Geochemistry and Acid Hydrometallurgy Accessibility of Uraninite from Mianhuakeng Granite-Hosted Uranium Deposit, South China
Abstract
1. Introduction
2. Materials and Methods
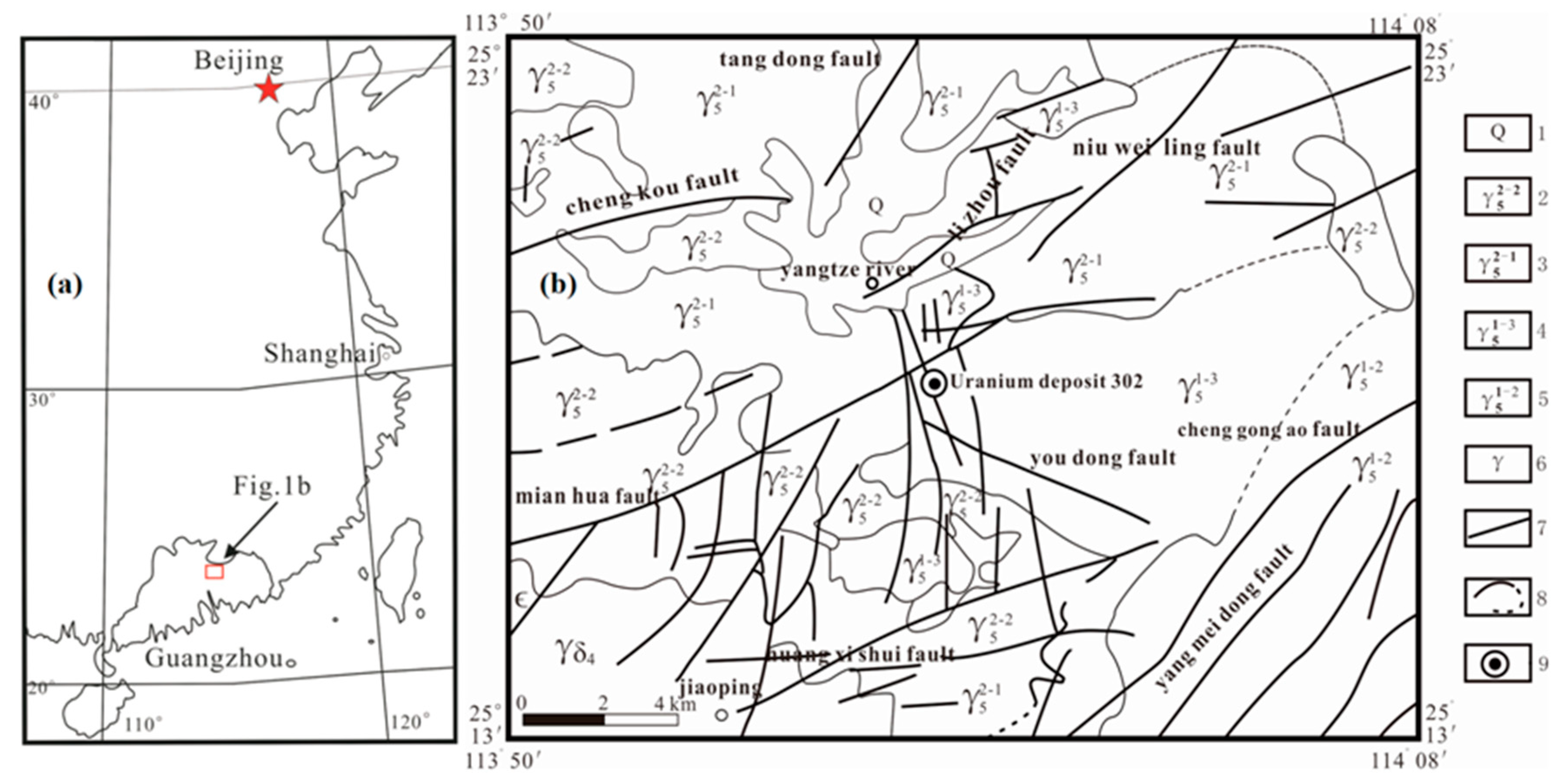
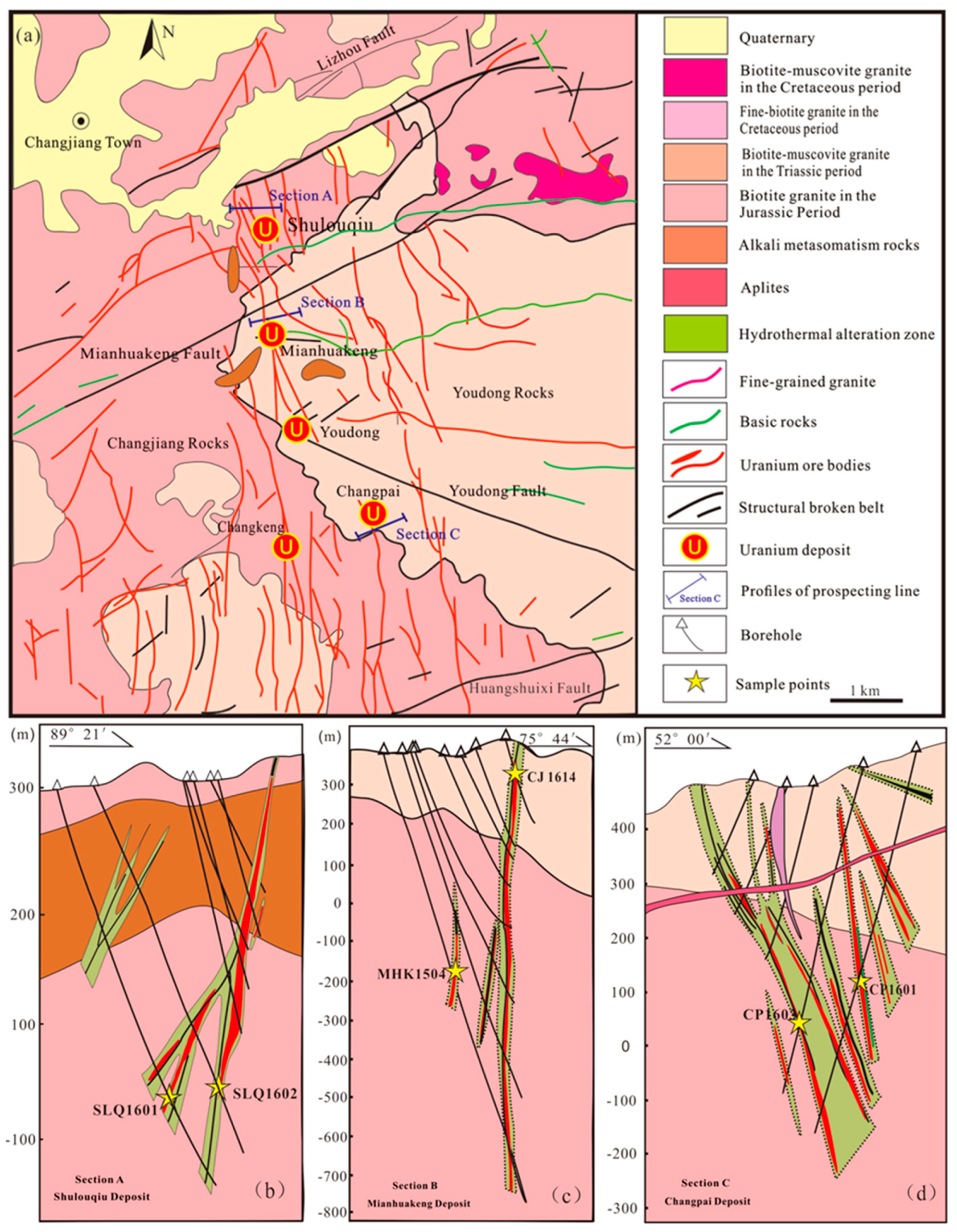
2.1. Geological Setting and MHK Uranium Deposit
2.2. Analytical Methods
3. Result
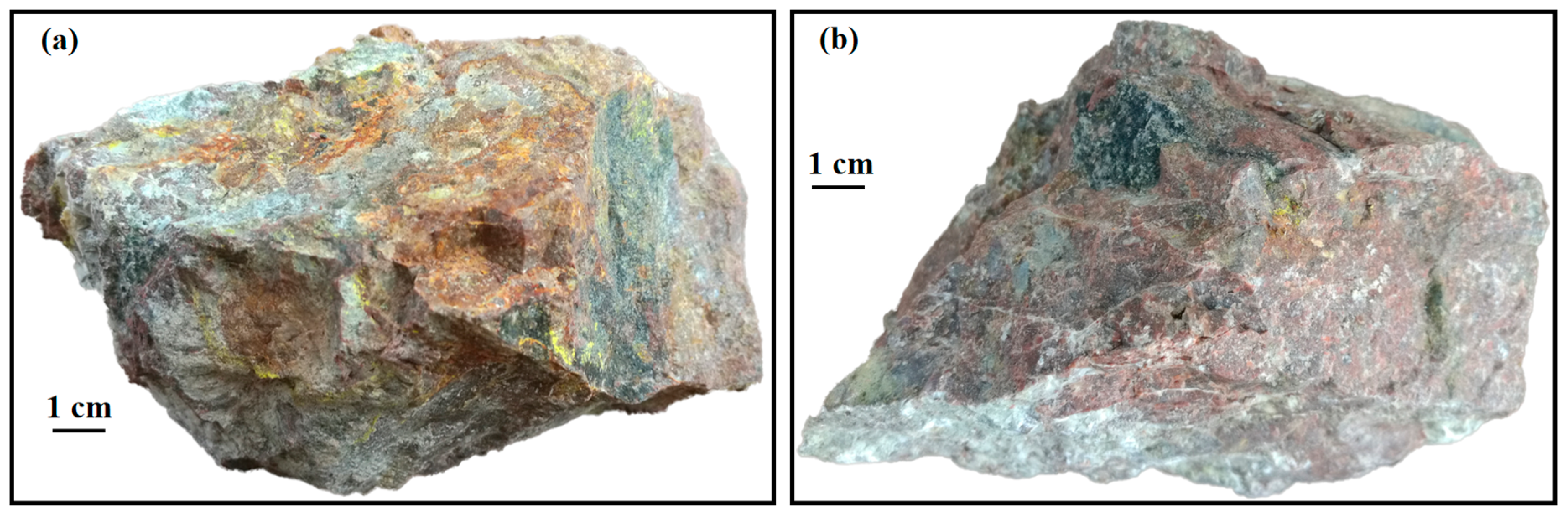
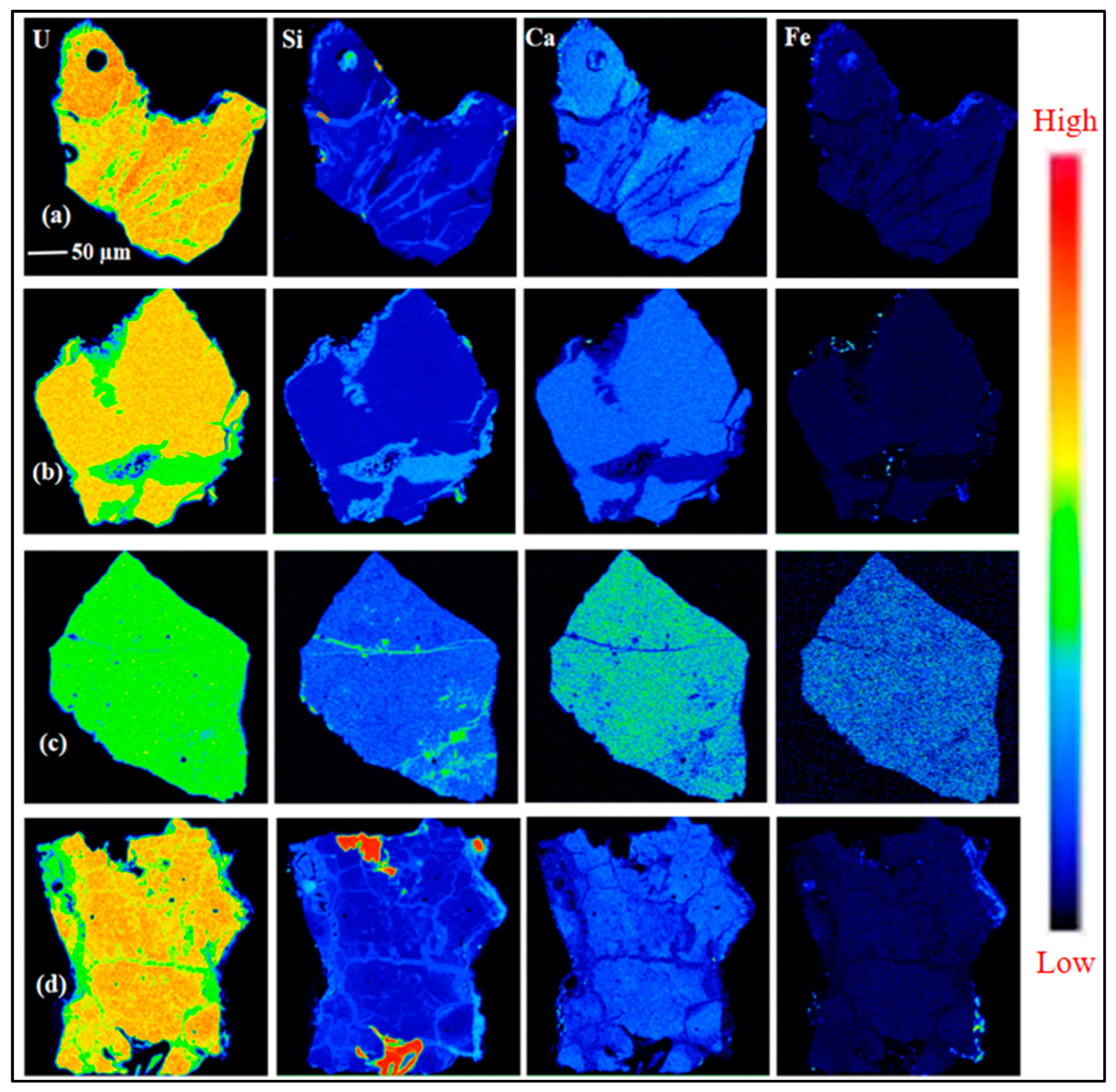
3.1. Petrography and Mineralogy of the Uraninite
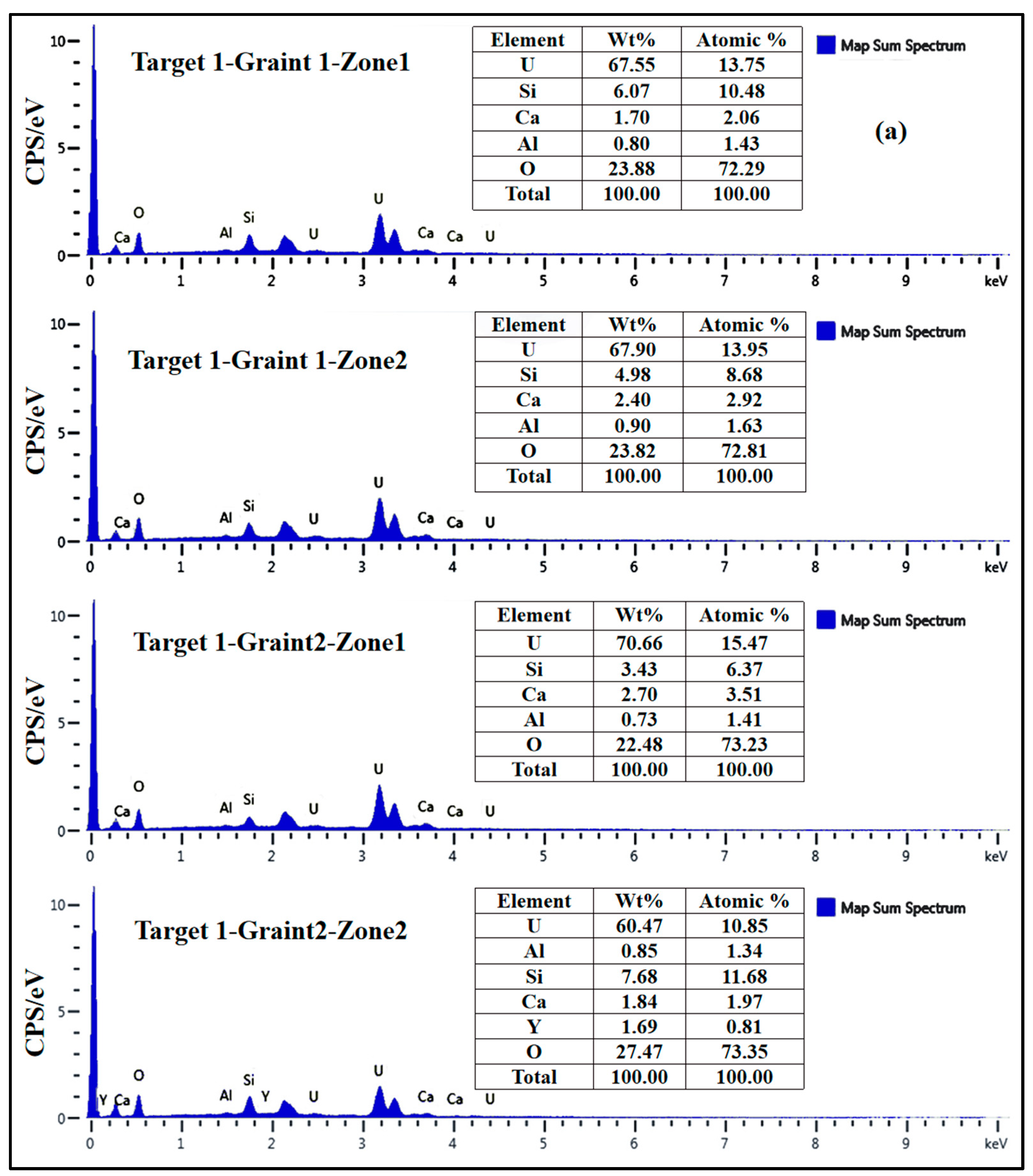
3.2. Geochemical Composition of Uraninite
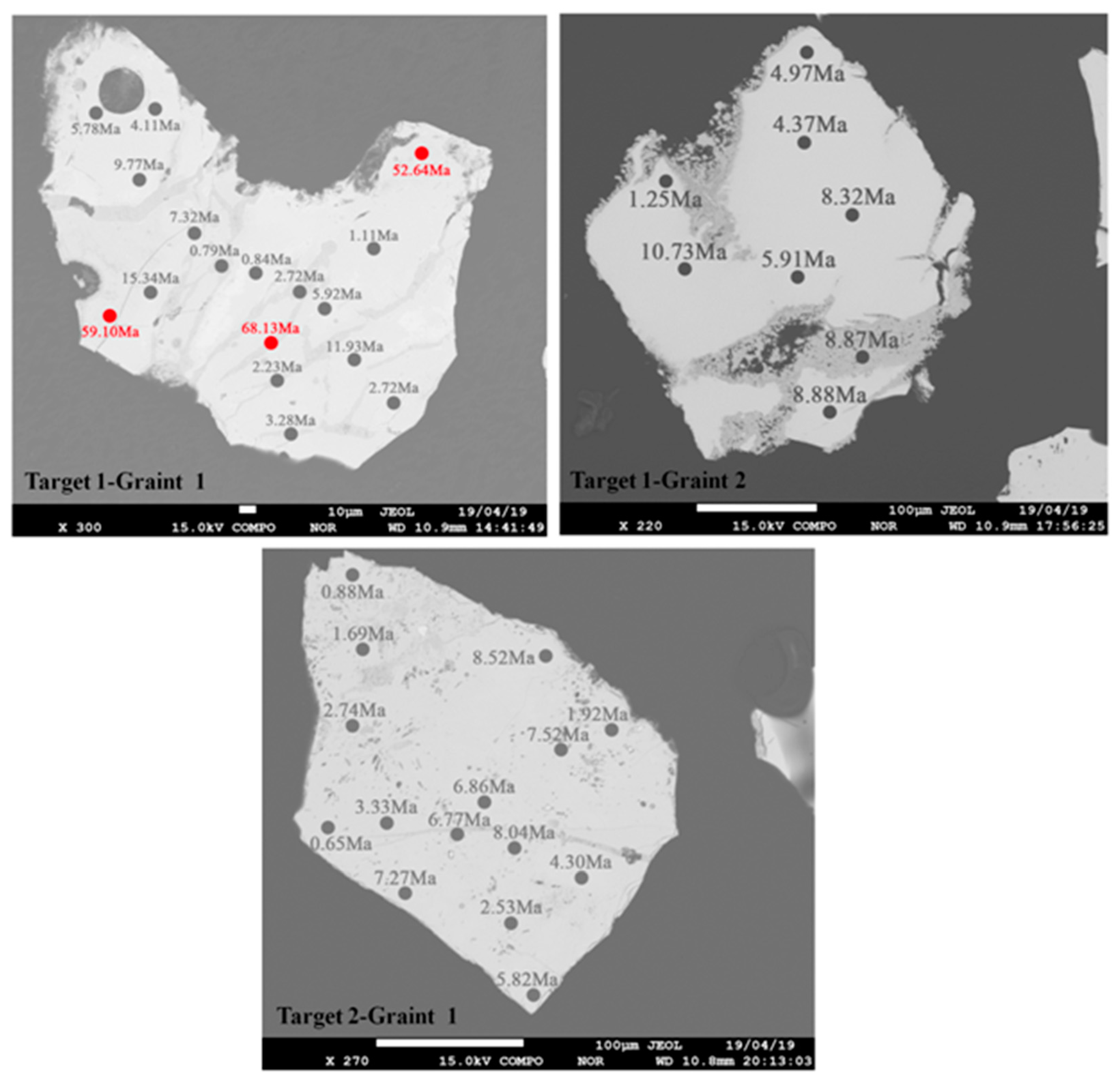
3.3. The Age of Uraninite from the MHK Uranium Deposit
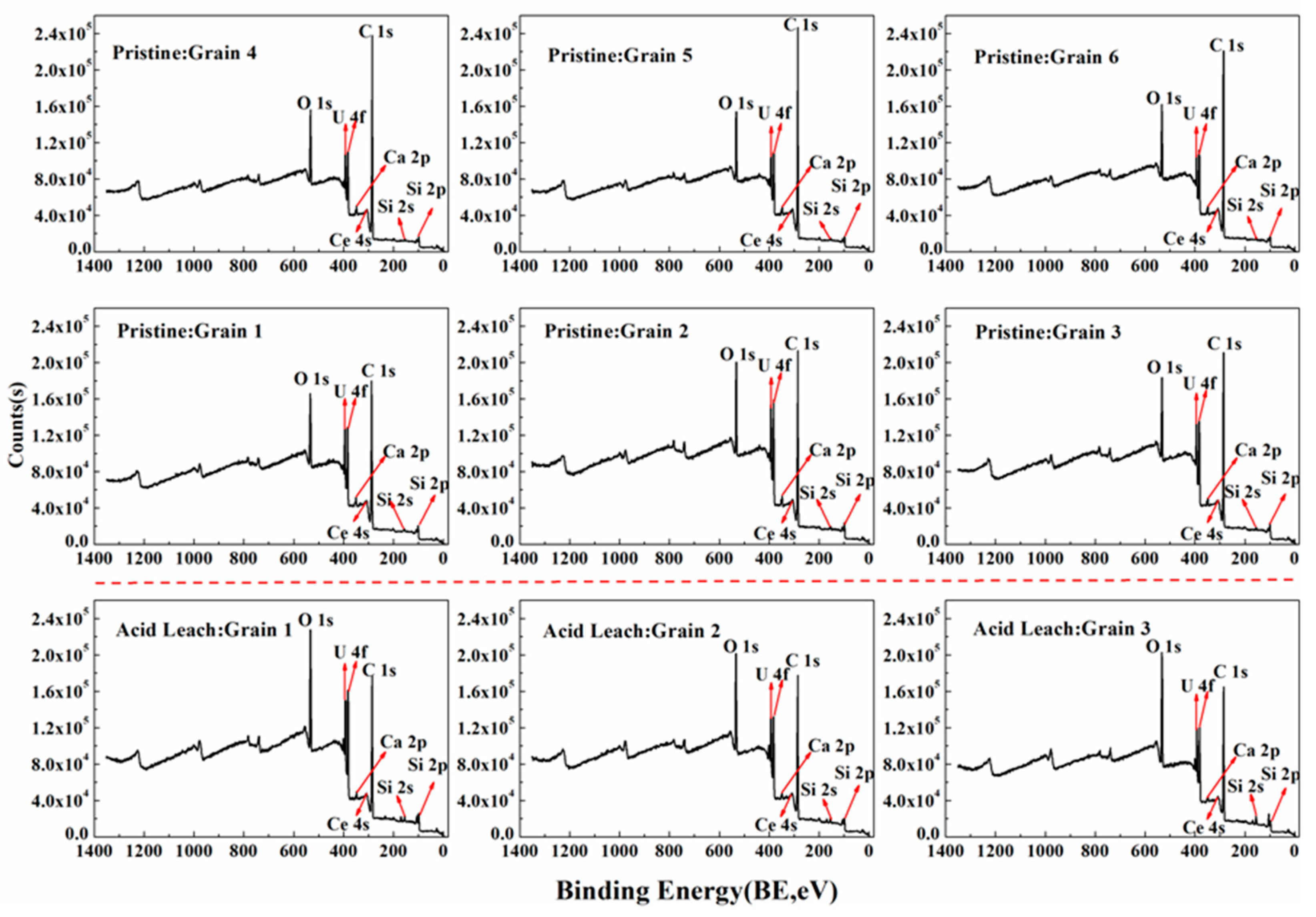
3.4. Acid Hydrometallurgy of Uraninite
4. Discussion
4.1. Chemical Composition of Uraninite
4.2. Pb Loss and Reliability of the Uraninite U–Th–Pb Chemical Ages
4.3. Acid Leaching Accessibility of Uraninite
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jungho, B. Do nuclear and renewable energy improve the environment? Empirical evidence from the United States. Ecol. Ind. 2016, 66, 352–356. [Google Scholar]
- Xing, W.L.; Wang, A.J.; Yan, Q.; Chen, S. A study of China’s uranium resources security issues: Based on analysis of China’s nuclear power development trend. Ann. Nucl. Energy 2017, 110, 1156–1164. [Google Scholar] [CrossRef]
- Mudd, G.M. The future of Yellowcake: A global assessment of uranium resources and mining. Sci. Total. Environ. 2014, 472, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Ilankoon, I.M.S.K.; Tang, Y.; Ghorbani, Y.; Northey, S.; Yellishetty, M.; Deng, X.Y.; McBride, D. The current state and future directions of percolation leaching in the Chinese mining industry: Challenges and opportunities. Miner. Eng. 2018, 125, 206–222. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Ram, R.; Pownceby, M.; Grocott, S.; Ring, B.; Tardio, J.; Jones, L. A review of acid leaching of uraninite. Hydrometallurgy 2015, 151, 10–24. [Google Scholar] [CrossRef]
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores—A brief overview. Hydrometallurgy 2016, 165, 206–212. [Google Scholar] [CrossRef]
- Wang, X.G.; Liu, Y.J.; Sun, Z.X.; Li, J.; Chai, L.Y.; Min, X.B.; Guo, Y.D.; Li, P.; Zhou, Z.K. Heap bioleaching of uranium from low-grade granite-type ore by mixed acidophilic microbes. J. Radioanal. Nucl. Chem. 2017, 314, 251–258. [Google Scholar] [CrossRef]
- Wang, X.G.; Li, P.; Liu, Y.J.; Sun, Z.X.; Chai, L.Y.; Min, X.B.; Guo, Y.D.; Zheng, Z.H.; Ke, Y.; Liang, Y.J. Uranium bioleaching from low-grade carbonaceous-siliceous-argillaceous type uranium ore using an indigenous Acidithiobacillus ferrooxidans. J. Radioanal. Nucl. Chem. 2018, 317, 1033–1040. [Google Scholar] [CrossRef]
