Molecular Dynamics Simulation of Cetyl Phosphate Adsorption in Flotation of Magnesite and Pertinent Chemical Aspects
Abstract
1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
2.2. pKa Measurements
2.3. Flotation Experiments
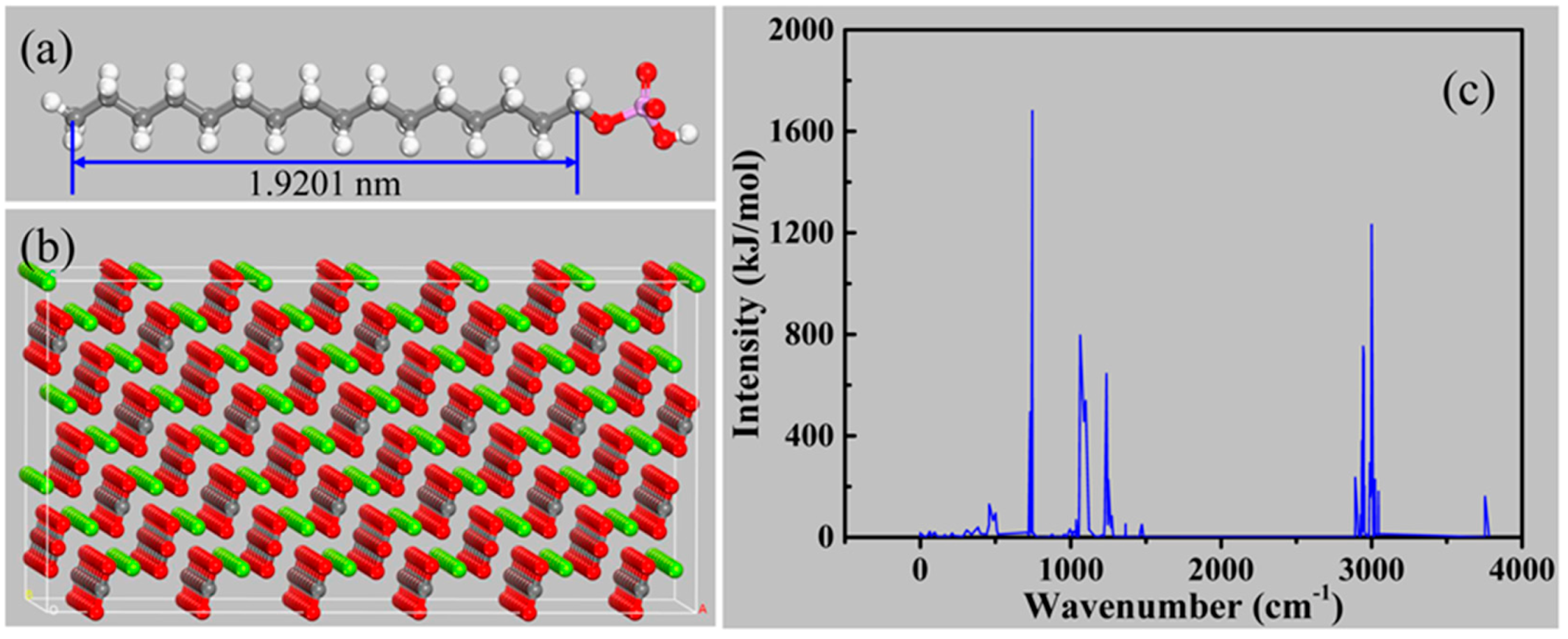
2.4. Molecular Dynamics Simulation
2.5. Zeta Potential and Wettability Measurements
3. Results and Discussion
3.1. Distribution of Species in PCP Solution
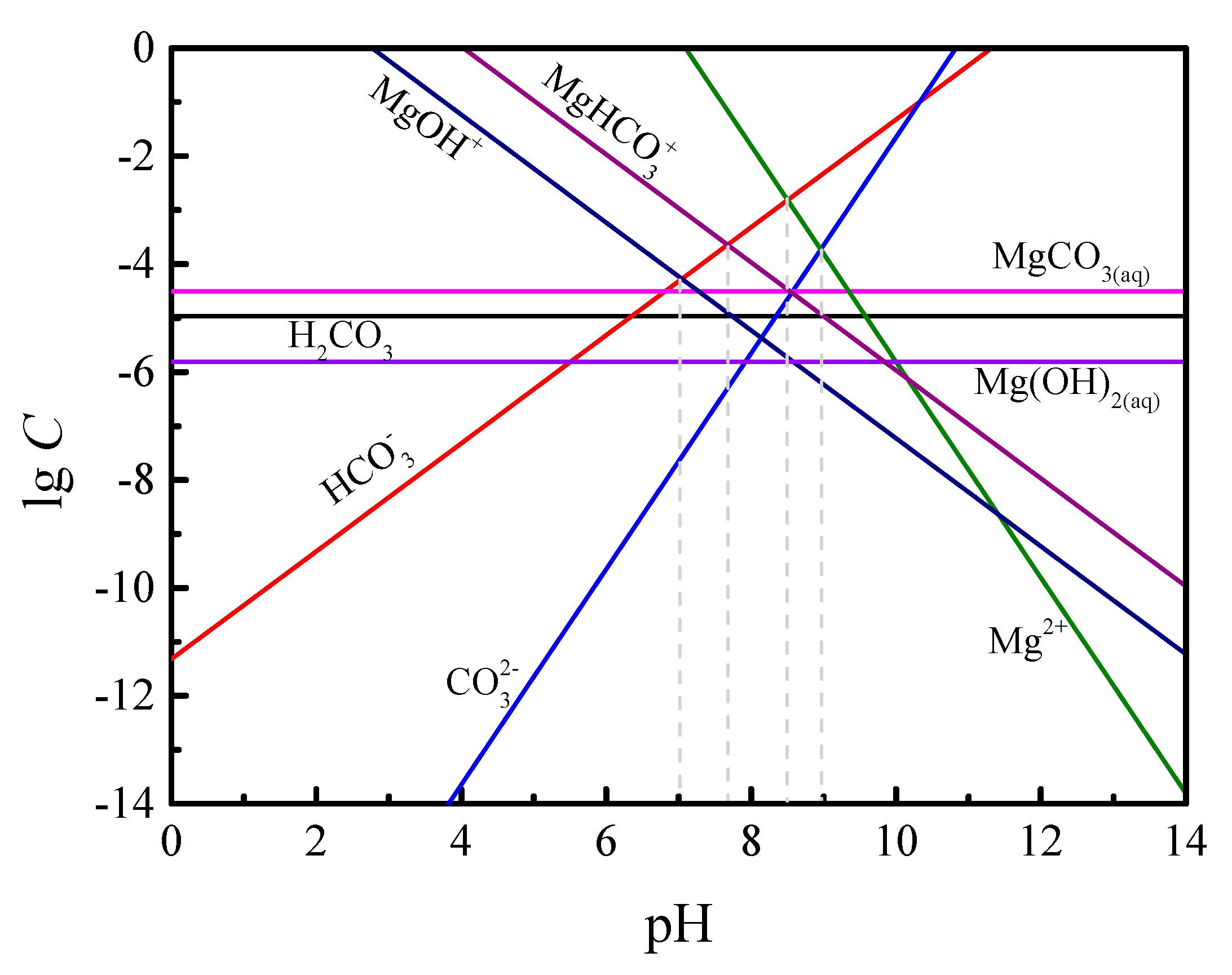
3.2. Distribution of Ionic Species of Magnesite
