Removal of Copper from Mining Wastewater Using Natural Raw Material—Comparative Study between the Synthetic and Natural Wastewater Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Characterization
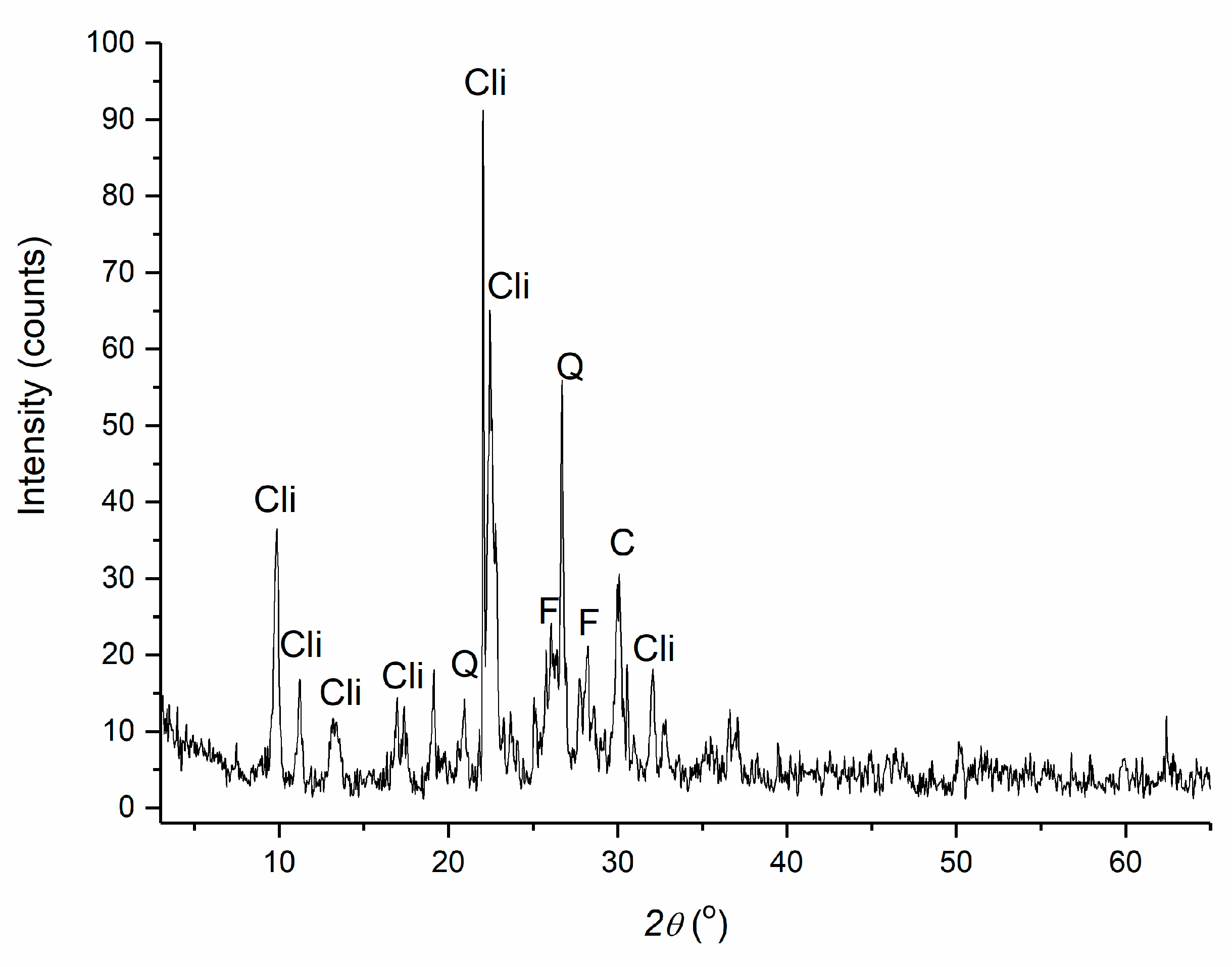
2.1.1. Clinoptilolite
- Below 0.043 mm—optimal for the zeolite characterization and investigations of maximum adsorption capacity.
- 0.6–0.8 mm—optimal for the flow-through experiments due to better hydraulic properties than smaller (micron) fractions.