- Wang, X.G.; Sun, Z.X.; Liu, Y.J.; Min, X.B.; Guo, Y.D.; Li, P.; Zheng, Z.H. Effect of particle size on uranium bioleaching in column reactors from a low-grade uranium ore. Bioresour. Technol. 2019, 281, 66–71. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Li, G.R.; Xu, L.L.; Liu, J.H.; Sun, Z.X.; Shi, W.J. Uranium recovery from sandstone-type uranium deposit by acid in-situ leaching-an example from the Kujieertai. Hydrometallurgy 2020, 191, 105–116. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Zhang, J.D.; Li, Z.Y.; Guo, Q.Y.; Song, J.Y.; Fan, H.H.; Liu, W.S.; Qi, F.C.; Zhang, M.L. Outline of uranium resources characteristics and metallogenetic regularity in China. Acta Geol. Sin. Engl. 2015, 89, 918–937. [Google Scholar]
- Zhong, F.J.; Pan, J.Y.; Qi, J.M.; Yan, J.; Liu, W.Q.; Li, H.D. New in-situ LA-ICP-MS U-Pb ages of uraninite from the Mianhuakeng uranium deposit, Northern Guangdong Province, China: Constraint on the metallogenic mechanism. Acta Geol. Sin. Engl. 2018, 92, 852–854. [Google Scholar] [CrossRef]
- Bonnetti, C.; Liu, X.D.; Mercadier, J.; Cuney, M.; Etienne, D.; Johan, V.; Liu, W.Q. The genesis of granite-related hydrothermal uranium deposits in the Xiazhuang and Zhuguang ore fields, North Guangdong Province, SE China: Insights from mineralogical, trace elements and U-Pb isotopes signatures of the U mineralisation. Ore Geol. Rev. 2018, 92, 588–612. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.Y.; Li, S.R.; Huang, G.L. Characteristics of uranium minerals in wall-rock alteration zones of the Mianhuakeng (No. 302) uranium deposit, northern Guangdong, South China. Acta Petrol. Sin. 2018, 34, 2657–2670. [Google Scholar]
- Luo, J.C.; Hu, R.Z.; Shi, S.H. Timing of uranium mineralization and geological implications of Shazijiang granite-hosted uranium deposit in Guangxi, South China: New constraint from chemical U-Pb Age. J. Earth Sci. 2015, 26, 911–919. [Google Scholar] [CrossRef]
- Luo, J.C.; Hu, R.Z.; Fayek, M.; Bi, X.W.; Shi, S.H.; Chen, Y.W. Newly discovered uranium mineralization at ~2.0 Ma in the Menggongjie granite-hosted uranium deposit, South China. J. Asian Earth Sci. 2017, 137, 241–249. [Google Scholar] [CrossRef]
- Hu, R.Z.; Bi, X.W.; Zhuo, M.F.; Peng, J.T.; Su, W.C.; Liu, S.; Qi, H.W. Uranium metallogenesis in South China and its relationship to crustal extension during the Cretaceous to Tertiary. Econ. Geol. 2008, 103, 583–598. [Google Scholar] [CrossRef]
