3.1. Characterization of the Sepiolite Samples
The X-ray diffractograms of the two sepiolite samples were similar and indicated the presence of only sepiolite, pointing out to a clay with monomineralic composition (
Figure 1).
Additionally, the chemical composition of the two sepiolite samples was also similar (
Table 1). The high content of Si and Mg evidenced the magnesium silicate nature of these raw materials. The SiO
2/MgO ratio was 2.41 for sepiolite 1 and 2.35 for sepiolite 2, which are common values for this type of mineral [
20]. Al and Fe were found, which are known to substitute Si and Mg in the crystalline structure. The XRF data are in agreement with results provided by Sabah et al. (2007) for other sepiolite samples from the same origin [
20].
The thermogravimetric analysis results of the two sepiolite samples were similar (
Figure 2), in agreement with the similar chemical composition. The first and major endothermic weight loss (≈11%) can be seen by increasing temperature up to ca. 100 °C; this is mainly due to the release of zeolitic water and hygroscopic water. A second endothermic weight loss (≈2.8–3.0%) is observed from ca. 100 to 280 °C, attributed to release of some Mg-coordinated water. The release of the remaining Mg-coordinated water is completed in a third stage, extending up to about 600 °C (≈2.8–3.1%). A final endothermic weight loss step from ca. 600 to 1000 °C (≈3.3–3.7%), occurs due to the dehydroxylation of the octahedrally coordinated hydroxyl groups [
21,
22,
23,
24]. The total thermogravimetric weight losses (≈20%) are in agreement with the loss on ignition values (
Table 1) presented above.
FTIR spectroscopy was also used to characterize the mineral (
Figure 3). The spectra of the two sepiolite samples were quite similar. The bands at 3688 and 3616 cm
−1 for sepiolite 1 and at 3685 and 3625 cm
−1 for sepiolite 2 are associated to the stretching of the OH coordinated with Mg [
25]. At 3557 and 3564 cm
−1 for sepiolite 1 and sepiolite 2, respectively, a band occurs related with the OH-stretching for water coordinated with Mg [
25]. For both samples, the diffuse region between 3400 and 3200 cm
−1 may include bands attributed to adsorbed, zeolitic, and coordinated water. In the 2370–2330 cm
−1 region, bands corresponding to ambient CO
2 are present. A group of bands related to OH bending are observed at 1660 (for sepiolite 1) and 1666 cm
−1 (for sepiolite 2) assigned to water strongly bonded to Mg [
25]; 1649 (sepiolite 1) and 1652 cm
−1 (sepiolite 2) related to zeolitic water [
26]; 1636 (sepiolite 1) and 1634 cm
−1 (sepiolite 2) attributed to adsorbed water [
26]. Bands at 1210, 1070 (shoulder), and 1000 cm
−1 in the case of sepiolite 1 and at 1209, 1078 (shoulder), and 1003 cm
−1 in the case of sepiolite 2 were assigned to Si–O stretching modes, whereas those at 969 (sepiolite 1) and 973 cm
−1 (sepiolite 2) were attributed to OH deformation in the Mg–OH or Al–OH bonds [
25].
Some physical parameters of the sepiolite samples were also determined, namely particle sizes and specific surface area. The results are summarized in
Table 2. Considering the results supplied by the manufacturing company, similar Dv10 and Dv50 size values are obtained for the two sepiolites; the apparent larger Dv90 of sepiolite 2 compared to sepiolite 1 is due to the presence of a few agglomerates with bigger dimensions in sepiolite 2 dispersion. The original materials, as supplied by Tolsa, did not undergo any optimized dispersion procedure for these measurements, being the starting point for the developed work discussed in
Section 3.2. Thus, the size values shown in
Table 2 correspond for sure to aggregated particles, and this state of the initial materials is, in our opinion, the reason for the formation of suspensions of poor stability. The more extensive disentanglement of the fiber bundles in sepiolite 2 compared to sepiolite 1 makes the surface more available and it results in a higher specific surface area.
The SEM microphotographs clearly showed the fibrous nature of the studied samples, composed by bundles, and individual long rods with thin diameter. No contaminants were detected in the samples (
Figure 4).