2.1.2. Wastewater
2.2. Adsorption Experiments
2.2.1. Batch System
2.2.2. Flow-through System
3. Results and Discussion
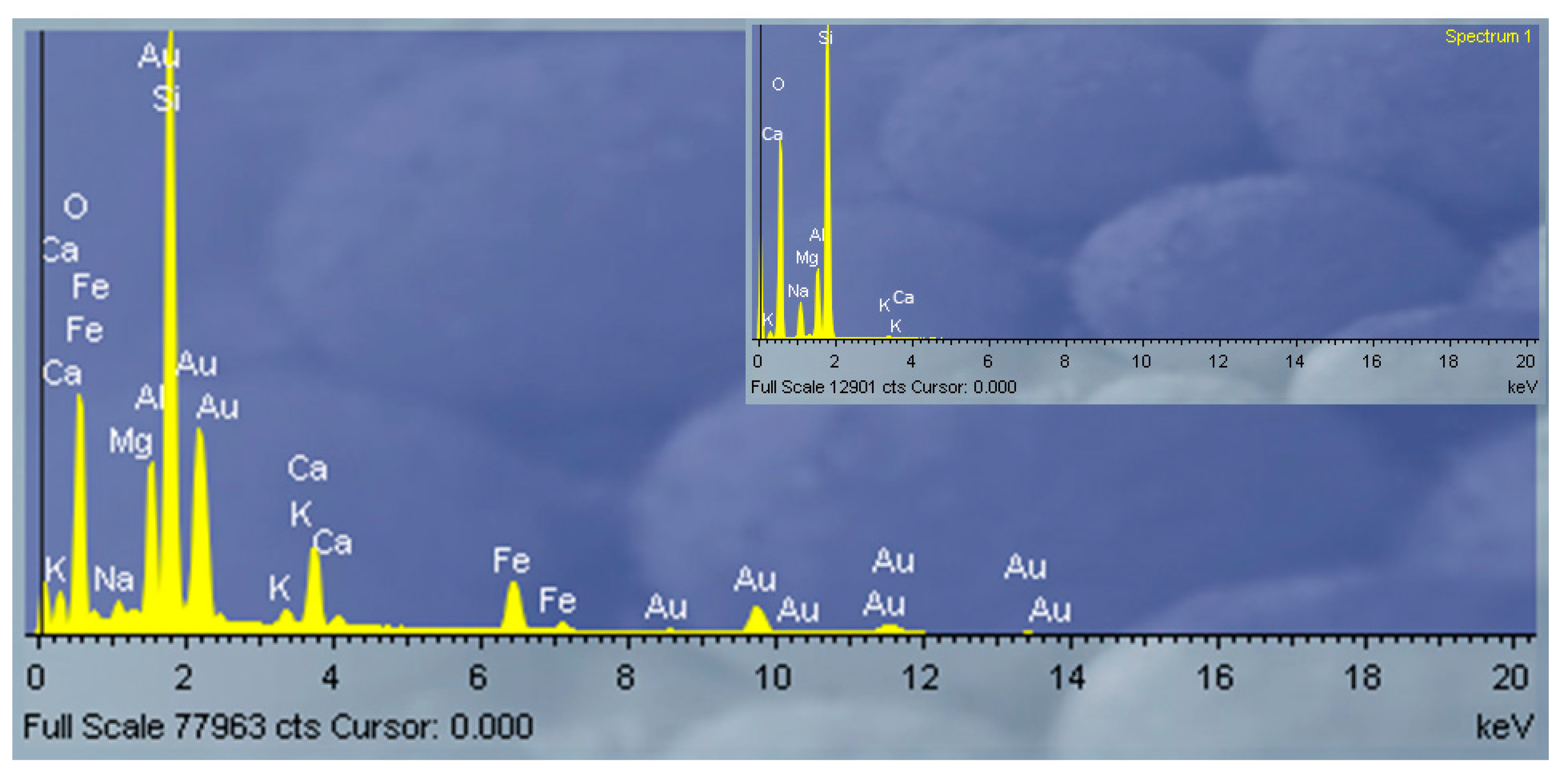
3.1. Clinoptilolite Characterization
3.2. Adsorption Experiments
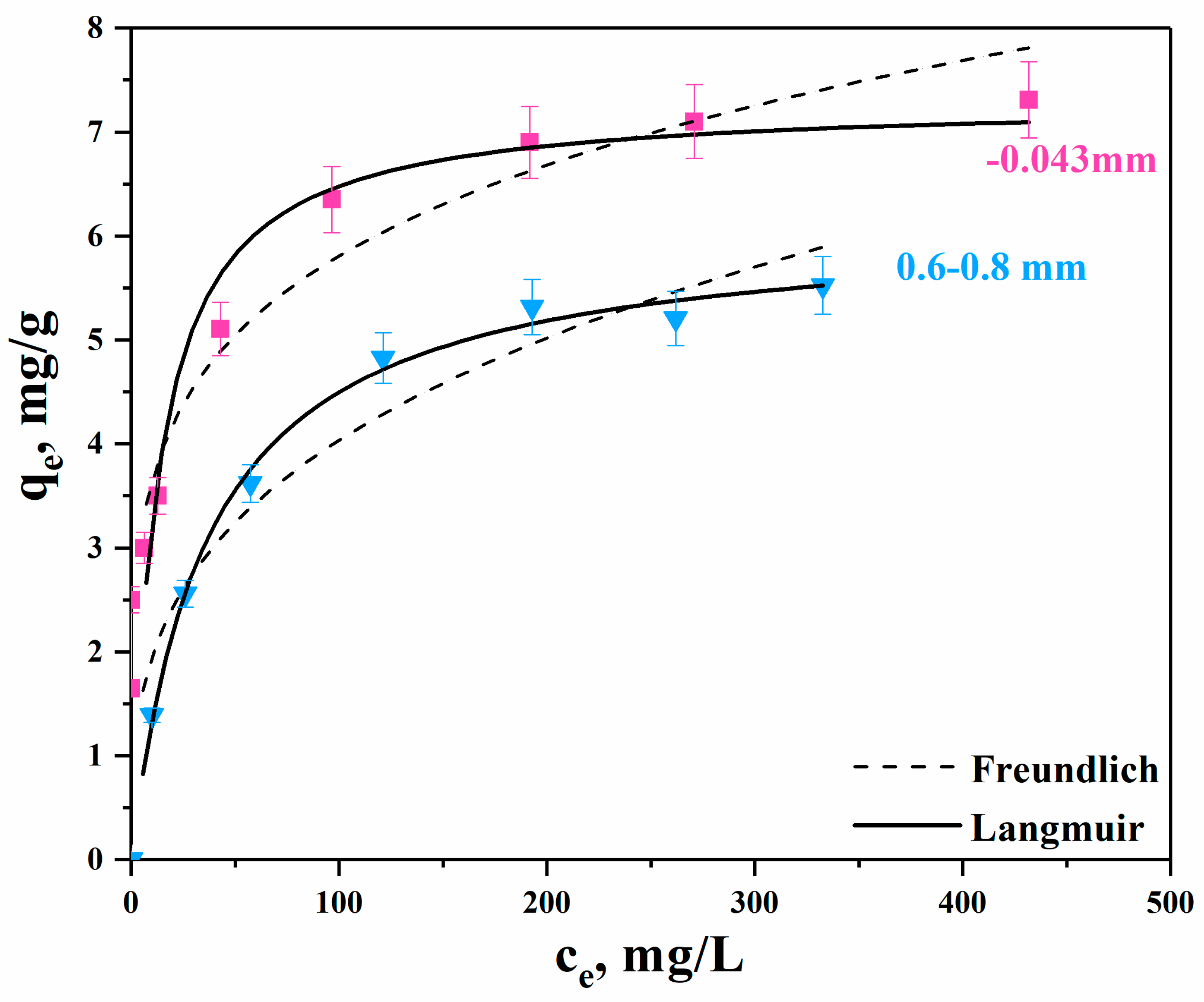
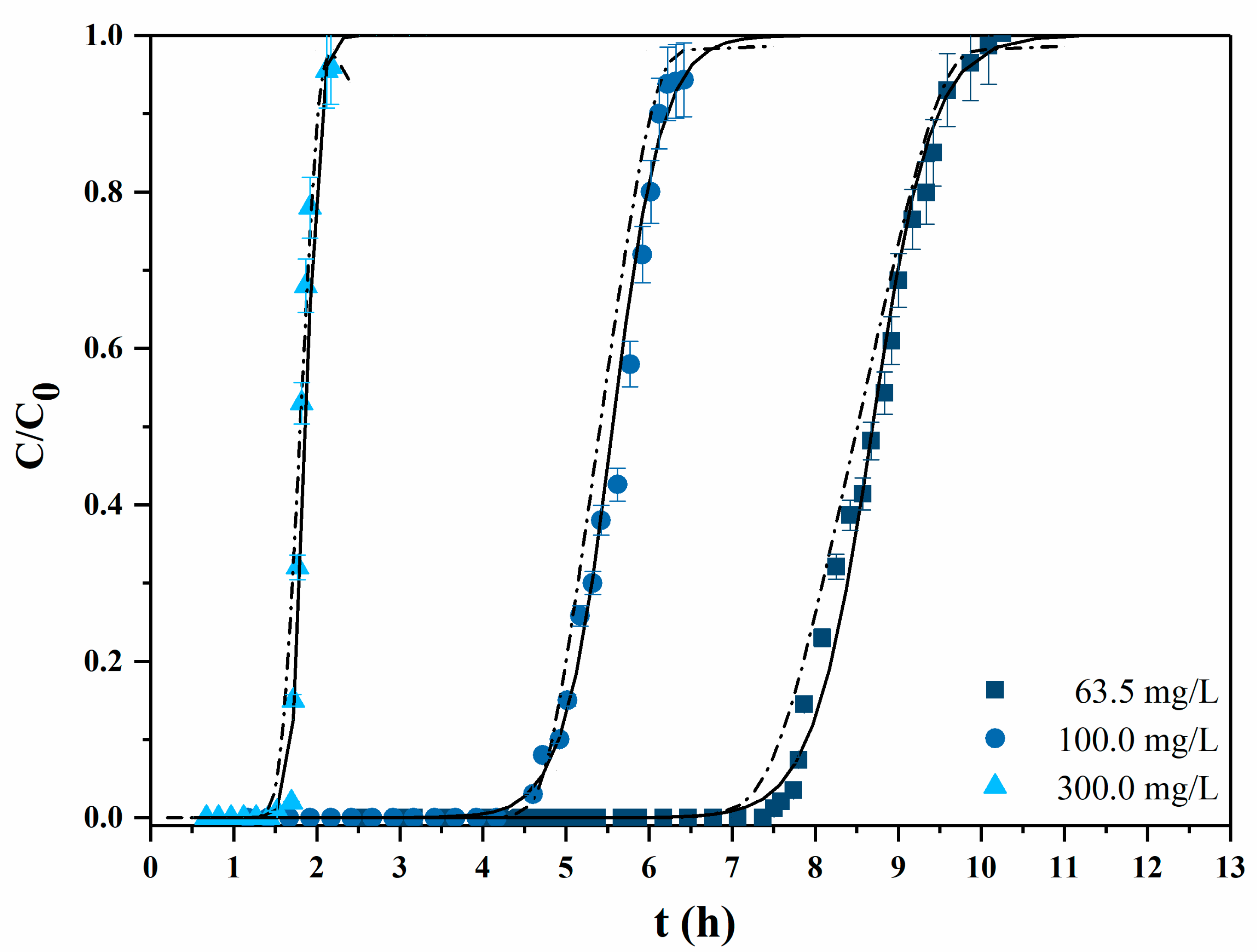
3.2.1. Experiments with Synthetic Solutions
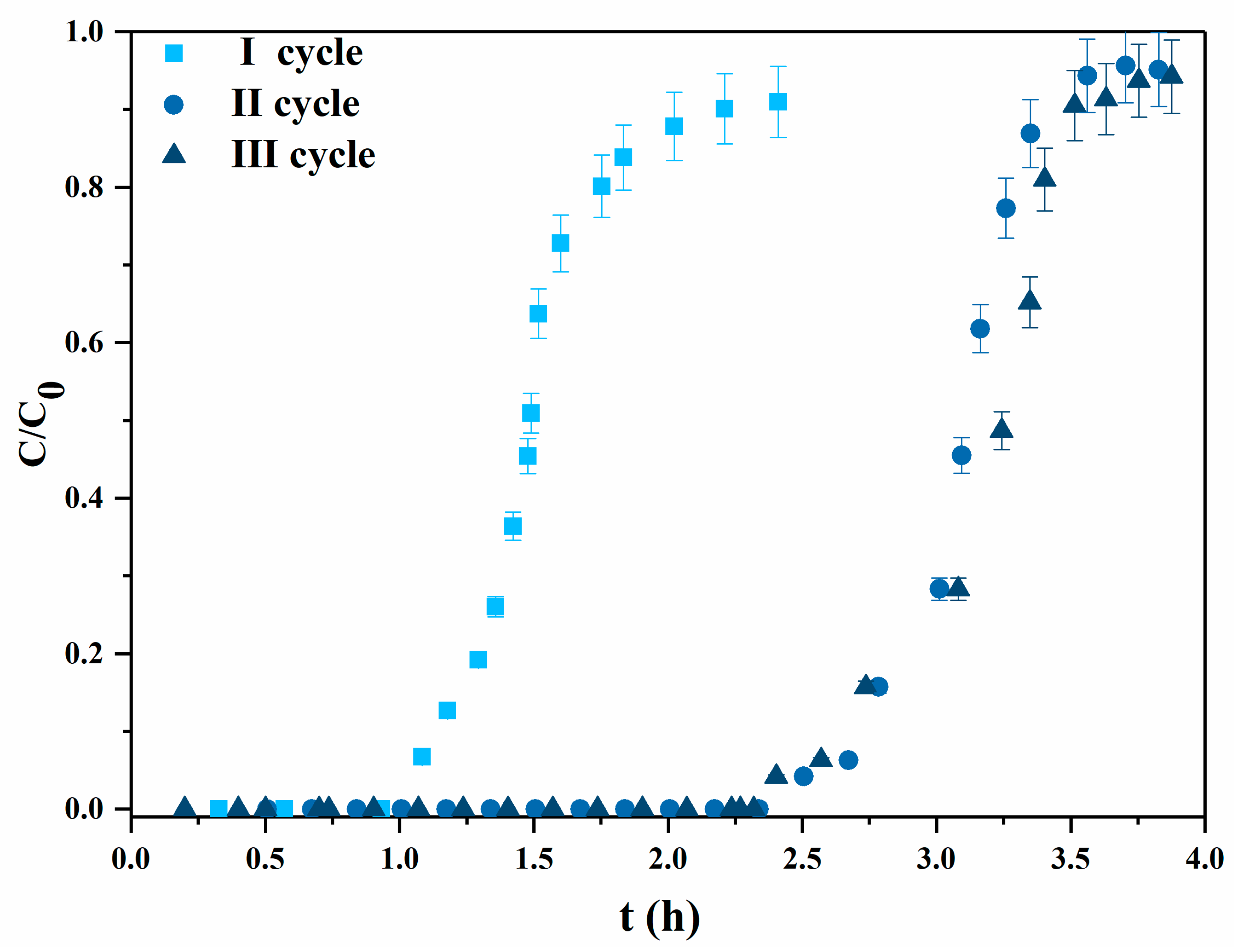
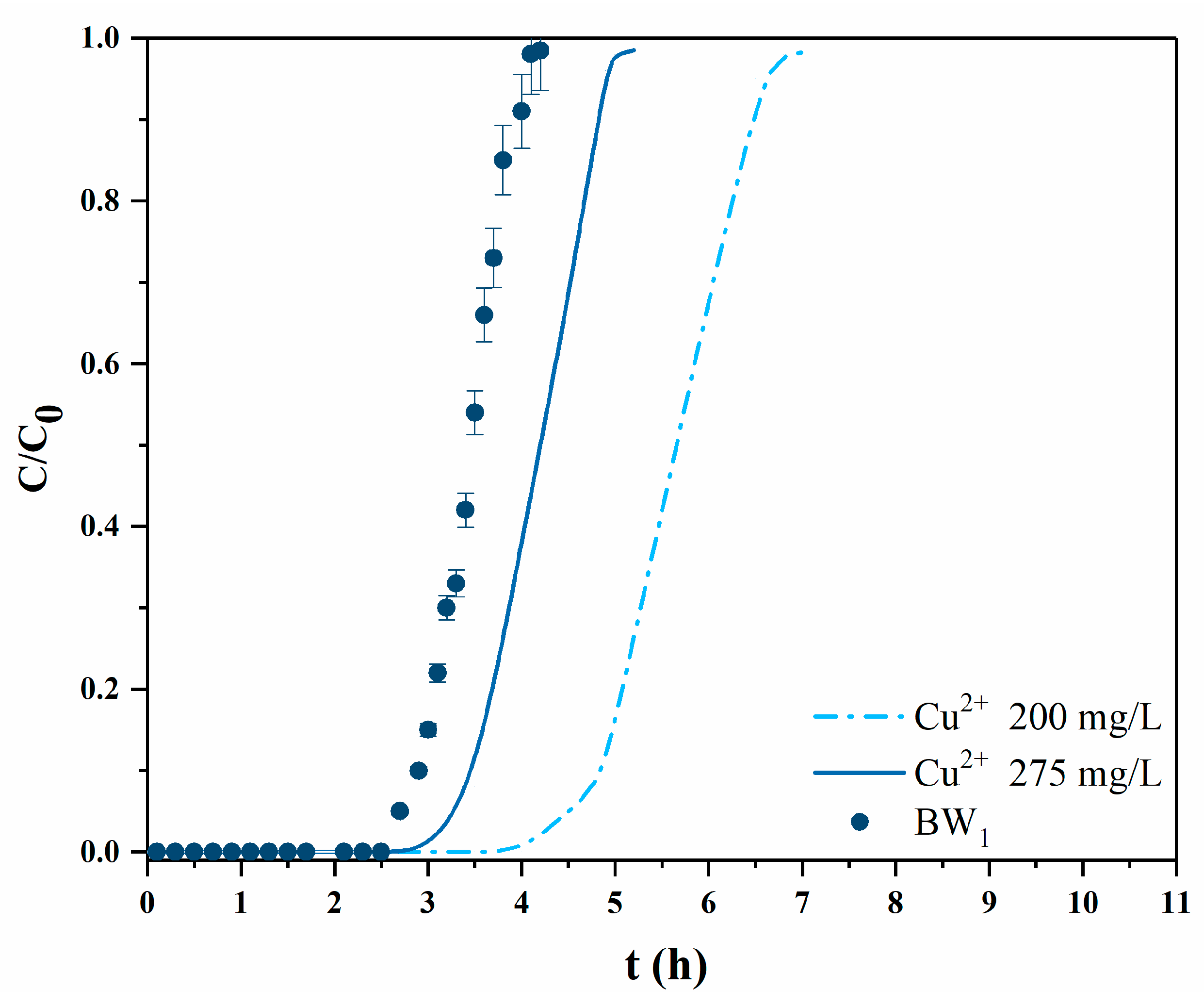
3.2.2. Experiments with Mining Wastewater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Šljivić, M.; Smičiklas, I.; Pejanović, S.; Plećaš, I. Comparative study of Cu2+ adsorption on a zeolite, a clay and a diatomite from Serbia. Appl. Clay Sci. 2009, 43, 33–40. [Google Scholar] [CrossRef]
- Esaifan, M.; Warr, L.N.; Grathoff, G.; Meyer, T.; Schafmeister, M.-T.; Kruth, A.; Testrich, H. Synthesis of hydroxy-sodalite/cancrinite zeolites from calcite-bearing kaolin for the removal of heavy metal ions in aqueous media. Minerals 2019, 9, 484. [Google Scholar] [CrossRef] [Green Version]
- Zayed, A.M.; Selim, A.Q.; Mohamed, E.A.; Wahed, M.S.A.; Seliem, M.K.; Sillanpää, M. Adsorption characteristics of Na-A zeolites synthesized from Egyptian kaolinite for manganese in aqueous solutions: Response surface modeling and optimization. Appl. Clay Sci. 2017, 140, 17–24. [Google Scholar] [CrossRef]
