Flotation Behavior of Malachite Using Hydrophobic Talc Nanoparticles as Collectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
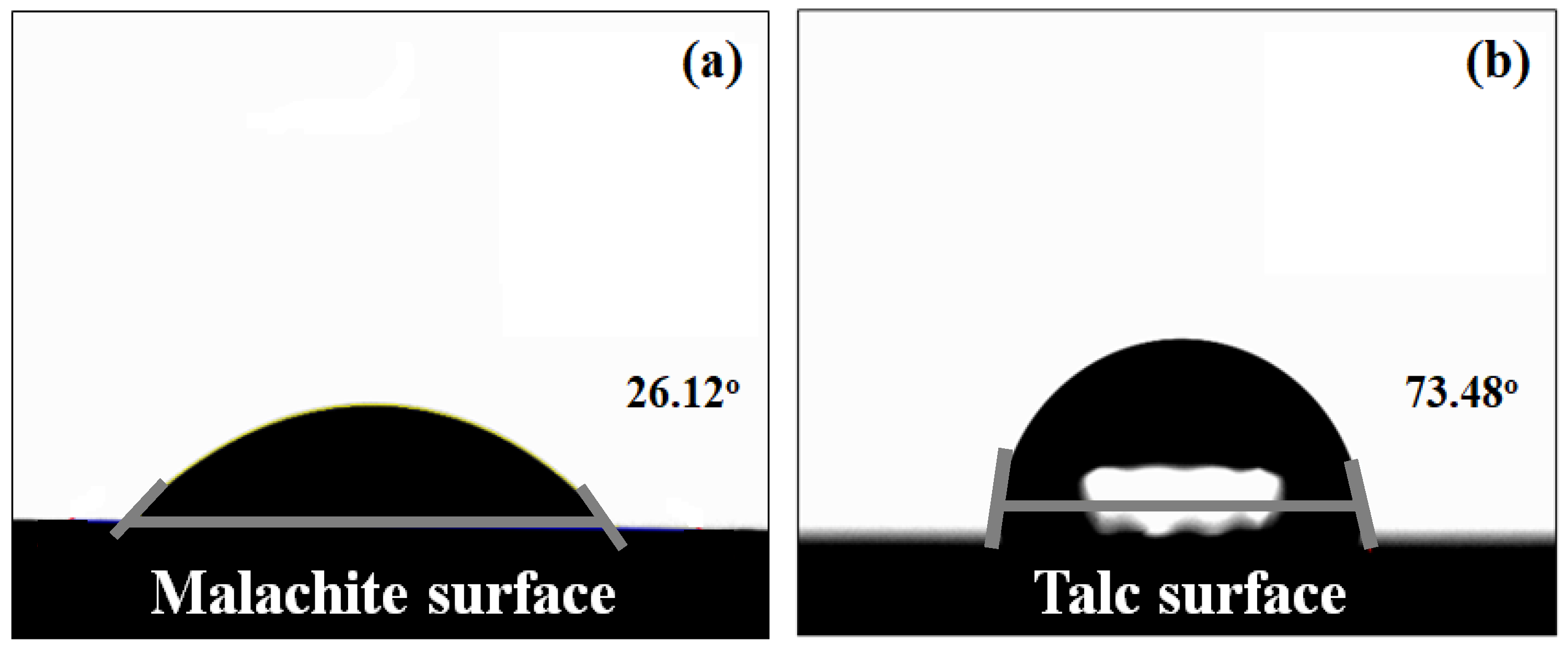
2.2. Zeta Potential and Contact Angle Measurements
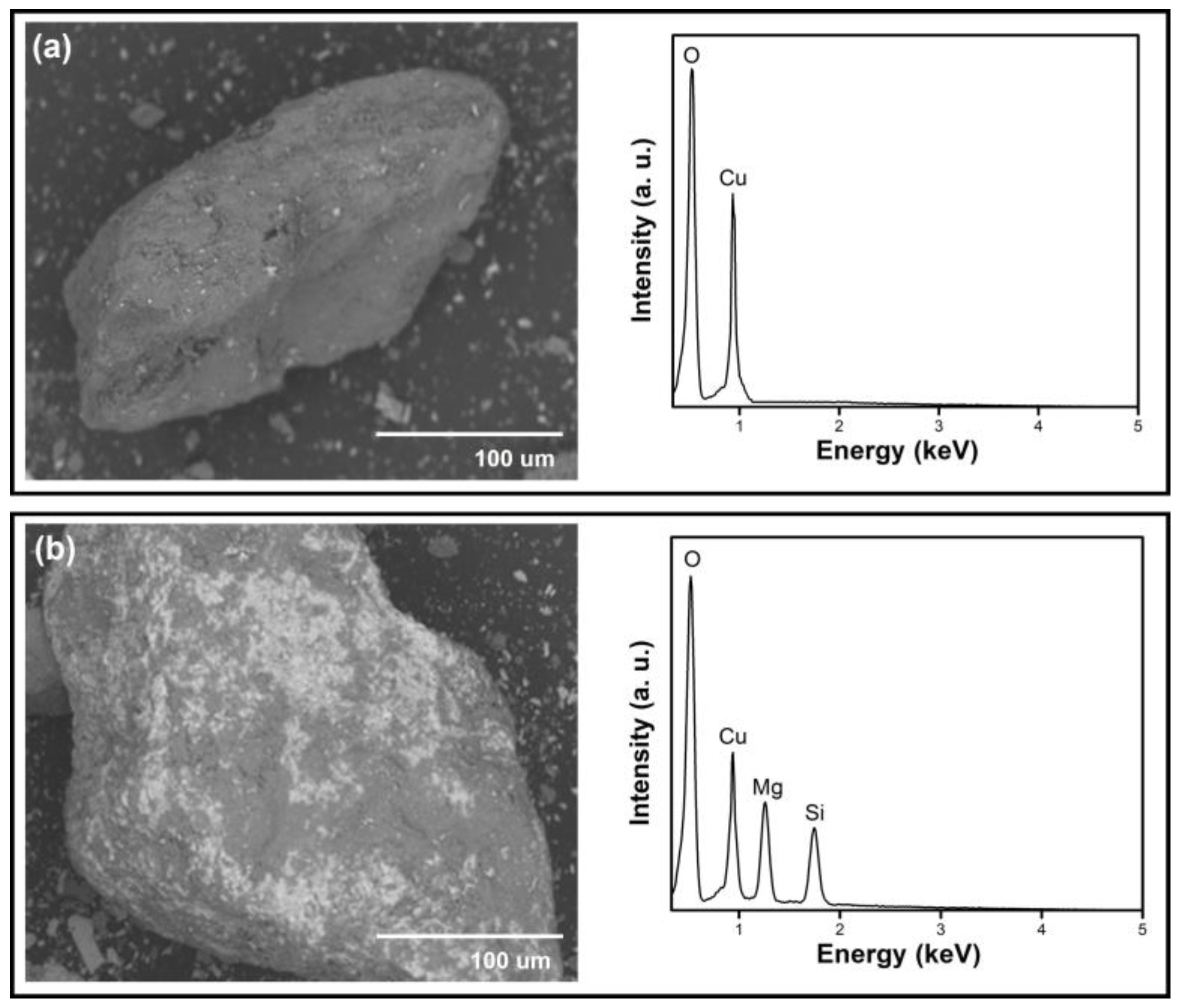
2.3. Deposition of TNs onto the Surface of the Malachite
2.4. Microflotation Tests
3. Results and Discussion
3.1. Effects of the Size of the TNs
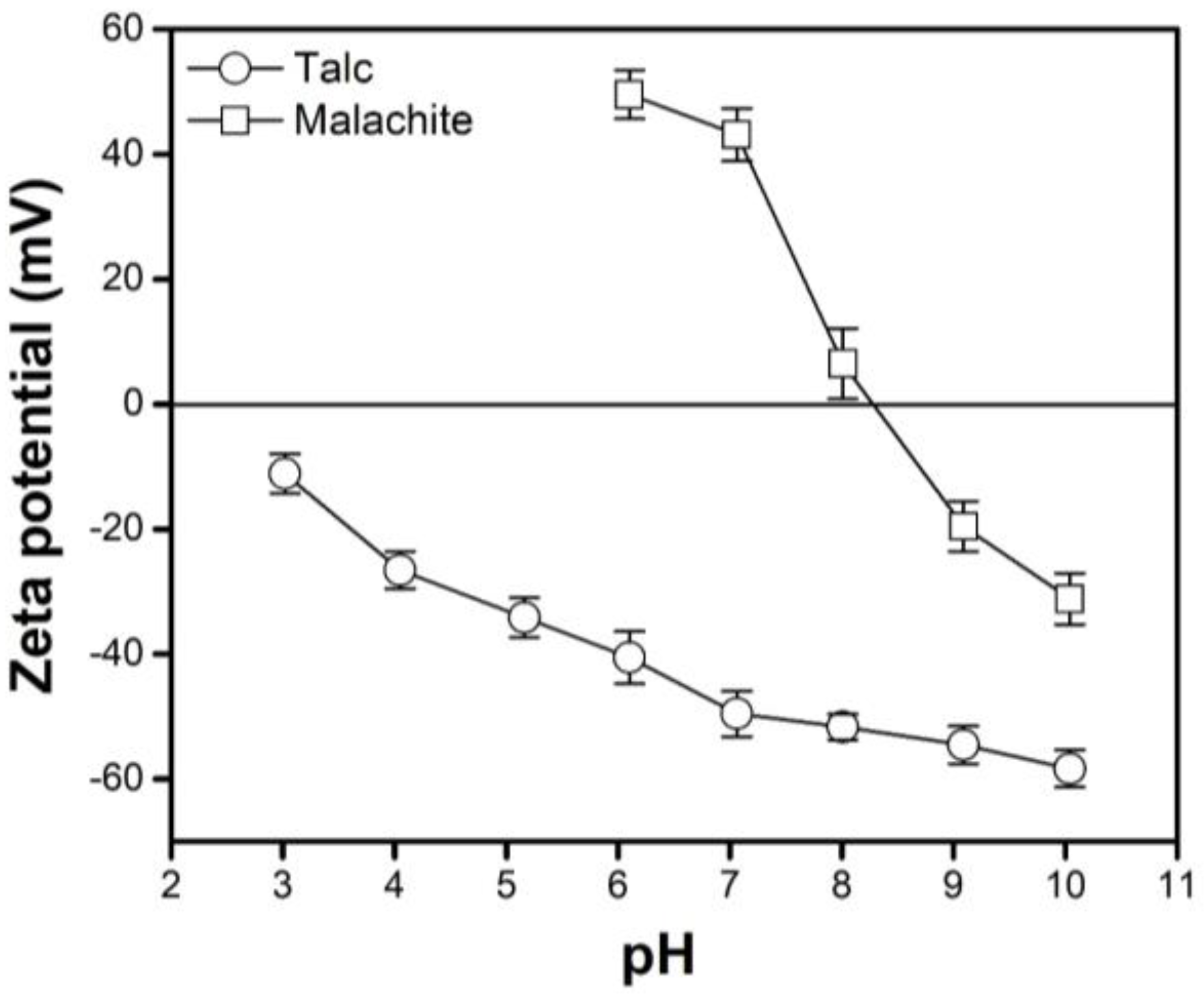
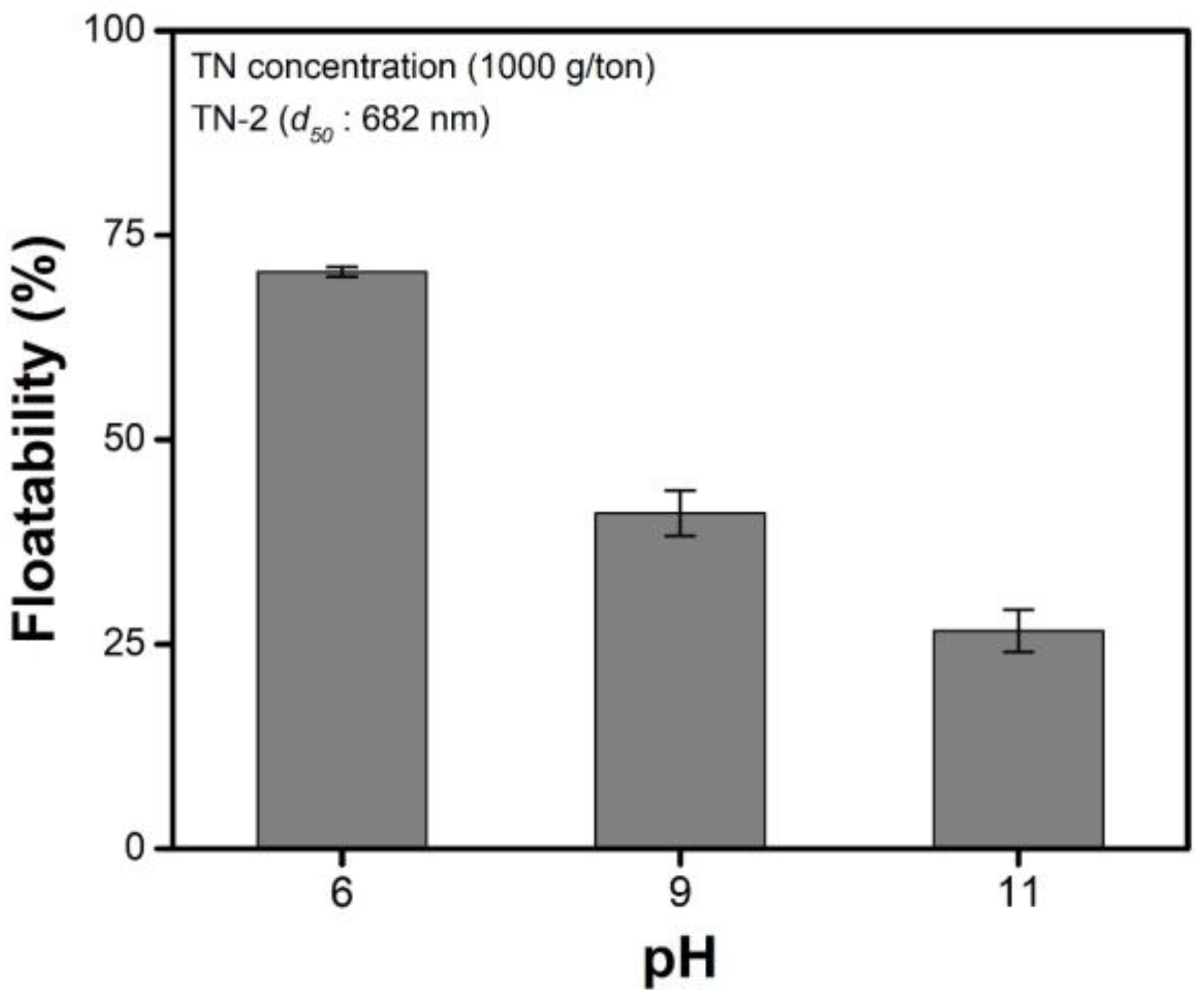
3.2. Effects of pH
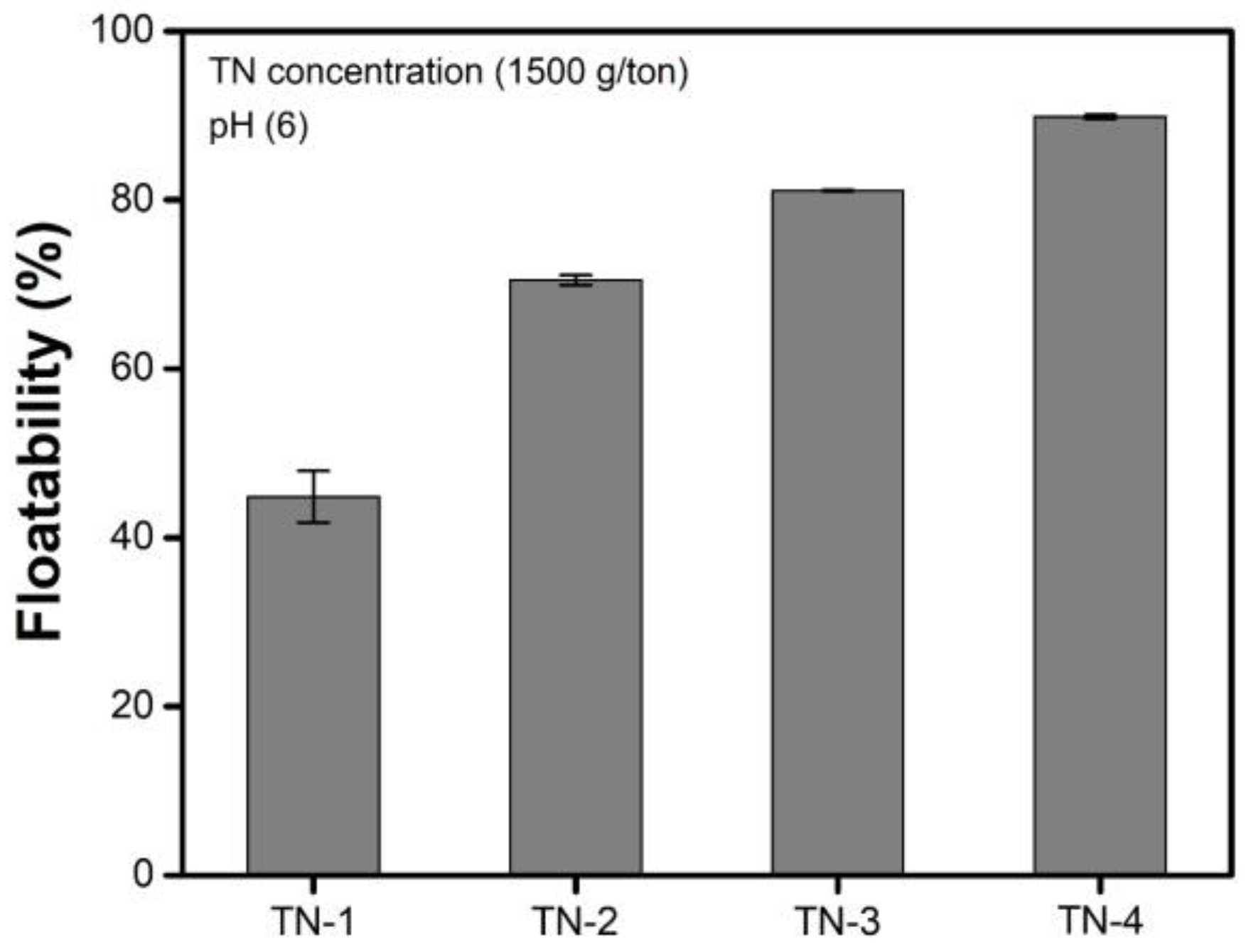
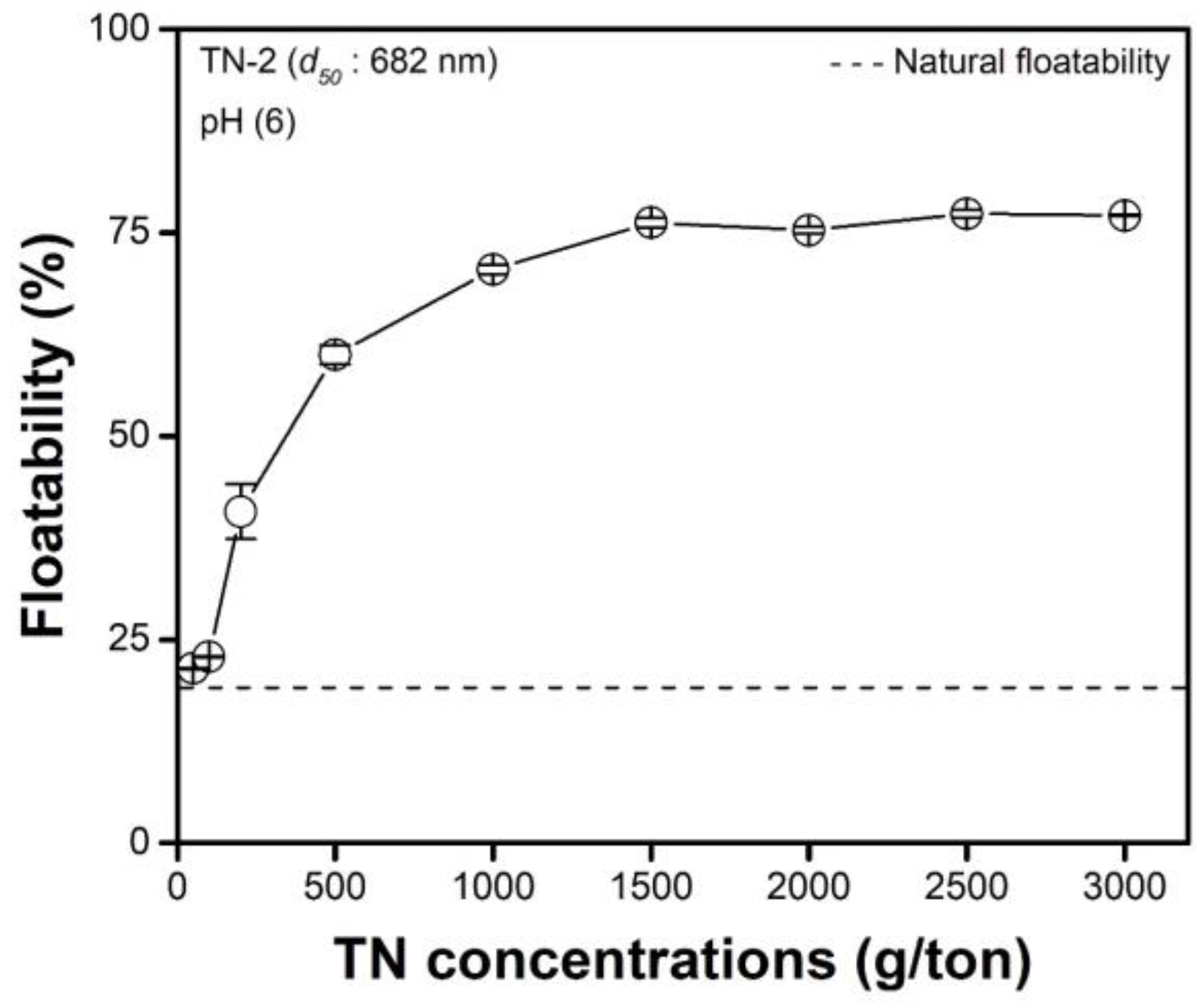
3.3. Effects of the Concentration of TNs
4. Conclusions
- The IEPs of the malachite and TNs were approximately pH 8–9 and pH < 3, respectively. The malachite was positively charged at pH 6, whereas the TNs were negatively charged at this pH value, indicating that the electrostatic interaction