelli, R.A. Immiscibility in the FeS3O4–FeCr2O4 spinel binary. J. Phys. Chem. Solids 2003, 64, 2081–2091. [Google Scholar] [CrossRef]




| wt. % | Mean | Min | Max |
|---|---|---|---|
| MgO | 4.29 | 2.58 | 5.53 |
| Al2O3 | 5.65 | 4.49 | 10.10 |
| SiO2 | 9.70 | 7.90 | 21.00 |
| P2O5 | 0.40 | 0.32 | 0.45 |
| CaO | 30.58 | 24.73 | 32.87 |
| TiO2 | 0.24 | 0.19 | 0.32 |
| V2O3 | 0.03 | 0.02 | 0.04 |
| Cr2O3 | 2.83 | 2.00 | 3.66 |
| MnO | 5.27 | 3.62 | 6.16 |
| FeO * | 29.86 | 21.64 | 34.49 |
| MoO3 | 0.05 | 0.05 | 0.07 |
| Initial Sample | Slag-13—Chemical Composition of the Different Grain Size Fractions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (ED-XRF) | (ICP-AES—Elemental Analyser) | ||||||||
| Grain Size (mm) | Total | >10 | 5–10 | 2.5–5 | 1.6–2.5 | 1.0–1.6 | 0.5–1.0 | 0.25–0.5 | <0.25 |
| Distribution | (100%) | (20%) | (18%) | (32%) | (6%) | (11%) | (8%) | (3%) | (2%) |
| (wt. %) | |||||||||
| MgO | 3.59 | 5.14 | 4.53 | 5.23 | 5.46 | 5.50 | 5.48 | 5.87 | 7.36 |
| Al2O3 | 4.73 | 4.43 | 4.43 | 4.42 | 4.80 | 4.88 | 5.37 | 6.66 | 6.93 |
| SiO2 | 8.25 | 9.15 | 7.89 | 7.76 | 7.63 | 7.25 | 7.78 | 7.62 | 6.61 |
| P2O5 | 0.41 | 0.54 | 0.38 | 0.39 | 0.37 | 0.35 | 0.32 | 0.27 | 0.17 |
| CaO | 31.27 | 31.69 | 29.47 | 31.27 | 30.74 | 29.65 | 29.43 | 31.53 | 32.37 |
| TiO2 | 0.25 | 0.34 | 0.30 | 0.28 | 0.29 | 0.28 | 0.28 | 0.24 | 0.19 |
| V2O3 | 0.03 | 0.11 | 0.10 | 0.10 | 0.09 | 0.09 | 0.09 | 0.07 | 0.05 |
| Cr2O3 | 2.75 | 1.87 | 2.35 | 2.14 | 1.57 | 2.64 | 2.25 | 1.88 | 1.27 |
| MnO | 5.38 | 6.48 | 5.80 | 5.91 | 5.40 | 5.23 | 4.85 | 3.45 | 2.51 |
| FeO * | 30.46 | 35.49 | 38.83 | 33.71 | 31.81 | 31.55 | 30.35 | 22.21 | 16.56 |
| MoO3 | 0.05 | N/A ** | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| C | N/A | 0.90 | 1.06 | 1.35 | 1.52 | 1.93 | 2.59 | 4.45 | 4.80 |
| LOI 110 °C | N/A | 0.44 | 0.68 | 0.74 | 0.81 | 0.65 | 0.77 | 1.11 | 1.57 |
| LOI 1000 °C | N/A | 0.42 | 2.04 | 4.93 | 7.43 | 9.03 | 10.32 | 16.71 | 21.99 |
| Phase | Formula | >10 mm | 10–5 mm | 5–2.5 mm | 2.5–1.6 mm | 1.6–1.0 mm | 1.0–0.5 mm | 0.5–0.25 mm | <0.25 mm |
|---|---|---|---|---|---|---|---|---|---|
| Wustite | FeO | +++ | ++ | + | + | + | + | + | - |
| Spinel solid solution | (Fe,Mg)(Fe,Mn,Cr)2O4 | + | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Chromite | Fe(Cr,Al)2O4 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Brownmillerite | Ca2(Fe,Al,Cr)2O5 | +++ | ++ | ++ | ++ | ++ | ++ | ++ | + |
| Calcium chromite | CaCr2O4 | + | + | + | + | + | + | + | ++ |
| Larnite | Ca2SiO4 | +++ | +++ | +++ | ++ | ++ | ++ | + | + |
| Calcite | CaCO3 | + | + | + | + | + | + | +++ | +++ |
| Quartz | SiO2 | - | - | + | + | + | ++ | ++ | ++ |
| Element | Mineral Phase | C3S | C2S | Brw | Chr | ss-Spl | Ca-Chr |
|---|---|---|---|---|---|---|---|
| Mg (at. %) | min–max | 0.00–0.10 | 0.00–0.38 | 0.12–0.51 | 5.10–7.50 | 0.32–43.51 | 0.29–0.97 |
| average | 0.05 | 0.03 | 0.27 | 6.70 | 10.96 | 0.63 | |
| std. Dev. | 0.04 | 0.16 | 0.13 | 0.75 | 9.03 | 0.28 | |
| Al (at. %) | min–max | 0.54–1.72 | 0.15–0.64 | 2.72–9.46 | 0.99–5.04 | 0–2.37 | 0.33–0.75 |
| average | 0.92 | 0.34 | 6.47 | 2.50 | 0.20 | 0.52 | |
| std. Dev. | 0.34 | 0.16 | 2.13 | 1.49 | 0.40 | 0.20 | |
| Si (at. %) | min–max | 9.54–10.35 | 12.69–13.65 | 0.03–2.12 | <DL * | <DL | 0.01–0.24 |
| average | 10.01 | 13.14 | 0.73 | <DL | <DL | 0.15 | |