- Bai, X.; Wen, S.; Feng, Q.; Liu, J.; Lin, Y.L.; Yao, Z.H. A New Technique for Recovering Copper From Complex Copper Oxide Ore by Flotation and Metallurgical Processing. JOM 2019, 71, 784–790. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Isa, I.M. Comparison study of copper ion adsorption on chitosan, Dowex A-1, and Zerolit 225. J. Appl. Polym. Sci. 1998, 67, 1067–1070. [Google Scholar] [CrossRef]
- Crini, G. Recent Developments in Polysaccharide-Based Materials Used as Adsorbents in Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Ajmal, M.; Rao, R.; Ahmad, J. Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni (II) from electroplating wastewater. J.Hazard Mater. 2000, 79, 117–131. [Google Scholar] [CrossRef]
- Amana, T.; Kazi, A.A.; Sabri, M.U.; Banoa, Q. Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surf. B 2008, 63, 116–121. [Google Scholar] [CrossRef]
- Passaglia, E.; Sheppard, R.A. The crystal chemistry of zeolites. In Natural Zeolites: Occurrence, Properties, Application; Bish, D.L., Ming, D.W., Eds.; The Mineralogical Society of America: Washington, DC, USA, 2001; pp. 66–116. [Google Scholar]
- Gottardi, G.; Galli, E. Natural Zeolites; Springer: Berlin, Germany, 1985; pp. 256–283. [Google Scholar]
- Mumpton, F.A.; Sand, L.B. Natural Zeolites: Occurrence, Properties and Use; Pergamon: New York, NY, USA, 1978; pp. 3–27. [Google Scholar]