between malachite and talc is favorable, resulting in a maximum amount of TNs deposited on the surface of malachite at pH 6. Based on this, the maximum floatability of malachite was observed at pH 6.
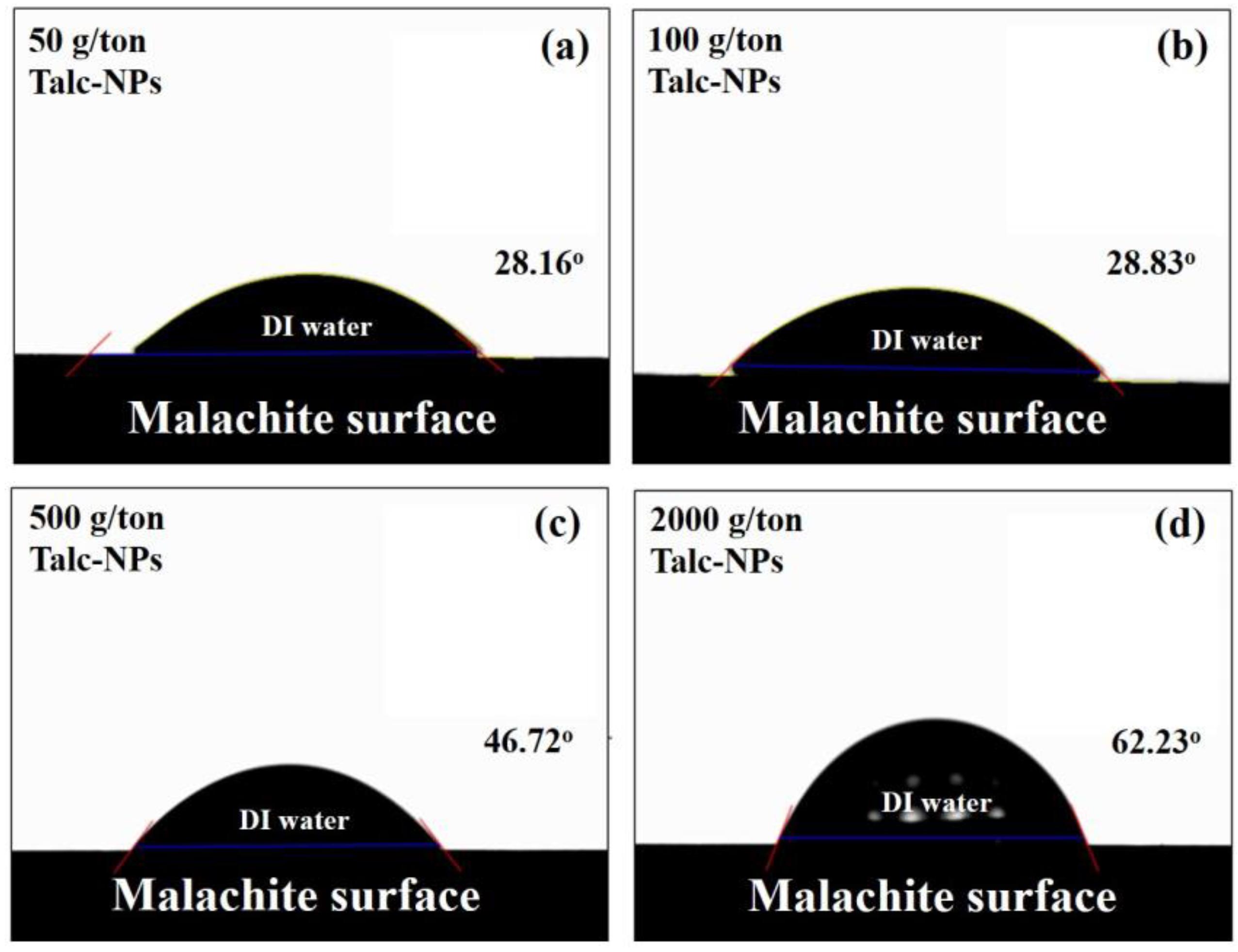
- The amount of TNs deposited on the surface of the malachite increased as the concentration of the TNs increased and the size of the TNs decreased. The trends in terms of the amount of TNs deposited on the surface of the malachite were highly consistent with the contact angle measurements. These two experimental results indicate that the higher the amount of TNs deposited on the surface of malachite, the greater the hydrophobicity of the surface of the TN-deposited malachite, resulting in enhanced malachite floatability.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, R.; Liu, Z.; Li, J.; Ao, S.; Pei, B.; Liu, D.; Li, J. Reexamining the role of ammonium ions in the sulfidization, xanthate-flotation of malachite. Minerals 2020, 10, 537. [Google Scholar] [CrossRef]