- Shi, S.H.; Hu, R.Z.; Wen, H.J.; Sun, R.L.; Wang, J.S.; Chen, H. Geochronology of the Shazijiang uranium ore deposit, Northern Guangxi, China: U-Pb ages of uraninite and their geological significance. Acta Geol. Sin. 2010, 84, 1175–1182, (In Chinese With English Abstract). [Google Scholar]
- Hu, H.; Wang, R.C.; Chen, W.F.; Chen, P.R.; Ling, H.F.; Zhang, G.M. Timing of hydrothermal activity associated with the Douzhashan uranium-bearing granite and its significance for uranium mineralization in Northeastern Guangxi, China. Chin. Sci. Bull. 2013, 58, 4319–4328. [Google Scholar] [CrossRef]
- Hu, R.Z.; Luo, J.C.; Chen, Y.W.; Pan, L.C. Several processes in the study of uranium deposits in South China. Acta Petrol. Sin. 2019, 35, 2625–2636, (In Chinese With English Abstract). [Google Scholar]
- Corcoran, L.; Simonetti, A.; Spano, T.L.; Lewis, S.R.; Dorais, C.; Simonetti, S.; Burns, P.C. Multivariate analysis based on geochemical, isotopic, and mineralogical compositions of uranium-rich samples. Minerals 2019, 9, 537. [Google Scholar] [CrossRef]
- Yan, S.; Boyanov, M.I.; Mishra, B.; Kemner, K.M.; O’Loughlin, E.J. U (VI) reduction by biogenic and abiotic hydroxycarbonate green rusts: Impacts on U (IV) speciation and stability over time. Environ. Sci. Technol. 2018, 52, 4601–4609. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M.; Ewing, R.C.; Sverjensky, D.A. Evolution of uranium and thorium minerals. Am. Mineral. 2009, 94, 1293–1311. [Google Scholar] [CrossRef]
- Li, Z.Y.; Huang, Z.Z.; Li, X.Z.; Guo, J.; Fan, C. The discovery of natural native uranium and its significance. Acta Geol. Sin. Engl. 2015, 89, 1561–1567. [Google Scholar]
- Luo, J.C.; Hu, R.Z.; Fayek, M.; Li, C.; Bi, X.W.; Abdu, Y.; Chen, Y.W. In-situ SIMS uraninite U-Pb dating and genesis of the Xianshi granite-hosted uranium deposit, South China. Ore Geol. Rev. 2015, 65, 968–978. [Google Scholar] [CrossRef]
- Luo, J.C.; Hu, R.Z.; Bi, X.W.; Chen, Y.W. REE characteristics of a new uranium mineral from the Xianshi uranium deposit, South China. Acta Geol. Sin. 2018, 92, 1667–1669. [Google Scholar] [CrossRef]
- Tan, Z.Y.; Zhang, D.C.; Kuang, Z.P.; Zhu, Q.Z.; Li, Y. Analysis of deep prospecting at Mianhuakeng uranium deposit in the southern Zhuguang massif in northern Guangdong. Uranium Min. Metall. 2015, 2, 22–30, (In Chinese With English Abstract). [Google Scholar]
- Li, L.L. Studying on Geological and Geochemical Characteristics of Uranium Ore from Typical Uranium Deposits in Zhuguang Area. Master’s Thesis, East China Institute of Technology, Nanchang, China, 2018. (In Chinese With English Abstract). [Google Scholar]