Zeta potential results for aqueous suspensions of clay are shown in
Figure 5. For sepiolite 1, zeta potential at the initial pH (≈8.5) is negative (ca. −12 mV) and tended to increase (in absolute value) with the increase of pH. Increasing pH until 12 highly increased the zeta potential to around −45 mV. On the other hand, zeta potential varied less in the region of pH 8.5 down to pH 3.0. At pH 3.7, the zeta potential was −3 mV. The isoelectric point was found at a pH value of 3.2–3.3. Under this condition, there was an equilibrium between positive and negative charges on the surface of sepiolite particles. The isoelectric point found for this sample is similar to that determined for a sample of similar origin, for which the authors determined an isoelectric point pH of ca. 3.4, at the same mineral content [
20]. For pH 2.6, the zeta potential achieved a relatively high positive value (+9 mV). The variation of zeta potential with pH is the expected for this type of mineral and can be rationalized in terms of the acid-base behavior of the surface hydroxyl groups (M–OH) of sepiolite. At pH values superior to the isoelectric point, the hydroxyl groups deprotonate releasing protons and forming negatively charged M–O
− species, providing a negative zeta potential. At pH values lower than the isoelectric point, they protonate to form M–OH
2+ species, providing positive zeta potential. In the isoelectric point, the concentration of M–O
− and M–OH
2+ is the same. Different isoelectric point values can be obtained depending on the sepiolite origin [
27,
28]. The presence of contaminants and defects in the crystalline structure of the sepiolite (e.g., isomorphic tetrahedral and octahedral substitutions) highly influence the pH value for the isoelectric point.
For sepiolite 2, the zeta potential results evidenced a trend similar to that found for sepiolite 1 (
Figure 5). However, the values achieved at each pH are somewhat different. At pH 8.5, the zeta potential was ca. −20 mV and increasing the pH till 12 increased it (in absolute value) to −56 mV. At pH 3.0, zeta potential was −4 mV. The isoelectric point was found to be at a pH near 2.5. At pH 1.6, zeta potential was +5 mV. Thus, in comparison to sepiolite 1, sepiolite 2 showed more negative values of zeta potential for the same pH and lower pH value for the isoelectric point. Since sepiolite 1 and 2 have similar mineralogical, chemical, and thermal properties, as demonstrated above, the different zeta potential values can be related to slightly different surface characteristics. The higher specific surface area of sepiolite 2 (