- Dmirkou, A.; Ioannou, A.; Doula, M. Preparation, characterization and sorption properties for phosphates of hematite, bentonite and bentonite–hematite systems. Adv. Colloid Inter. Sci. 2002, 97, 37–61. [Google Scholar] [CrossRef]
- Eljamal, O.; Shubair, T.; Tahara, A.; Sugihara, Y.; Matsunaga, N.J. Iron based nanoparticles-zeolite composites for the removal of cesium from aqueous solutions. Mol. Liq. 2019, 277, 613–623. [Google Scholar] [CrossRef]
- Shubair, T.; Eljamal, O.; Tahara, A.; Sugihara, Y.; Matsunaga, N.J. Preparation of new magnetic zeolite nanocomposites for removal of strontium from polluted waters. Mol. Liq. 2019, 288, 111026. [Google Scholar] [CrossRef]
- Doula, M. Synthesis of a clinoptilolite–Fe system with high Cu sorption capacity. Chemosphere 2007, 67, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Dmirkou, A.; Theodoropoulou, E. Phosphate sorption by hematite and kaolinite-hematite (k-h) system as described by isotherms. Soil Sci. Plant Anal. 1996, 27, 1925–1947. [Google Scholar] [CrossRef]
- Morturano, P.; Drozdova, L.; Pirngruber, D.; Kogelbauer, A.; Prins, R. The mechanism of formation of the Fe species in Fe/ZSM-5 prepared by CVD. Phys. Chem. Chem. Phys. 2001, 3, 5585–5595. [Google Scholar] [CrossRef]
- Milićević, S.; Milošević, V.; Povrenović, D.; Stojanović, J.; Martinović, S.; Babić, B. Removal of heavymetals from aqueous solutions by using natural and Fe (III) oxyhydroxide clinoptilolite. Clays Clay Min. 2013, 61, 508–517. [Google Scholar] [CrossRef]