| std. Dev. | 0.26 | 0.36 | 0.50 | 0.34 | |||
| P (at. %) | min–max | 0.21–0.55 | 0.32–1.06 | 0.00–0.36 | <DL | <DL | <DL |
| average | 0.35 | 0.68 | 0.04 | <DL | <DL | <DL | |
| std. Dev. | 0.12 | 0.23 | 0.07 | ||||
| Ca (at. %) | min–max | 30.32–32.04 | 26.95–28.31 | 22.06–24.39 | 0.37–0.97 | 0.18–7.49 | 14.25–16.33 |
| average | 31.36 | 27.93 | 23.22 | 0.60 | 1.87 | 14.93 | |
| std. Dev. | 0.63 | 0.47 | 0.52 | 0.23 | 2.01 | 0.73 | |
| Ti (at. %) | min–max | <DL | <DL | 0.04–1.55 | 0.02–0.08 | <DL | <DL |
| average | <DL | <DL | 0.70 | 0.04 | <DL | <DL | |
| std. Dev. | 0.33 | 0.02 | |||||
| V (at. %) | min–max | <DL | <DL | 0.04–0.98 | 0.03–0.13 | <DL | <DL |
| average | <DL | <DL | 0.18 | 0.06 | <DL | <DL | |
| std. Dev. | 0.19 | 0.04 | |||||
| Cr (at. %) | min–max | <DL | <DL | 0.22–6.64 | 21.77–26.63 | 0–6.38 | 22.43–25.10 |
| average | <DL | <DL | 3.39 | 24.88 | 0.94 | 24.43 | |
| std. Dev. | 1.79 | 1.74 | 1.23 | 1.03 | |||
| Mn (at. %) | min–max | 0.27–0.74 | 0.03–0.47 | 0.24–1.26 | 2.65–5.70 | 0.96–16.12 | 0.06–0.24 |
| average | 0.46 | 0.14 | 2.59 | 3.50 | 8.43 | 0.12 | |
| std. Dev. | 0.16 | 0.15 | 0.24 | 0.91 | 3.53 | 0.07 | |
| Fe (at. %) | min–max | 0.90–1.46 | 0.25–0.91 | 5.20–17.13 | 2.47–7.39 | 4.97–47.73 | 2.48–3.36 |
| average | 1.18 | 0.40 | 11.13 | 4.80 | 27.23 | 2.79 | |
| std. Dev. | 0.16 | 0.23 | 3.95 | 1.21 | 8.66 | 0.32 | |
| Number of analyses | 9 | 7 | 24 | 12 | 81 | 6 | |
| Point Number | Mg (at. %) | Al (at. %) | Si (at. %) | Ca (at. %) | Cr (at. %) | Mn (at. %) | Fe (at. %) | Identified Mineral |
|---|---|---|---|---|---|---|---|---|
| 1 | 14.32 | 0.90 | 0.02 | 0.50 | 1.69 | 8.81 | 23.03 | jacobsite |
| 2 | 13.85 | 0.39 | 0.03 | 0.60 | 1.29 | 9.06 | 24.28 | jacobsite |
| 3 | 0.38 | 0.64 | 12.69 | 26.95 | 0.04 | 0.47 | 0.91 | larnite |
| 5 | 1.35 | 0.00 | 0.02 | 0.43 | 0.00 | 5.11 | 43.09 | jacobsite |
| 7 | 6.11 | 0.10 | 0.02 | 0.43 | 0.02 | 9.97 | 33.35 | jacobsite |
| 8 | 4.62 | 0.15 | 0.03 | 2.63 | 0.06 | 7.21 | 35.27 | jacobsite |
| 9 | 0.75 | 0.13 | 0.01 | 11.31 | 0.16 | 2.12 | 35.32 | harmunite |
| 11 | 18,00 | 0.00 | 0.02 | 0.50 | 0.44 | 10.68 | 20.14 | maghemite |
| 12 | 11.20 | 0.03 | 0.00 | 2.06 | 0.66 | 8.98 | 26.90 | maghemite |
| 13 | 5.45 | 0.02 | 0.02 | 12.47 | 1.53 | 4.96 | 25.15 | harmunite |
| 14 | 0.18 | 0.12 | 0.01 | 21.74 | 3.47 | 1.18 | 22.3 | harmunite |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbelin, M.; Bascou, J.; Lavastre, V.; Guillaume, D.; Benbakkar, M.; Peuble, S.; Baron, J.-P. Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques. Minerals 2020, 10, 705. https://doi.org/10.3390/min10080705
Herbelin M, Bascou J, Lavastre V, Guillaume D, Benbakkar M, Peuble S, Baron J-P. Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques. Minerals. 2020; 10(8):705. https://doi.org/10.3390/min10080705
Chicago/Turabian StyleHerbelin, Maud, Jérôme Bascou, Véronique Lavastre, Damien Guillaume, Mhammed Benbakkar, Steve Peuble, and Jean-Philippe Baron. 2020. "Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques" Minerals 10, no. 8: 705. https://doi.org/10.3390/min10080705
APA StyleHerbelin, M., Bascou, J., Lavastre, V., Guillaume, D., Benbakkar, M., Peuble, S., & Baron, J.-P. (2020). Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques. Minerals, 10(8), 705. https://doi.org/10.3390/min10080705