Table 2) affords a higher exposure of silanol groups on the external surface of the particles which generates more negative values of zeta potential.
3.2. Characterization of the Sepiolite Suspensions: Effects of Dispersing Equipment and Dispersant
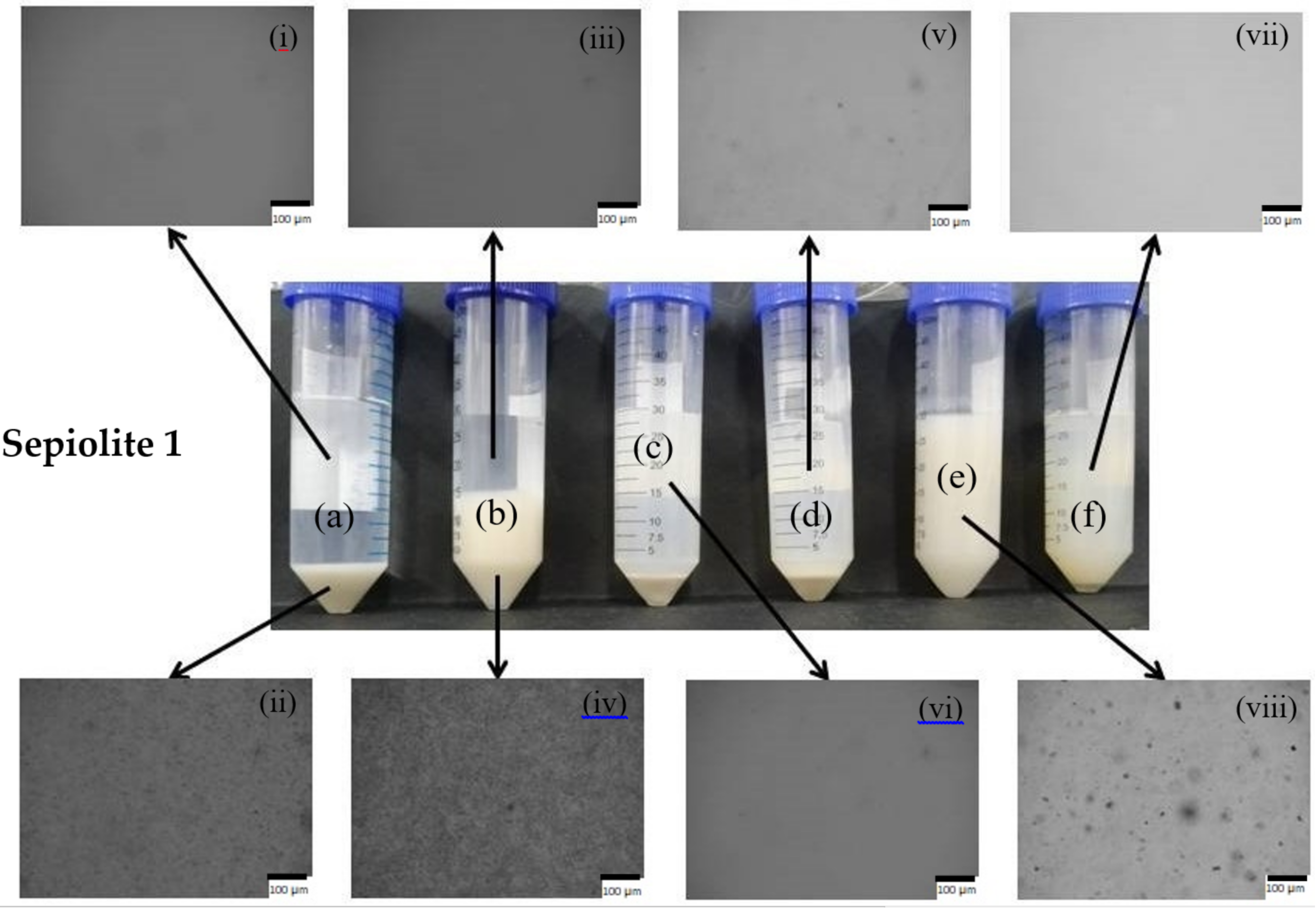
Images of the formed suspensions of sepiolite 1 and sepiolite 2 (at the natural pH of the suspensions), in the presence of sodium polyphosphate and without addition of dispersing agent, using different dispersing equipment, are presented in
Figure 6.
As can be macroscopically observed, the use of sepiolite 2 resulted, overall, in suspensions of better stability, being only observed phase separation for the samples produced using the magnetic stirrer and a slight sedimentation of clay particles for the sample produced with the high-speed disperser. All the other three samples appeared stable for a long period of time, 90 days. Less stable suspensions were formed when sepiolite 1 was used, being only observed macroscopically uniform samples with the use of the sonicator as dispersion equipment. This is certainly related with the different pre-treatments used to prepare the two samples of sepiolite; the pre-treatment used to produce sepiolite 2 allows a better and slightly easier dispersion of the clay individual fibers, even if these have a longer length compared to sepiolite 1.
Additionally, in the insets, which correspond to microscopic observations of the samples it is also possible to observe clear differences. For the case of sepiolite 1 suspensions prepared using the magnetic stirrer and high-shear disperser, the top phase microscopic image shows a clear image, being the mineral-rich phase concentrated in the bottom of the flask, even when the dispersing agent, polyphosphate, was used (
Figure 6, images (i), (iii), (v), and (vi)). On the other hand, for the suspension of sepiolite 2 prepared with the high-shear disperser, the top phase of suspension (c) shows some dispersed particles, and the bottom phase is smaller than the observed for sepiolite 1. When polyphosphate was added to the suspension of sepiolite 2, using the high-shear disperser, a homogeneous suspension was formed, some particles of relatively large dimensions being observed in suspension. Comparing the macroscopic aspect and the microscopic observation of suspensions of sepiolite 2 prepared with the high-speed disperser (
Figure 6, samples c and d), it is reasonable to say that part of the mineral was disaggregated into small particles, not visible in the optical microscope, only a small sediment of mineral particles being observed in the absence of polyphosphate.