3.3. Microflotation Results
3.4. Electrokinetic Behavior and Wettability
3.5. Molecular Modeling Studies
3.5.1. Adsorption Characteristics of CP Species
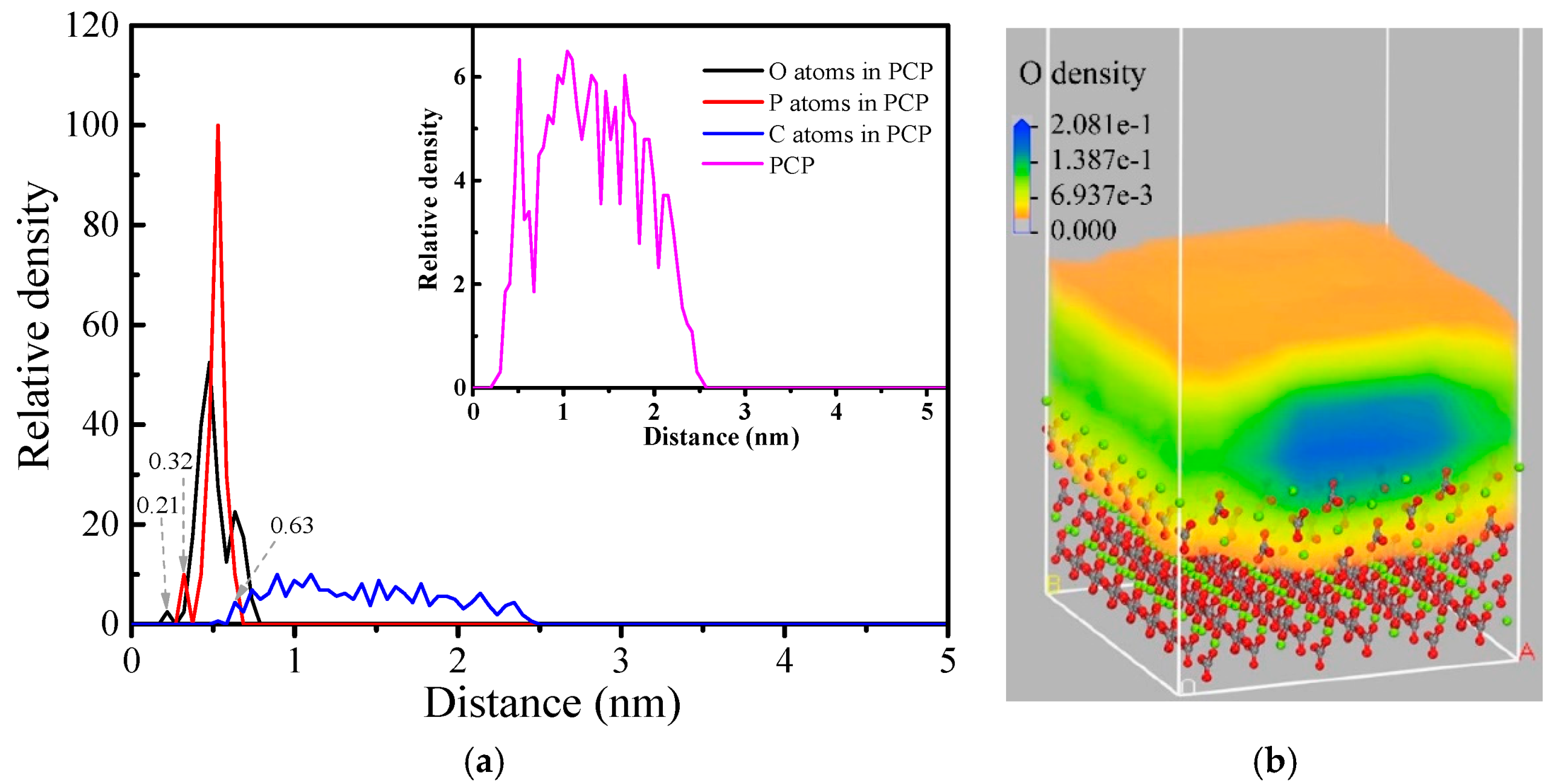
3.5.2. Relative Density Distribution and Density Field
4. Summary and Conclusions
- Based on the results of flotation experiments, it is clear that PCP is a promising collector for magnesite with excellent recoveries even at a low concentration (60 mg/L).
- Results from chemical computations confirmed that the monoanionic forms of CP are the dominant species within the pH range used in this study. The measured isoelectric point of magnesite was pH 7.3, which is the pH range predictable from the analysis of chemical equilibria diagrams (7.0).
- The adsorption of CP− on the magnesite surface causes significant negative shifts in the zeta potentials and results in the development of a significant level of hydrophobicity on magnesite. It is more likely that the reason for these results is the strong and stable interactions between the magnesite surface and CP species.