- Li, J.H.; Xia, Z.Q. Typical deposit type and diagenetic metallogenic sequence model of Taoshan—Zhuguangshan uranium metallogenic belt. Mineral. Depos. 2012, S1, 205–206, (In Chinese With English Abstract). [Google Scholar]
- Huang, G.L.; Yin, Z.P.; Ling, H.H.; Deng, P.; Zhu, B.; Shen, W.Z. Formation age, geochemical characteristics and genesis of uraninite from No. 302 uranium deposit in northern Guangdong. Mineral Depos. 2010, 29, 352–360, (In Chinese With English Abstract). [Google Scholar]
- Zhang, C.; Cai, Y.Q.; Xu, H.; Dong, Q.; Liu, J.L.; Hao, R.X. Mechanism of mineralization in the Changjiang uranium ore field, South China: Evidence from fluid inclusions, hydrothermal alteration, and H-O isotopes. Ore. Geol. Rev. 2017, 86, 225–253. [Google Scholar] [CrossRef]
- Kotzer, T.G.; Kyser, T.K. O, U and Pb isotopic and chemical variations in uraninite: Implications for determining. Am. Mineral. 1993, 78, 11–12. [Google Scholar]
- Hu, H.; Wang, R.C.; Chen, W.F.; Ding, H.H.; Chen, P.R.; Ling, H.F. Study on uranium resource minerals of Douzhashan uranium-bearing granite, Northeastern Guangxi. Geol. Rev. 2012, 58, 1056–1068. [Google Scholar]
- Huang, G.L.; Liu, X.Y.; Sun, L.Q.; Li, Z.S.; Zhang, S.J. Zircon U-Pb dating, geochemical characteristic and genesis of the Changjiang granite in Northern Guangdong. Acta Geol. Sin. 2014, 88, 836–849. [Google Scholar]
- Gao, F.; Lin, J.R.; Zhong, Q.L.; Guo, S.Y.; Pang, Y.Q.; Rong, J.S.; Hu, Z.H. The wall rock alteration and its geochemical characteristics of uranium deposit No. 302. Uranium Geol. 2011, 5, 274–281, (In Chinese With English Abstract). [Google Scholar]
- Gao, X.; Shen, W.Z.; Liu, L.L.; Yao, W.; Zhu, B.; Huang, G.L.; Li, Q.Y. Geochemical characteristics and causes of wall rock alteration in the No. 302 uranium deposit, northern Guangdong. Acta Petrol. Mineral. 2011, 30, 71–82. [Google Scholar]
- Li, Z.Y.; Zhang, J.D.; Jian, X.F.; Cai, Y.Q.; Guo, Q.Y.; Zhu, P.F. Uranium metallogenic regionalization and resource potential in China. Acta Geol. Sin. Engl. 2012, 82, 547–554. [Google Scholar]
- Li, X.H. Cretaceous magmatism and lithospheric extension in Southeast China. J. Asian Earth Sci. 2000, 18, 293–305. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.Y.; Li, X.F.; Li, S.R.; Santosh, M.; Huang, G.L. Zircon U-Pb geochronology and geochemistry of granites in the Zhuguangshan complex, South China: Implications for uranium mineralization. Lithos. 2018, 308, 19–33. [Google Scholar] [CrossRef]