With ultrasonication, stable suspensions with solely sepiolite in water and also with the addition of polyphosphate (
Figure 6, samples e and f) were prepared. However, the sample containing only sepiolite 1 (no dispersant) showed some large particles in suspension, contrary to the samples prepared with sepiolite 1 or sepiolite 2 with dispersing agent, which presented a clear image in the optical microscope. The presence of the dispersing agent can introduce extra charges on the surface of the particles, resulting in an improved disaggregation and stability of the suspensions. Sepiolite 2, without added dispersing agent, already showed a clear image in the microscope, confirming that it is easier to disperse than sepiolite 1.
3.2.1. Effect of Mechanical Treatment
In order to have a better elucidation of the state of the sepiolite aqueous suspensions, dynamic light scattering and zeta potential measurements were performed. The results obtained for the size and zeta potential of the prepared suspensions are shown in
Table 3,
Table 4,
Table A1 and
Table A2 (
Appendix A).
As shown in
Table 3 and
Table 4, typically the disaggregation and dispersion stability increase as the power of the dispersion equipment is increased. In the case of sepiolite 1, for the samples where the magnetic stirrer was employed, only one stable dispersion was obtained (at pH 12 with CMC), contrary to the suspensions prepared using the ultrasonicator where all preparations (excluding the sample with alginate at the initial pH) showed stability for a long period of time. The same trend was observed for the sepiolite 2, only three stable suspensions being prepared (at pH 12) using the magnetic stirrer, whereas using the ultrasonicator almost all the prepared suspensions (excluding with alginate at pH 3 and 8) were stable. With the high-speed disperser equipment, the results were, in general, intermediate between those above mentioned, being not very effective, as ultrasonication, to generate stable colloidal dispersions of sepiolite. These results are in agreement with previous conclusions from other authors [
9].
This factor can be rationalized in the following way: the increase in the power of the dispersing equipment results in higher disaggregation of the clay micro and nanoparticles (aggregates and bundles), leading to particles of smaller size and thus the formation of more stable suspensions.
3.2.2. Effect of the pH
An increase in the suspension pH is expected to improve the dispersibility and colloidal stability of the sepiolite particles, due to an increase in the negative charge of the clay particles (
Figure 5).
Table 3,
Table 4,
Table A1 and
Table A2 evidence that the disaggregation and dispersion stability increased with the rise of the suspension pH from 3 to 12: an increment in the number of stable suspensions, i.e., six suspensions over the 15 investigated at pH 3, to nine suspensions over the 15 investigated at pH 12, was observed when working with sepiolite 1 and from 7 to 12, over the 15 investigated, in the case of sepiolite 2.
The increase in the negative surface charge of the clay particles is derived from the presence of hydroxyl groups or breakage of M–O–M (M = Si or Mg) at highly alkaline pH [
29]. This increase in the surface charge helps the disaggregation and the stabilization of the particles, avoiding also the particle re-aggregation that introduces instability on the systems. Even though no strict defined relationship between zeta potential values and colloidal stability of systems can be assumed, it is known that a very small absolute zeta potential value, lower than ±5.0 mV, is a driving force for flocculation and instability of the particles in solution, and only above a value of ±30 mV the systems tend to be stable [
30]. Thus, an increase in the absolute value obtained by pH increment will be favorable to enhance the stability of the suspensions.
The combination of high pH (pH 12) and ultrasonication led to 100% stable suspensions either for the sepiolite 1 or sepiolite 2. At pH 12 and with the high-shear disperser most of the prepared suspensions were also stable. Even with only magnetic stirring at pH 12, it was possible to obtain a few stable suspensions if an appropriate chemical dispersant (e.g., CMC for both sepiolite samples) was used. These suspensions stayed stable for more than three months, contrary to most of the suspensions at pH 3. Additionally, the particle size of the suspensions tendentiously decreased with pH rise and the polydispersity index decreased, indicating a suspension with a narrower distribution of the particle size (
Table 3 and
Table 4). Besides the increase in the surface charge of the clay particles, the dispersing agents used also suffer changes as the pH is increased, as discussed in the next section.