- Milićević, S.; Martinović, S.; Milošević, V.; Stojanović, J.; Povrenović, D. Differences in coating mechanism of structurally different aluminosilicates observed through the thermal analysis. J. Therm. Anal. Calorim 2018, 134, 1011–1019. [Google Scholar] [CrossRef]
- Schollenberger, C.J.; Simon, R.H. Determination of exchange capacity and exchangeable bases in soil-ammonium acetate method. Soil Sci. 1945, 59, 13–24. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; Garcia-Sanchez, A.; Querol, X. Purification of metal electroplating waste waters using zeolites. Water Res. 2003, 37, 4855. [Google Scholar] [CrossRef]
- Abdel Salam, O.E.; Reiad, N.A.; ElShafei, M.M. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Lemić, J.B.; Kovačević, D.; Tomašević-Čanović, M.; Kovačević, D.; Stanić, T.; Pfend, R. Removal of atrazine, lindane and diazinone from water by organo-zeolites. Water Res. 2006, 40, 1079–1085. [Google Scholar] [CrossRef]
- Stanić, T. Adsorption of Arsenate on Modified Clinoptilolites. Master’s Thesis, Unversity of Belgrade, Belgrade, Serbia, 2008. [Google Scholar]
- Langmuir, I.J. The adsorption of gases on plane surfaces of glass, mica and platinum. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Khayyun, T.S.; Mseer, A.H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Adsorption in Solutions. Phy. Chem. 1926, 57, 384–410. [Google Scholar]