- Martz, P.; Mercadier, J.; Perret, J.; Villeneuve, J.; Deloule, E.; Cathelineau, M.; Quirt, D.; Doney, A.; Ledru, P. Post-crystallization alteration of natural uraninites: Implications for dating, tracing, and nuclear forensics. Geochim. Cosmochim. Acta 2019, 249, 138–159. [Google Scholar] [CrossRef]
- Alexandre, P.; Kyser, T.K. Effects of cationic substitutions and alteration in uraninite, and implications for the dating of uranium deposits. Can. Mineral. 2005, 43, 1005–1017. [Google Scholar] [CrossRef]
- Fayek, M.; Harrison, T.M.; Ewing, R.C.; Grove, M.; Coath, C.D. O and Pb isotopic analyses of uranium minerals by ion microprobe and U-Pb ages from the Cigar Lake deposit. Chem. Geol. 2002, 185, 205–225. [Google Scholar] [CrossRef]
- Bowles, J.F.W. Age dating of individual grains of uraninite in rocks from electron microprobe analyses. Chem. Geol. 1990, 83, 47–53. [Google Scholar] [CrossRef]
- Pal, D.C.; Rhede, D. Geochemical dating of uraninite in the Jaduguda uranium deposit, Singhbhum Shear Zone, India-implications for uranium mineralization and geochemical evolution of uraninite. Econ. Geol. 2013, 108, 1499–1515. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Ewing, R.C. Fate of trace elements during alteration of uraninite in a hydrothermal vein-type U-deposit from Marshall Pass, Colorado, USA. Geochim. Cosmochim. Acta 2007, 71, 4954–4973. [Google Scholar] [CrossRef]
- Moudler, J.F.; Stickle, W.F. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Teterin, Y.A.; Popel, A.J.; Maslakov, K.I.; Teterin, A.Y.; Ivanov, K.E.; Kalmykov, S.N.; Springell, R.; Scott, T.B.; Farnan, I. XPS study of ion irradiated and unirradiated UO2 thin films. Curr. Org. Chem. 2016, 55, 8059–8070. [Google Scholar] [CrossRef]
- Kempe, U. Precise electron microprobe age determination in altered uraninite: Consequences on the intrusion age and the metallogenic significance of the Kirchberg Granite (Erzgebirge, Germany). Contrib. Mineral. Petr. 2003, 145, 107–118. [Google Scholar] [CrossRef]
- Mukhopadhyay, J.; Mishra, B.; Chakrabarti, K.; De, S.; Ghosh, G. Uraniferous paleoplacers of the Mesoarchean Mahagiri Quartzite, Singhbhum craton, India: Depositional controls, nature and source of 3.0 Ga detrital uraninites. Ore. Geol. Rev. 2016, 72, 1290–1306. [Google Scholar] [CrossRef]
- Frimmel, H.E.; Schedel, S.; Brätz, H. Uraninite chemistry as forensic tool for provenance analysis. Appl. Geochem. 2014, 48, 104–121. [Google Scholar] [CrossRef]
- Cuney, M. Evolution of uranium fractionation processes through time: Driving the secular variation of uranium deposit types. Econ. Geol. 2010, 105, 553–569. [Google Scholar] [CrossRef]