- According to the simulations, the O atoms in the polar phosphate head group are the primary bonding atoms, which explains the floatability of magnesite with CP. The chemisorption of CP was supported by a large concentration of adsorbed species in the immediate vicinity of the magnesite surface, with the expected orientation of cetyl groups towards the bulk solution and hence the air-solution interface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, W.Z.; Tang, Y. Interactive effect of minerals on complex ore flotation: A brief review. Int. J. Miner. Metall. Mater. 2020, 27, 571–583. [Google Scholar] [CrossRef]
- Yao, J.; Yin, W.Z.; Gong, E.P. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 84–93. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. Reverse flotation of magnesite by dodecyl phosphate from dolomite in the presence of sodium silicate. Sep. Sci. Technol. 2004, 39, 377–390. [Google Scholar] [CrossRef]
- Doerner, H.A.; Dwight, L. Concentration of low-grade magnesite ores by flotation. US Bur. Mines Bull. 1938, 1, 1–30. [Google Scholar]
- Matis, K.A.; Gallios, G.P. Anionic flotation of magnesium carbonates by modifiers. Int. J. Miner. Process. 1989, 25, 261–274. [Google Scholar] [CrossRef]
- Sahoo, H.; Sinha, N.; Rath, S.S.; Das, B. Ionic liquids as novel quartz collectors: Insights from experiments and theory. Chem. Eng. J. 2015, 273, 46–54. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Zhong, H.; Wang, S.; Xia, L.; Zou, W.; Liu, G. Investigations on reverse cationic flotation of iron ore by using a Gemini surfactant: Ethane-1,2-bis(dimethyl-dodecyl- ammonium bromide). Chem. Eng. J. 2014, 257, 218–228. [Google Scholar] [CrossRef]
- Florek, I. The effects of radiation pretreatment on the floatability of magnesite and siderite. Miner. Eng. 1995, 8, 329–331. [Google Scholar] [CrossRef]
- Brando, P.R.G.; Poling, G.W. Anionic flotation of magnesite. Can. Metall. Q. 1982, 3, 211–220. [Google Scholar] [CrossRef]
- Wonyen, D.; Kromah, V.; Gibson, B.; Nah, S.; Chelgani, S.A. Review of flotation separation of Mg carbonates (Dolomite and Magnesite). Minerals 2018, 8, 354. [Google Scholar] [CrossRef]
- Liu, W.; McDonald IV, L.W.; Wang, X.; Miller, J.D. Bastnaesite flotation chemistry issues associated with alkyl phosphate collectors. Miner. Eng. 2018, 127, 286–295. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G. Flotation performances and adsorption mechanism of α-hydroxyoctyl phosphinic acid to cassiterite. Appl. Surf. Sci. 2015, 353, 856–864. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. Effect of solution chemistry on flotability of magnesite and dolomite. Int. J. Miner. Process. 2004, 74, 343–357. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J. Mechanism of flotation using citric acid for separating monazite from bastnaesite. Nonferrous Met. 1989, 41, 33–40. [Google Scholar]
- Zhou, G.; Luo, J. Mechanism of flotation using mono-alkyl ester phosphoric acid for bastnaesite. J. Chin. Rare Earth Soc. 1990, 8, 261–264. [Google Scholar]
- Zhang, H.; Liu, W.; Han, C.; Hao, H. Effects of monohydric alcohols on the flotation of magnesite and dolomite by sodium oleate. J. Mol. Liq. 2018, 249, 1060–1067. [Google Scholar] [CrossRef]
- Paschek, D.; Engels, T.; Geiger, A.; von Rybinski, W. MD-simulation study of the hydrophobic hydration of nonionic surfactants. Colloids Surf. A 1999, 489–500. [Google Scholar] [CrossRef]
- de Leeuw, N.H.; Cooper, T.G. A computational study of the surface structure and reactivity of calcium fluoride. J. Mater. Chem. 2003, 13, 93–101. [Google Scholar] [CrossRef]
- Yin, X.; Miller, J.D. Wettability of kaolinite basal planes based on surface force measurements using atomic force microscopy. Min. Metall. Explor. 2012, 29, 13–19. [Google Scholar] [CrossRef]
- Domínguez, H. Self-aggregation of the SDS surfactant at a solid-liquid interface. J. Phys. Chem. B 2007, 111, 4054–4059. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Han, C.; Wei, D. Intensify dodecylamine adsorption on magnesite and dolomite surfaces by monohydric alcohols. Appl. Surf. Sci. 2018, 444, 729–738. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, W.; Kelebek, S. Magnesite-dolomite separation using potassium cetyl phosphate as a novel flotation collector and related surface chemistry. Appl. Surf. Sci. 2020, 508, 145191. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, W.; Kelebek, S. Selective flotation of magnesite from calcite using potassium cetyl phosphate as a collector in the presence of sodium silicate. Miner. Eng. 2020, 146, 106154. [Google Scholar] [CrossRef]
- Reijenga, J.; van Hoof, A.; van Loon, A.; Teunissen, B. Development of methods for the determination of pKa values. Anal. Chem. Insights. 2013, 8, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Avdeef, A.; Comer, J.E.A.; Thomson, S.J. pH-Metric log P. 3. glass electrode calibration in methanol-water, Applied to pKa determination of water-insoluble substances. Anal. Chem. 1993, 65, 42–49. [Google Scholar] [CrossRef]
- Qiang, Z.; Adams, C. Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res. 2004, 38, 2874–2890. [Google Scholar] [CrossRef]
- BIOVIA Materials Studio 2017 (17.1.0.48). Available online: http://accelrys.com/products/collaborative-science/biovia-materials-studio/ (accessed on 12 April 2020).