- Park, K.; Park, S.; Choi, J.; Kim, G.; Tong, M.; Kim, H. Influence of excess sulfide ions on the malachite-bubble interaction in the presence of thiol-collector. Sep. Purif. Technol. 2016, 168, 1–7. [Google Scholar] [CrossRef]
- Clark, D.W.; Newell, A.J.H.; Chilman, G.F.; Capps, P.G. Improving flotation recovery of copper sulphides by nitrogen gas and sulphidisation conditioning. Miner. Eng. 2000, 13, 1197–1206. [Google Scholar] [CrossRef]
- Tijsseling, L.T.; Dehaine, Q.; Rollinson, G.K.; Glass, H.J. Flotation of mixed oxide sulphide copper-cobalt minerals using xanthate, dithiophosphate, thiocarbamate and blended collectors. Miner. Eng. 2019, 138, 246–256. [Google Scholar] [CrossRef]
- Webb, M.; Ruber, H.; Leduc, G. The toxicity of various mining flotation reagents to rainbow trout (Salmo gairdneri). Water Res. 1976, 10, 303–306. [Google Scholar] [CrossRef]
- Okibe, N.; Johnson, D.B. Toxicity of flotation reagents to moderately thermophilic bioleaching microorganisms. Biotechnol. Lett. 2002, 24, 2011–2016. [Google Scholar] [CrossRef]
- Dong, X.; Price, M.; Dai, Z.; Xu, M.; Pelton, R. Mineral-mineral particle collisions during flotation remove adsorbed nanoparticle flotation collectors. J. Colloid Interface Sci. 2017, 504, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Abarca, C.; Yang, S.; Pelton, R.H. Towards high throughput screening of nano-particle flotation collectors. J. Colloid Interface Sci. 2015, 460, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pelton, R. Nanoparticle flotation collectors II: The role of nanoparticle hydrophobicity. Langmuir 2011, 27, 11409–11415. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pelton, R.; Montgomery, M.; Cui, Y. Nanoparticle flotation collectors III: The role of nanoparticle diameter. ACS Appl. Mater. Interfaces 2012, 4, 4882–4890. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pelton, R.; Abarca, Z.; Dai, Z.; Montgomery, M.; Xu, M.; Bos, J.-A. Towards nanoparticle flotation collectors for pentlandite separation. Int. J. Miner. Process. 2013, 123, 137–144. [Google Scholar] [CrossRef]
- Yang, S.; Razavizadeh, B.B.M.; Pelton, R.; Bruin, G. Nanoparticle flotation collectors-The influence of particle softness. ACS Appl. Mater. Interfaces 2013, 5, 4836–4842. [Google Scholar] [CrossRef] [PubMed]
- Hajati, A.; Shafaei, S.Z.; Noaparast, M.; Farrokhpay, S.; Aslani, S. Novel application of talc nanoparticles as collector in flotation. RSC Adv. 2016, 6, 98096–98103. [Google Scholar] [CrossRef]
- Kim, H.; You, J.; Gomez-Flores, A.; Solongo, S.K.; Hwang, G.; Zhao, H.; Lee, B.; Choi, J. Malachite flotation using carbon black nanoparticles as collectors: Negative impact of suspended nanoparticle aggregates. Miner. Eng. 2019, 137, 19–26. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, X.; Liu, G.; Liu, J. Novel chelating surfactant 5-heptyl-1,2,4-triazole-3-thione: Its synthesis and flotation separation of malachite against quartz and calcite. Miner. Eng. 2019, 131, 342–352. [Google Scholar] [CrossRef]
- Kursun, H.; Ulusoy, U. Influence of shape characteristics of talc mineral on the column flotation behavior. Int. J. Miner. Process. 2006, 78, 262–268. [Google Scholar] [CrossRef]
- Ohshima, H. Electrokinetics of soft particles. Colloid Polym. Sci. 2007, 285, 1141–1421. [Google Scholar] [CrossRef]
- Choi, J.; Choi, S.Q.; Park, K.; Han, Y.; Kim, H. Flotation behavior of malachite in mono- and di-valent salt solutions using sodium oleate as a collector. Int. J. Miner. Process. 2016, 146, 38–45. [Google Scholar] [CrossRef]