- Evins, L.Z.; Jensen, K.A.; Ewing, R.C. Uraninite recrystallization and Pb loss in the Oklo and Bangombé natural fission reactors, Gabon. Geochim. Cosmochim. Acta 2005, 69, 1589–1606. [Google Scholar] [CrossRef]
- Clourier, J.; Kyser, K.; Olivo, G.R.; Alexandre, P.; Halaburda, J. The millennium uranium deposit, Athabasca basin, Saskatchewan, Canada: An atypical basement-hosted unconformity-related uranium deposit. Econ. Geol. 2009, 104, 815–840. [Google Scholar] [CrossRef]
- Kister, P.; Cuney, M.; Golubev, V.N.; Royer, J.J.; Veslud, C.L.C.D.; Rippert, J.C. Radiogenic lead mobility in the Shea Creek unconformity-related uranium deposit (Saskatchewan, Canada): Migration pathways and Pb loss quantification. Geochemistry. 2004, 336, 205–215. [Google Scholar] [CrossRef]
- Suzuki, K.; Kato, T. CHIME dating of monazite, xenotime, zircon and polycrase: Protocol, pitfalls and chemical criterion of possibly discordant age data. Gondwana Res. 2008, 14, 569–586. [Google Scholar] [CrossRef]
- Janeczek, J.; Ewing, R.C. Mechanisms of lead release from uraninite in the natural fission reactors in Gabon. Geochim. Cosmochim. Acta 1995, 59, 1917–1931. [Google Scholar] [CrossRef]
- Alexander, P.; Kyser, K.; Layton-matthews, D.; Joy, B. Chemical compositions of natural uraninite. Can. Mineral. 2015, 53, 595–622. [Google Scholar] [CrossRef]
- René, M.; Dolnícek, Z.; Sejkora, J.; Škácha, P.; Šrein, V. Uraninite, coffinite and ningyoite from vein-type uranium deposits of the Bohemian Massif (central European Variscan belt). Minerals. 2019, 9, 123. [Google Scholar] [CrossRef]
- Syverson, D.D.; Etschmann, B.; Liu, W.; Ram, R.; Mei, Y.; Lanzirotti, T.; Mercadier, J.; Brugger, J. Oxidation state and coordination environment of Pb in U-bearing minerals. Geochim. Cosmochim. Acta 2019, 265, 109–131. [Google Scholar] [CrossRef]
- Edwards, C.; Oliver, A. Uranium processing: A review of current methods and technology. JOM. 2000, 52, 12–20. [Google Scholar] [CrossRef]
- Youlton, B.; Kinnaird, J. Gangue-reagent interactions during acid leaching of uranium. Miner. Eng. 2013, 52, 62–73. [Google Scholar] [CrossRef]
- Ram, R.; Charalambous, F.; McMaster, S.; Pownceby, M.; Tardio, J.; Bhargava, S. Chemical and micro-structural characterisation studies on natural uraninite and associated gangue minerals. Miner. Eng. 2013, 45, 159–169. [Google Scholar] [CrossRef]
- Ulrich, K.U.; Ilton, E.S.; Veeramani, H.; Sharp, J.O.; Rizlan, B.L.; Schofield, E.J.; Bargar, J.R.; Giammar, D.E. Comparative dissolution kinetics of biogenic and chemogenic uraninite under oxidizing conditions in the presence of carbonate. Geochim. Cosmochim. Acta 2009, 73, 6065–6083. [Google Scholar] [CrossRef]