- Maslen, E.N.; Streltsov, V.A.; Streltsova, N.R. X-ray study of the electron density in magnesite MgCO3. Acta Cryst. B 1993, 49, 980–984. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 1–10. [Google Scholar] [CrossRef]
- Kitao, O.; Miura, N.; Ushiyama, H. Molecular mechanics with QEq-CS (charge equilibration method generalized for charge separation system). J. Mol. Struct. THEOCHEM 1999, 461, 239–247. [Google Scholar] [CrossRef]
- Labík, S.; Smith, W.R. Scaled particle theory and the efficient calculation of the chemical potential of hard spheres in the NVT ensemble. Mol. Simul. 1994, 12, 23–31. [Google Scholar] [CrossRef]
- Kraska, T. Molecular-dynamics simulation of argon nucleation from supersaturated vapor in the NVE ensemble. J. Chem. Phys. 2006, 124, 054507. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Kelebek, S.; Yin, W. Surface chemistry of magnesite and calcite flotation and molecular dynamics simulation of their cetyl phosphate adsorption. Colloids Surf. A 2020, 603, 125246. [Google Scholar] [CrossRef]
- Babić, S.; Horvat, A.J.; Pavlović, D.M.; Kaštelan-Macan, M. Determination of pKa values of active pharmaceutical ingredients. Trends Anal. Chem. 2007, 26, 1043–1061. [Google Scholar] [CrossRef]
- Hu, Y.H. Research on Solution Chemistry and Flotability of Salt-Type Minerals. Ph.D. Thesis, Central South University, Changsha, China, 1989. [Google Scholar]
- Wang, D.Z.; Hu, Y.H. Solution Chemistry of Flotation; Hunan Science and Technology Press: Beijing, China, 1988; pp. 235–238. [Google Scholar]
- Pokrovsky, O.S.; Schott, J.; Thomas, F.; Mielczarski, J. Surface speciation of Ca and Mg carbonate minerals in aqueous solutions: A combined potentiometric, electrokinetic, and DRIFT surface spectroscopy approach. Mineral. Mag. 1998, 62, 1196–1197. [Google Scholar] [CrossRef]
- Gence, N. Wetting behavior of magnesite and dolomite surfaces. Appl. Surf. Sci. 2006, 252, 3744–3750. [Google Scholar] [CrossRef]
- Spiritu, E.R.L.; Naseri, S.; Waters, K.E. Surface chemistry and flotation behavior of dolomite, monazite and bastnäsite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A 2018, 546, 254–265. [Google Scholar] [CrossRef]






| MgO | CaO | SiO2 | TFe | Al2O3 |
|---|---|---|---|---|
| 46.87 | 0.32 | 0.84 | 0.12 | 0.03 |
| Mineral | Values | a (nm) | b (nm) | c (nm) | α (°) | β (°) | γ (°) |
|---|---|---|---|---|---|---|---|
| Magnesite | Experimental 1 | 0.464 | 0.464 | 1.503 | 90 | 90 | 120 |
| Optimized | 0.469 | 0.469 | 1.515 | 90 | 90 | 120 |
| Samples | pH 7.0 | pH 9.0 | pH 11.0 |
|---|---|---|---|
| Magnesite | 0 1 | 0 | 0 |
| Magnesite + PCP | 41.5 | 39.9 | 37.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Yao, J.; Yin, W.; Kelebek, S. Molecular Dynamics Simulation of Cetyl Phosphate Adsorption in Flotation of Magnesite and Pertinent Chemical Aspects. Minerals 2020, 10, 761. https://doi.org/10.3390/min10090761
Tang Y, Yao J, Yin W, Kelebek S. Molecular Dynamics Simulation of Cetyl Phosphate Adsorption in Flotation of Magnesite and Pertinent Chemical Aspects. Minerals. 2020; 10(9):761. https://doi.org/10.3390/min10090761
Chicago/Turabian StyleTang, Yuan, Jin Yao, Wanzhong Yin, and Sadan Kelebek. 2020. "Molecular Dynamics Simulation of Cetyl Phosphate Adsorption in Flotation of Magnesite and Pertinent Chemical Aspects" Minerals 10, no. 9: 761. https://doi.org/10.3390/min10090761
APA StyleTang, Y., Yao, J., Yin, W., & Kelebek, S. (2020). Molecular Dynamics Simulation of Cetyl Phosphate Adsorption in Flotation of Magnesite and Pertinent Chemical Aspects. Minerals, 10(9), 761. https://doi.org/10.3390/min10090761