- Gu, G.; Chen, Z.; Zhao, K.; Song, S.; Li, S.; Wang, C. The effect of a novel depressant on the separation of talc and copper-nickel sulfide ore. Physicochem. Probl. Miner. Process. 2019, 55, 116–127. [Google Scholar]


| Sample | Amounts of TNs Deposited on the Malachite Surface (mg/g) |
|---|---|
| TN-1 | 0.863 ± 0.16 |
| TN-2 | 1.671 ± 0.17 |
| TN-3 | 1.809 ± 0.14 |
| TN-4 | 1.998 ± 0.11 |
| Sample | Amounts of TNs Deposited on the Malachite Surface (mg/g) |
|---|---|
| 6 | 1.671 ± 0.17 |
| 9 | 0.954 ± 0.19 |
| 11 | 0.799 ± 0.15 |
| TN Concentration (g/ton) | Amounts of TNs Deposited on the Malachite Surface (mg/g) |
|---|---|
| 50 | 0.023 ± 0.03 |
| 100 | 0.026 ± 0.06 |
| 200 | 0.834 ± 0.11 |
| 500 | 1.189 ± 0.14 |
| 1000 | 1.671 ± 0.17 |
| 1500 | 1.981 ± 0.09 |
| 2000 | 1.983 ± 0.13 |
| 2500 | 1.979 ± 0.11 |
| 3000 | 1.973 ± 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Seo, J.; Kim, S.B.; Kim, W. Flotation Behavior of Malachite Using Hydrophobic Talc Nanoparticles as Collectors. Minerals 2020, 10, 756. https://doi.org/10.3390/min10090756
Choi J, Seo J, Kim SB, Kim W. Flotation Behavior of Malachite Using Hydrophobic Talc Nanoparticles as Collectors. Minerals. 2020; 10(9):756. https://doi.org/10.3390/min10090756
Chicago/Turabian StyleChoi, Junhyun, Joobeom Seo, Sang Bae Kim, and Wantae Kim. 2020. "Flotation Behavior of Malachite Using Hydrophobic Talc Nanoparticles as Collectors" Minerals 10, no. 9: 756. https://doi.org/10.3390/min10090756
APA StyleChoi, J., Seo, J., Kim, S. B., & Kim, W. (2020). Flotation Behavior of Malachite Using Hydrophobic Talc Nanoparticles as Collectors. Minerals, 10(9), 756. https://doi.org/10.3390/min10090756