- Broczkowski, M.E.; Noel, J.J.; Shoesmith, D.W. The influence of temperature on the anodic oxidation/dissolution of uranium dioxide. Electrochim. Acta 2007, 52, 7386–7395. [Google Scholar] [CrossRef]
- Gaulard-Balandret, C.; Larabigruet, N.; Miserque, F.; Radwan, J.; Ferry, C.; Chausse, A. Kinetics of oxidation and dissolution of uranium dioxide in aqueous acid solutions. Electrochim. Acta 2012, 83, 471–477. [Google Scholar] [CrossRef]
- Ram, R.; Charalambous, F.A.; McMaster, S.; Pownceby, M.I.; Tardio, J.; Bhargava, S.K. An investigation on the dissolution of synthetic and natural uraninite ores. Miner. Eng. 2013, 50–51, 83–92. [Google Scholar] [CrossRef]
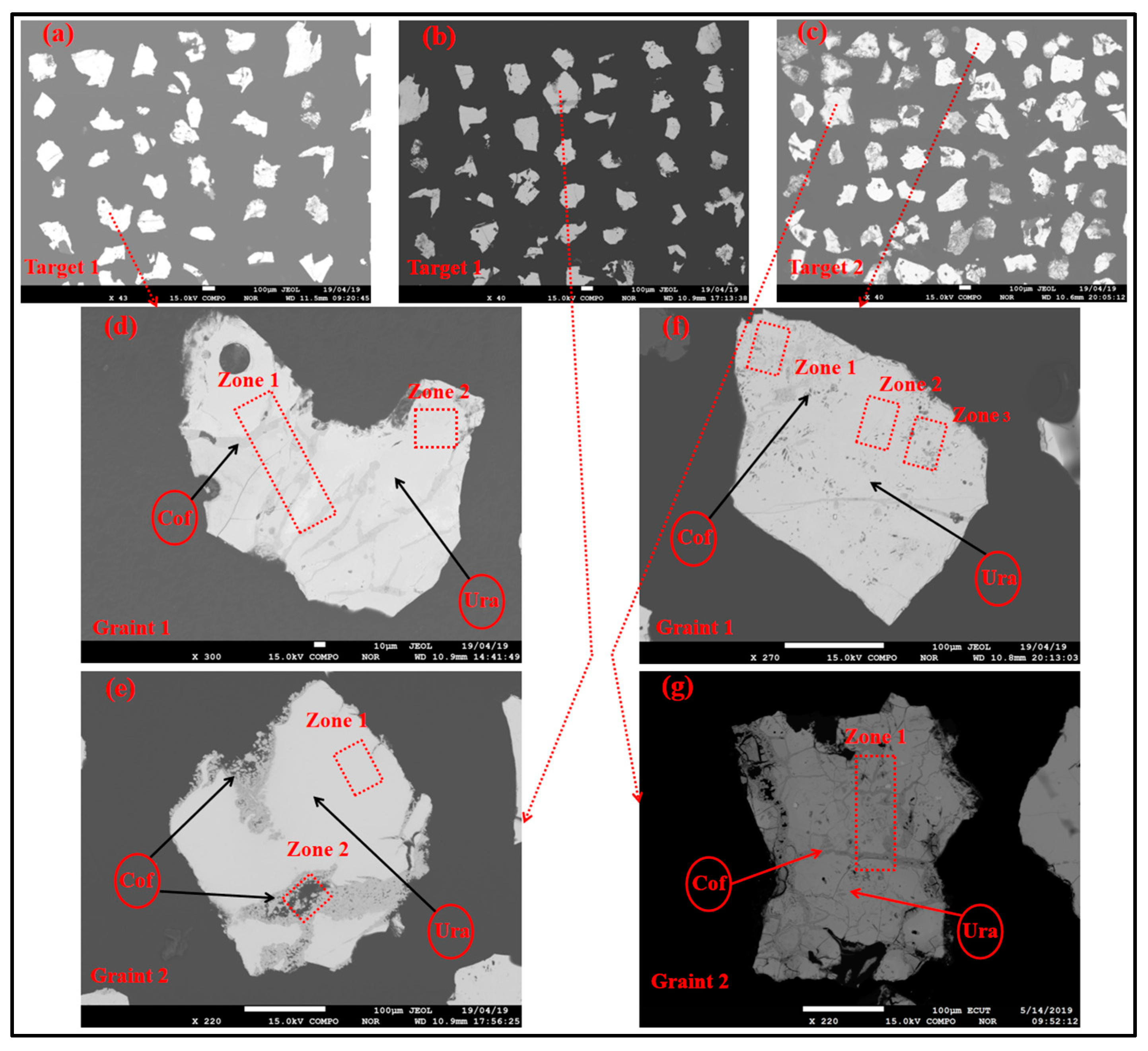

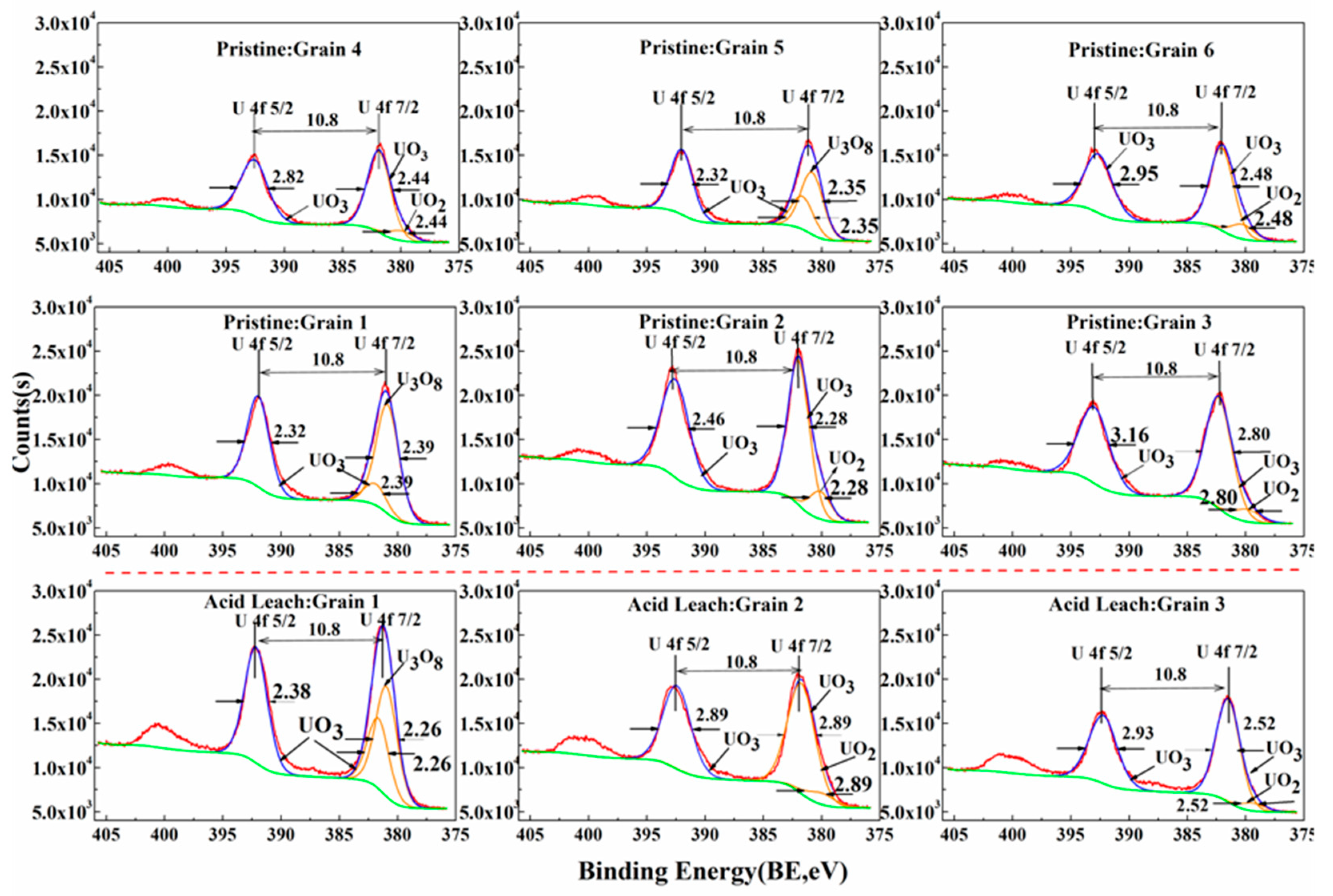
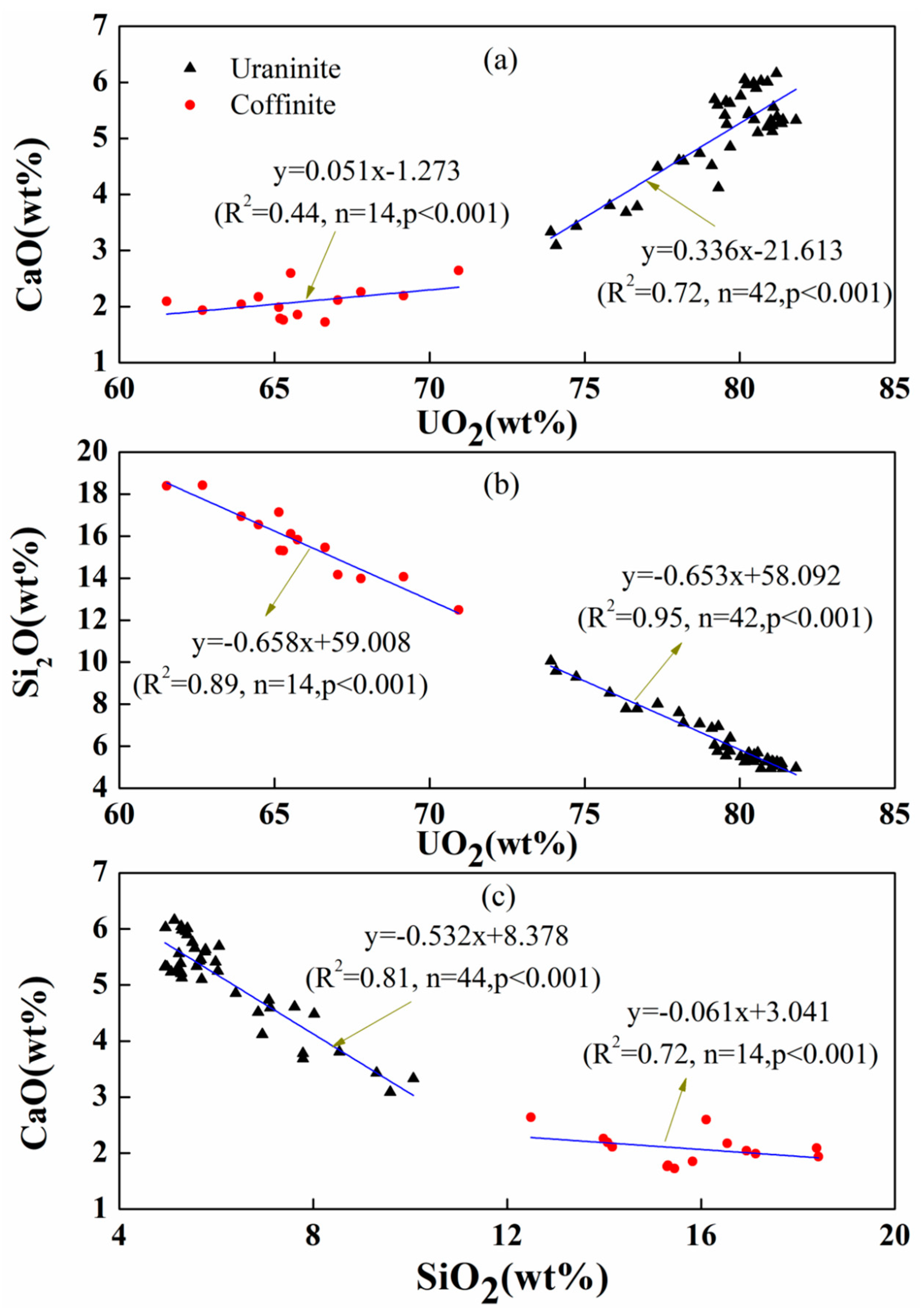
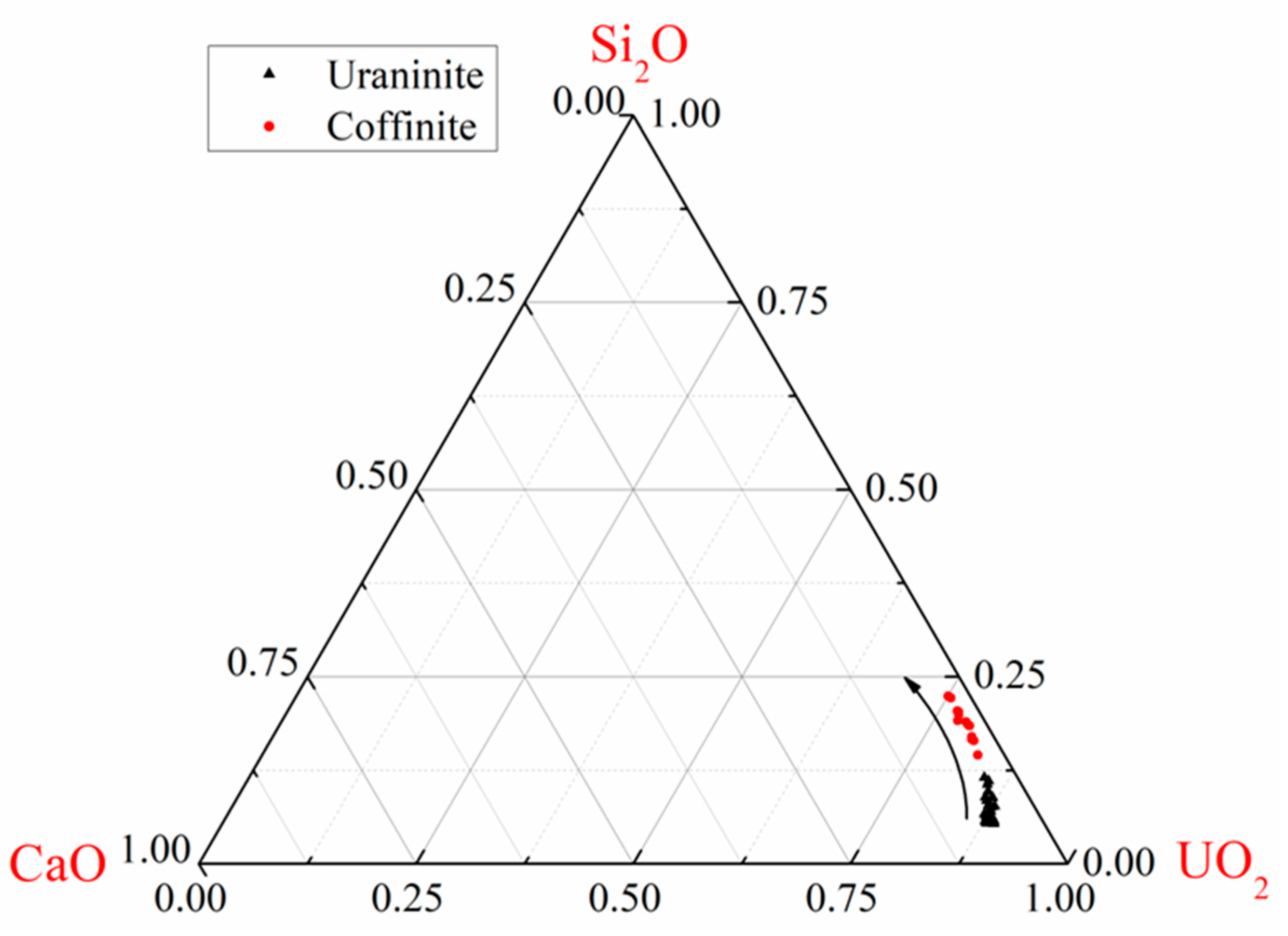
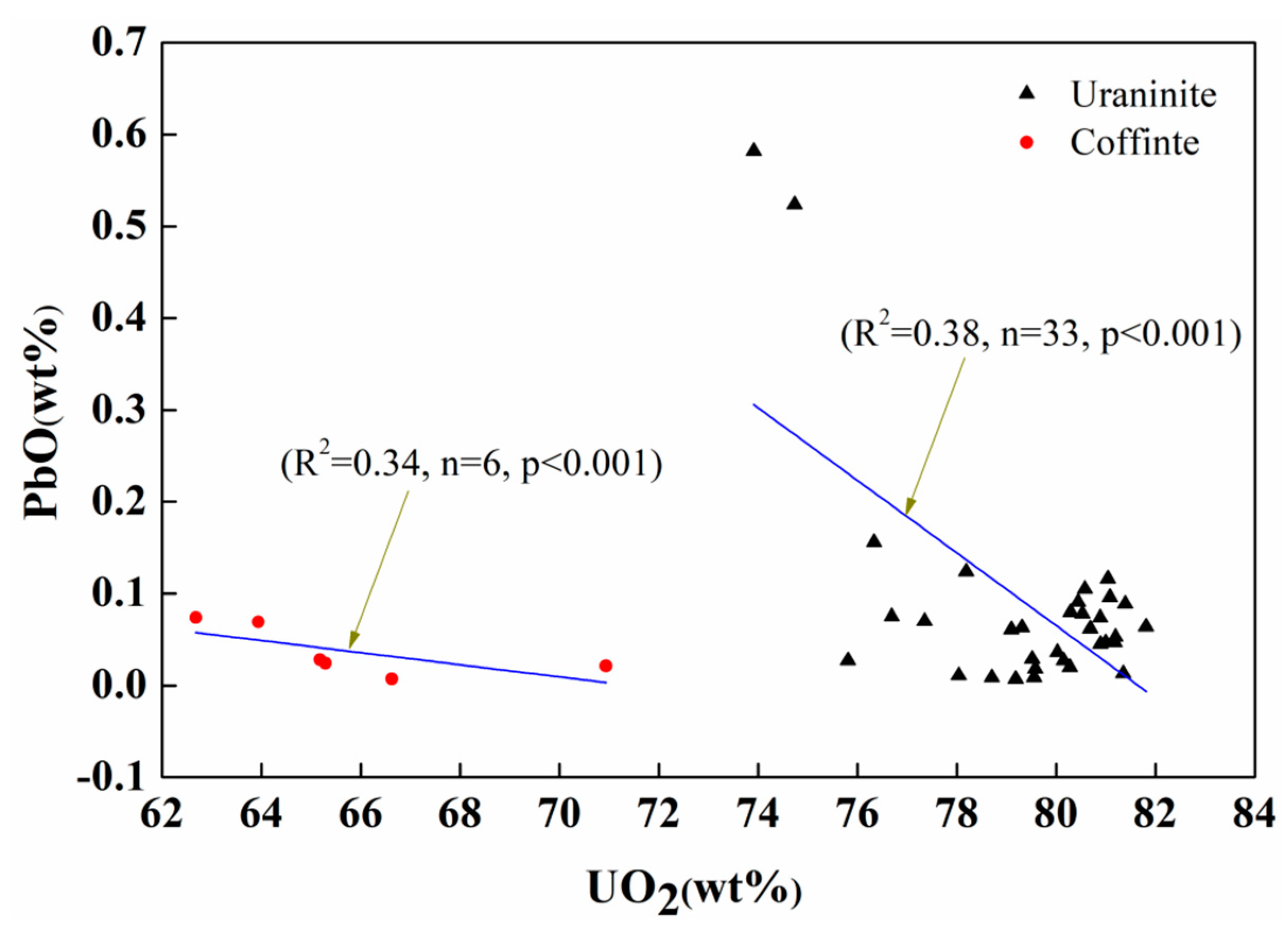

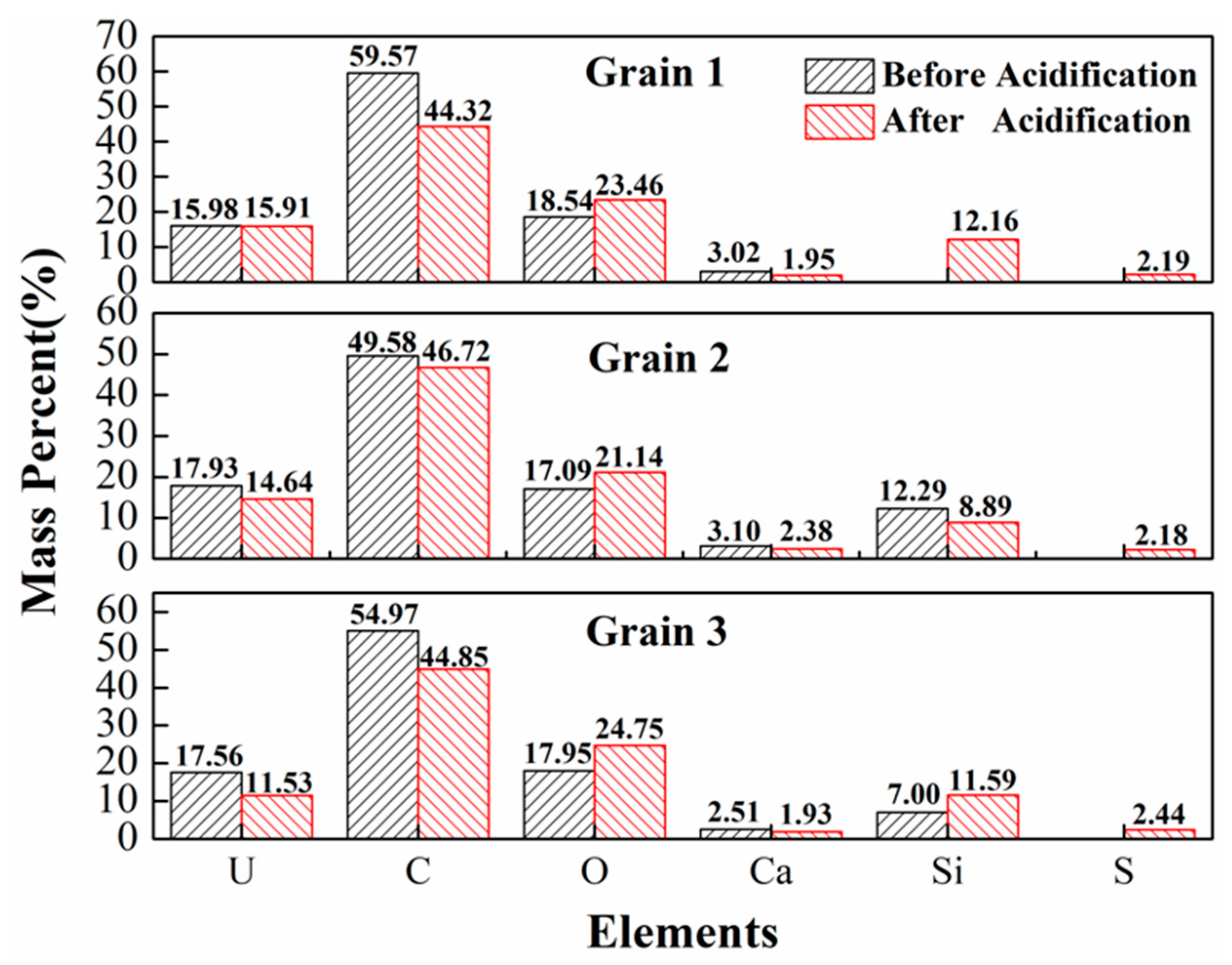

| Spot No. | Uranium Mineral | Number of Measuring Points | Characteristic Value | UO2 | SiO2 | CaO | FeO | Al2O3 | Ce2O3 | Na2O | TiO2 | P2O5 | PbO | MgO | ZrO2 | NiO | K2O | ThO2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1, 1-2 | Uraninite | 25 | Maximum | 81.81 | 10.07 | 5.56 | 0.72 | 1.06 | 0.52 | 0.30 | 0.33 | 0.08 | 0.58 | 0.03 | 0.04 | 0.05 | n.d | n.d | 93.85 |
| Minimum | 73.91 | 4.94 | 3.09 | 0.33 | 0.47 | 0.13 | 0.06 | 0.09 | 0.01 | 0.01 | n.d | n.d | n.d | n.d | n.d | 90.00 | |||
| Average | 79.15 | 6.52 | 4.69 | 0.52 | 0.64 | 0.31 | 0.15 | 0.21 | 0.04 | 0.12 | 0.01 | 0.02 | 0.02 | n.d | n.d | 92.41 | |||
| Standard Deviation | 2.51 | 1.60 | 0.76 | 0.11 | 0.18 | 0.10 | 0.05 | 0.09 | 0.02 | 0.16 | 0.01 | 0.02 | 0.02 | n.d | n.d | 1.30 | |||
| Coffinite | 11 | Maximum | 70.94 | 18.42 | 2.64 | 0.30 | 1.24 | 0.60 | 0.21 | 0.14 | 0.53 | 0.61 | 0.09 | 0.03 | 0.03 | n.d | n.d | 87.95 | |
| Minimum | 61.52 | 12.49 | 1.72 | 0.06 | 0.69 | 0.13 | n.d | 0.03 | 0.07 | n.d | n.d | 0.02 | 0.01 | n.d | n.d | 83.71 | |||
| Average | 65.71 | 15.64 | 2.08 | 0.15 | 0.98 | 0.35 | 0.07 | 0.06 | 0.27 | 0.13 | 0.03 | 0.03 | 0.02 | n.d | n.d | 85.38 | |||
| Standard Deviation | 2.51 | 1.77 | 0.32 | 0.08 | 0.17 | 0.16 | 0.07 | 0.05 | 0.17 | 0.24 | 0.03 | 0.01 | 0.01 | n.d | n.d | 1.21 | |||
| 2-1 | Uraninite | 17 | Maximum | 81.18 | 8.02 | 6.16 | 0.77 | 0.55 | 0.48 | 0.23 | 0.15 | 0.06 | 0.09 | 0.04 | 0.05 | 0.09 | n.d | n.d | 93.92 |
| Minimum | 77.35 | 4.95 | 4.49 | 0.36 | 0.39 | 0.17 | 0.07 | 0.07 | 0.01 | 0.01 | 0.01 | 0.01 | n.d | n.d | n.d | 91.43 | |||
| Average | 79.93 | 5.70 | 5.67 | 0.59 | 0.46 | 0.30 | 0.14 | 0.10 | 0.03 | 0.05 | 0.02 | 0.03 | 0.04 | n.d | n.d | 93.01 | |||