- Choi, Y.L.; Choi, J.S.; Lingamdinne, L.P.; Chang, Y.Y.; Koduru, J.R.; Ha, J.H.; Yang, J.K. Removal of U (VI) by sugar-based magnetic pseudo–graphene oxide and its application to authentic groundwater using electromagnetic system. Environ. Sci. Pollut. Res. 2019, 26, 22323–22337. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K.J. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X. Kinetics and thermodynamics of efficient phosphorus removal by a composite fiber. Appl. Sci. 2019, 9, 2220. [Google Scholar] [CrossRef] [Green Version]
- Vukojević Medvidović, N.; Perić, J.; Trgo, M. Column performance in lead removal from aqueous solutions by fixed bed of natural zeolite–clinoptilolite. Sep. Purif. Technol. 2006, 49, 237–244. [Google Scholar] [CrossRef]
- Tasić, Ž.; Bogdanović, G.; Antonijević, M. Application of natural zeolite in wastewater treatment: A review. J. Min. Metall. A Mining 2019, 55, 67–79. [Google Scholar] [CrossRef]
- Ambrozova, P.; Kynicky, J.; Urubek, T.; Nguyen, V.D. Synthesis and modification of clinoptilolite. Molecules 2017, 22, 1107. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, P.; Sprynskyy, M.; Terzyk, A.P.; Lebedynets, M.; Namiesnik, J.; Buszewski, B.J. Porous structure of natural and modified clinoptilolites. Colloid Interface Sci. 2006, 297, 77–85. [Google Scholar] [CrossRef]
