Geochemical Constraints on Mantle Melting and Magma Genesis at Pohnpei Island, Micronesia
Abstract
1. Introduction
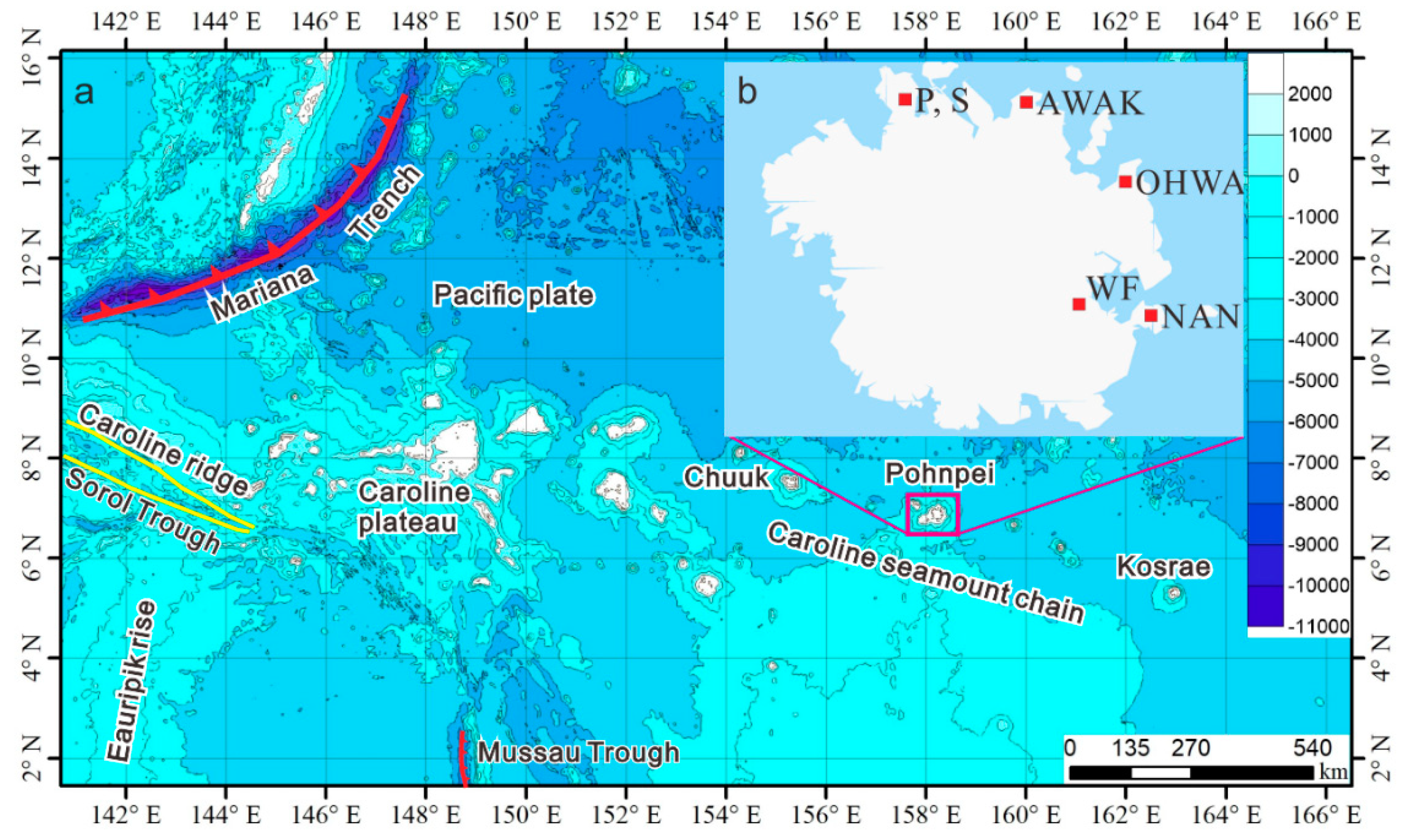
2. Geological Background