| Standard Deviation | 0.87 | 0.68 | 0.41 | 0.11 | 0.06 | 0.08 | 0.04 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 | 0.04 | n.d | n.d | 0.62 | |||
| Coffinite | 3 | Maximum | 69.16 | 17.13 | 2.19 | 0.19 | 0.96 | 0.54 | n.d | n.d | 0.25 | n.d | 0.05 | n.d | n.d | n.d | n.d | 87.32 | |
| Minimum | 63.93 | 14.07 | 1.99 | 0.10 | 0.46 | 0.12 | n.d | n.d | 0.10 | n.d | 0.02 | n.d | n.d | n.d | n.d | 83.95 | |||
| Average | 66.08 | 16.05 | 2.07 | 0.14 | 0.74 | 0.33 | n.d | n.d | 0.18 | n.d | 0.03 | n.d | n.d | n.d | n.d | 85.67 | |||
| Standard Deviation | 2.74 | 1.71 | 0.11 | 0.05 | 0.25 | 0.21 | n.d | n.d | 0.08 | n.d | 0.02 | n.d | n.d | n.d | n.d | 1.68 |
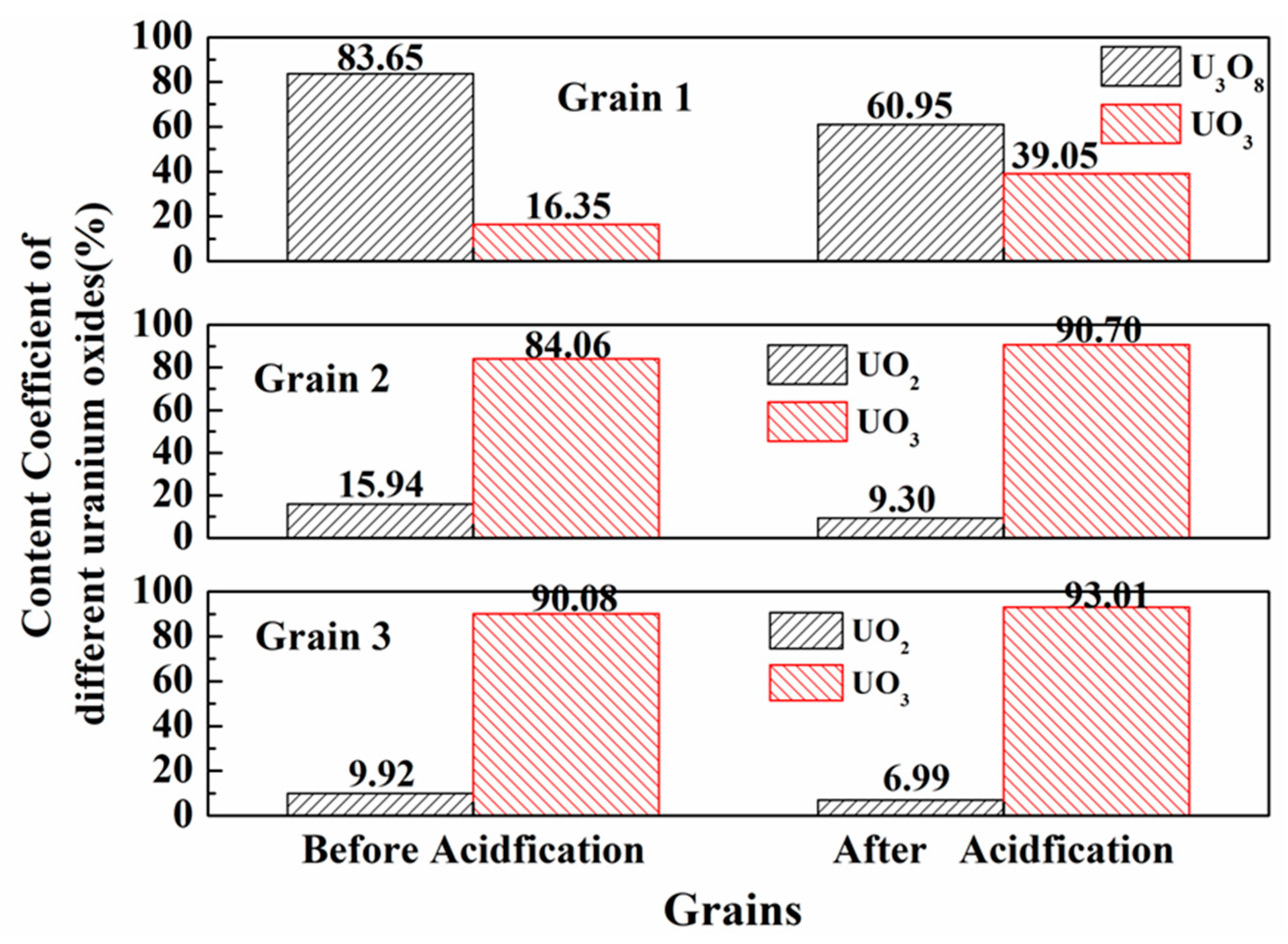
| Spot No. | Oxygen Coefficient, k0 | Mass Percentage, % | ||
|---|---|---|---|---|
| UO2 | U3O8 | UO3 | ||
| Grain 1 (pristine) | 2.72 | n.d | 83.65 | 16.35 |
| Grain 1 (acid leaching) | 2.79 | n.d | 60.95 | 39.05 |
| Grain 2 (pristine) | 2.84 | 15.94 | n.d | 84.06 |
| Grain 2 (acid leaching) | 2.91 | 9.30 | n.d | 90.70 |
| Grain 3 (pristine) | 2.90 | 9.92 | n.d | 90.08 |
| Grain 3 (acid leaching) | 2.93 | 6.99 | n.d | 93.01 |
| Grain 4 (pristine) | 2.89 | 10.89 | n.d | 89.11 |
| Grain 5 (pristine) | 2.78 | n.d | 65.32 | 34.68 |
| Grain 6 (pristine) | 2.85 | 15.15 | n.d | 84.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Sun, Z.; Li, G.; Liu, Y.; Zhou, Z.; Wang, X.; Zheng, Z.; Zhou, Y.; Zhao, K.; Xiang, L.; et al. Geochemistry and Acid Hydrometallurgy Accessibility of Uraninite from Mianhuakeng Granite-Hosted Uranium Deposit, South China. Minerals 2020, 10, 747. https://doi.org/10.3390/min10090747
Wang J, Sun Z, Li G, Liu Y, Zhou Z, Wang X, Zheng Z, Zhou Y, Zhao K, Xiang L, et al. Geochemistry and Acid Hydrometallurgy Accessibility of Uraninite from Mianhuakeng Granite-Hosted Uranium Deposit, South China. Minerals. 2020; 10(9):747. https://doi.org/10.3390/min10090747
Chicago/Turabian StyleWang, Jian, Zhanxue Sun, Guangrong Li, Yajie Liu, Zhongkui Zhou, Xuegang Wang, Zhihong Zheng, Yipeng Zhou, Kai Zhao, Ling Xiang, and et al. 2020. "Geochemistry and Acid Hydrometallurgy Accessibility of Uraninite from Mianhuakeng Granite-Hosted Uranium Deposit, South China" Minerals 10, no. 9: 747. https://doi.org/10.3390/min10090747
APA StyleWang, J., Sun, Z., Li, G., Liu, Y., Zhou, Z., Wang, X., Zheng, Z., Zhou, Y., Zhao, K., Xiang, L., & Wei, J. (2020). Geochemistry and Acid Hydrometallurgy Accessibility of Uraninite from Mianhuakeng Granite-Hosted Uranium Deposit, South China. Minerals, 10(9), 747. https://doi.org/10.3390/min10090747