3.2.3. Effect of the Dispersants
The dispersing agents are species able to interact with the clay particles and change some surface properties, leading to an improvement in dispersibility and colloidal stability. In the present work, four different dispersing/stabilizing agents were studied: two synthetic ones, sodium polyphosphate and hydrophobically modified poly(sodium acrylate), and two bio-based ones, sodium carboxymethylcellulose and sodium alginate. It was observed that among all the studied dispersing agents the CMC is the one that provided a higher number of stable dispersions of all the preparations performed, enabling the preparation of stable dispersions in a wide range of conditions. Polyphosphate as chemical dispersant also provided good results in terms of stability of the sepiolite suspensions. However, the particle size of the clay in the CMC stabilized suspensions was tendentiously higher than that obtained when polyphosphate was used, in the cases where it was possible to compare the particle size. On the other hand, poorer results were obtained with alginate, for which the obtained data indicate that this biopolymer is less effective to stabilize sepiolite suspensions in water (under the tested conditions).
The difference in the results obtained with CMC and alginate can be attributed to a better interaction of the CMC polymer with the clay particles, derived from the relatively low degree of substitution of the cellulose derivative (less charged). The reported values of the pKa for CMC (degree of substitution of 1.2) and alginate are quite similar, ca. 4.6 and 4.5, respectively [
31]. It has also been reported that the degree of substitution of CMC affects slightly the pKa value, with a higher substitution by carboxymethyl groups leading to a higher pKa. Thus, being the degree of substitution low (0.7), it is expected that the pKa value of the CMC used in the present work is slightly lower but not significantly different of that of alginate. Additionally, alginate possesses in its structure blocks of guluronate monomers (besides mannuronate) that due to their axial-axial conformation reduce the flexibility of the polymer chain; the mannuronate blocks are more flexible and with a structure similar to CMC structure in its conformation [
32]. The lower degree of substitution of CMC (less electrostatic repulsion with the sepiolite fibers), in the one hand, and, on the other hand, its semi-flexible chain leading to a more extended conformation of the polymer chain at low pH values, can explain the better results obtained when CMC was used. Moreover, the aggregation and gelation of alginate polymer chains has been reported at pH values of ca. 3 [
33], thus the polymer-polymer interaction being favored instead of polymer–clay interaction, resulting in poor dispersion and stabilization of clay suspension when alginate was used. Finally, it can be hypothesized that CMC is more apt to interact with sepiolite particles, via Van der Waals forces and hydrogen bonds, to produce stable suspensions [
34].
From the synthetic options studied, polyphosphate demonstrated to be the more effective, presenting better results than CMC in the case of sepiolite 2, but worse results in the case of sepiolite 1. Polyphosphate has a very low pKa value, being ionized and negatively charged in the entire range of studied pH [
35]. It was expected that this compound could act as a good dispersing/stabilizing agent in the studied range. It is important to highlight that phosphates are traditionally used as dispersing agents for clay suspensions [
36]. However, for sepiolite 1, it seems that the highly negative charge density of the polyphoshate did not favor the interaction with the clay, unless ultrasonication is applied in the system. For sepiolite 2, particularly at low pH, the results with polyphosphate were even better than the obtained when CMC was used. This observation reveals a better interaction of phosphate anions with the low charged clay particles at low pH.
The results obtained for polyacrylate (HM-PAA) as dispersant and stabilizer were quite unexpected. It was found that this polymer can have a good performance acting as disperser of sepiolite, being less effective as stabilizer of the suspensions. These results can be explained by two factors: the pKa of the polymer is higher than that of the other three dispersing agents studied, ca. 5.8 [
31]; secondly, the polymer possesses hydrophobic modification, which leads to a shift in the expansion of the polymer chain [
37]. These two factors can explain the poor stabilization effect observed for low pH values, having been typically observed suspension gelation with time, and a slightly better performance at pH 12, where the polymer is fully ionized and extended, being able to interact and stabilize the clay particles. It is important however to note that the lowest particle size obtained for the sonicated sepiolite 1 suspensions was obtained using HM-PAA at pH 8, with a particle size value of ca. 230 nm. This can be attributed to the excellent affinity of this kind of polymer, amphiphilic polymers, to interact for instance with surfactants [
38] and other compounds containing both hydrophilic and hydrophobic domains, as is the case of the sepiolite clays [
6,
39,
40]. In summary, the combination of an adequate dispersing agent with suitable dispersing equipment and suspension pH led to a significant improvement in disaggregation, dispersion, and stability of sepiolite suspensions in aqueous medium.