- Vučinić, D. Physicochemical Properties of the Clinoptilolite Deposit “Zlatokop”—Vranje. Master’s Thesis, University of Belgrade, Belgrade, Serbia, 1985. [Google Scholar]
- Nimibofa, A.; Ebelegi, A.; Donbebe, W. Modelling and Interpretation of Adsorption Isotherms. J. Chemistry 2017, 2017, 3039817. [Google Scholar]


| Content % | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | IOL | |
| AAS | 66.09 | 13.06 | 0.21 | 3.54 | 0.24 | 2.83 | 0.46 | 13.54 |
| EDS | 67.89 | 13.70 | 0.85 | 3.97 | 0.67 | 1.26 | 0.80 | 10.86 |
| Freundlich | R2 | KF (L/mg) × 10−3 | β |
| −0.043 mm Cli | 0.951 | 1.31 | 0.203 |
| 0.6–0.8 mm Cli | 0.935 | 0.87 | 0.315 |
| Langmuir | R2 | qm (mg/g) | KL (L/mg) |
| −0.043 mm Cli | 0.964 | 7.30 | 0.08 |
| 0.6–0.8 mm Cli | 0.994 | 6.10 | 0.03 |
| c0 (mg/L) | Q (ml/min) | qb (mg/g) | qS (mg/g) | η (%) | |
|---|---|---|---|---|---|
| I | 63.5 | 3 | 4.13 | 7.37 | 56.1 |
| II | 63.5 | 3 | 9.78 | 12.00 | 81.5 |
| III | 63.5 | 3 | 9.97 | 11.87 | 83.9 |
| IV | 63.5 | 1 | 9.91 | 11.24 | 88.1 |
| V | 100.0 | 1 | 9.72 | 11.62 | 83.6 |
| VI | 300.0 | 1 | 9.78 | 12.19 | 80.2 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| pH | 1.89 | 2.43 | 2.87 | 4.75 | 3.65 | 3.89 | 3.68 | 4.22 |
| [Cu2+], mg/L | 198 | 180 | 70.8 | 44.1 | 1269.6 | 1928 | 963 | 338 |
| Title | Cu | Fe | Zn | Mn | Na | K | Ca | Mg | pH |
|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | |||||||||
| BW | 205.0 | 14.6 | 27.6 | 45.1 | 84.7 | 1.27 | 480.0 | 442.0 | 2.89 |
| BW1 | 201.0 | 1.21 | 25.3 | 43.1 | 85.2 | 91.5 | 476.0 | 445.0 | 4.5 |
| Cli | 151.3 | / | 19.8 | 40.9 | 90.3 | 108.3 | 490.0 | 456.0 | 4.7 |
| c0 (Cu2+) (mg/L) | Q (cm3/min) | qb (mg/g) | qs (mg/g) | η (%) |
|---|---|---|---|---|
| BW1 200.0 | 1 | 5.84 | 7.05 | 87.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milićević, S.; Vlahović, M.; Kragović, M.; Martinović, S.; Milošević, V.; Jovanović, I.; Stojmenović, M. Removal of Copper from Mining Wastewater Using Natural Raw Material—Comparative Study between the Synthetic and Natural Wastewater Samples. Minerals 2020, 10, 753. https://doi.org/10.3390/min10090753
Milićević S, Vlahović M, Kragović M, Martinović S, Milošević V, Jovanović I, Stojmenović M. Removal of Copper from Mining Wastewater Using Natural Raw Material—Comparative Study between the Synthetic and Natural Wastewater Samples. Minerals. 2020; 10(9):753. https://doi.org/10.3390/min10090753
Chicago/Turabian StyleMilićević, Sonja, Milica Vlahović, Milan Kragović, Sanja Martinović, Vladan Milošević, Ivana Jovanović, and Marija Stojmenović. 2020. "Removal of Copper from Mining Wastewater Using Natural Raw Material—Comparative Study between the Synthetic and Natural Wastewater Samples" Minerals 10, no. 9: 753. https://doi.org/10.3390/min10090753