3. Samples and Analytical Method
4. Results
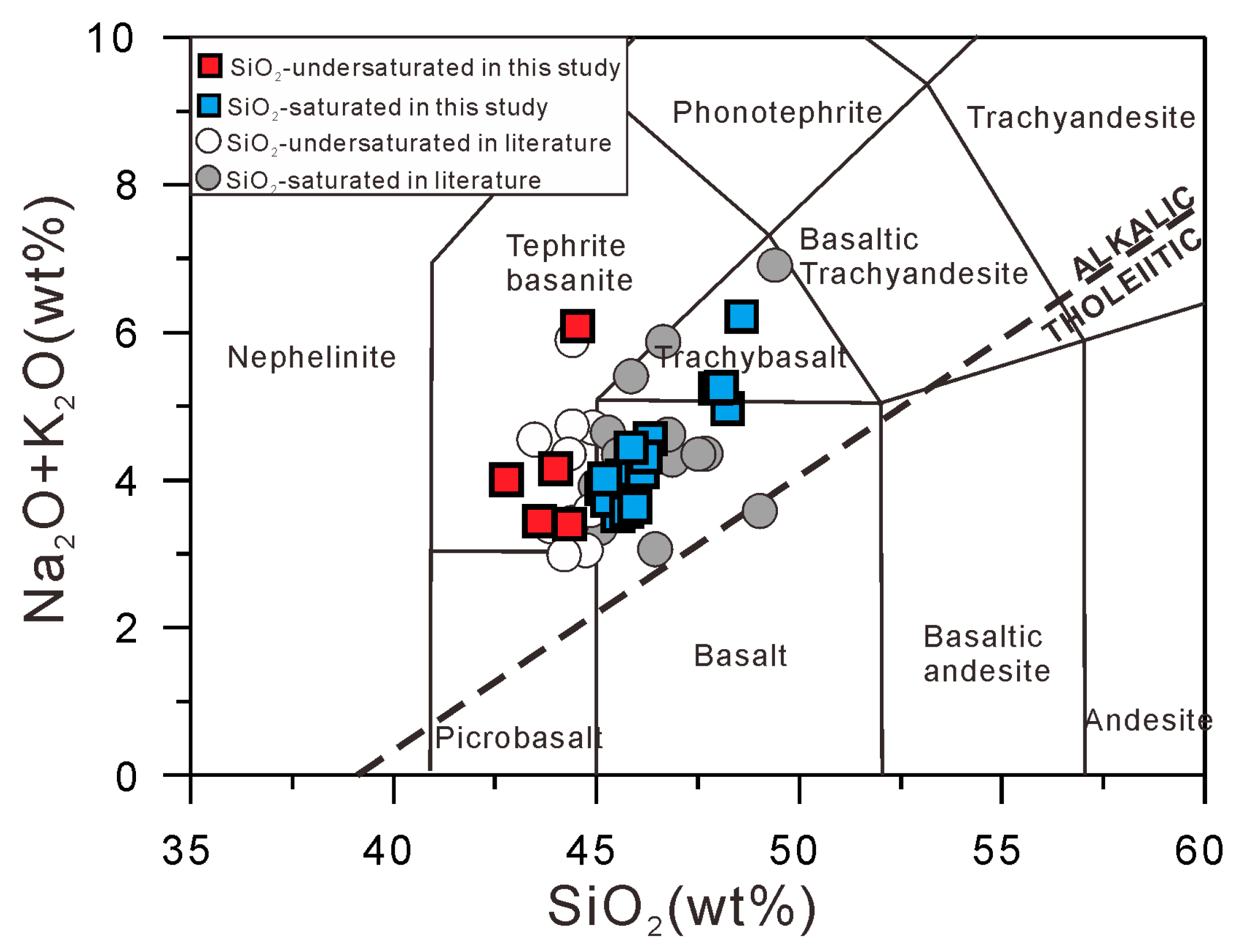
4.1. Whole-Rock Major- and Trace-Element Compositions
4.1.1. Major Elements
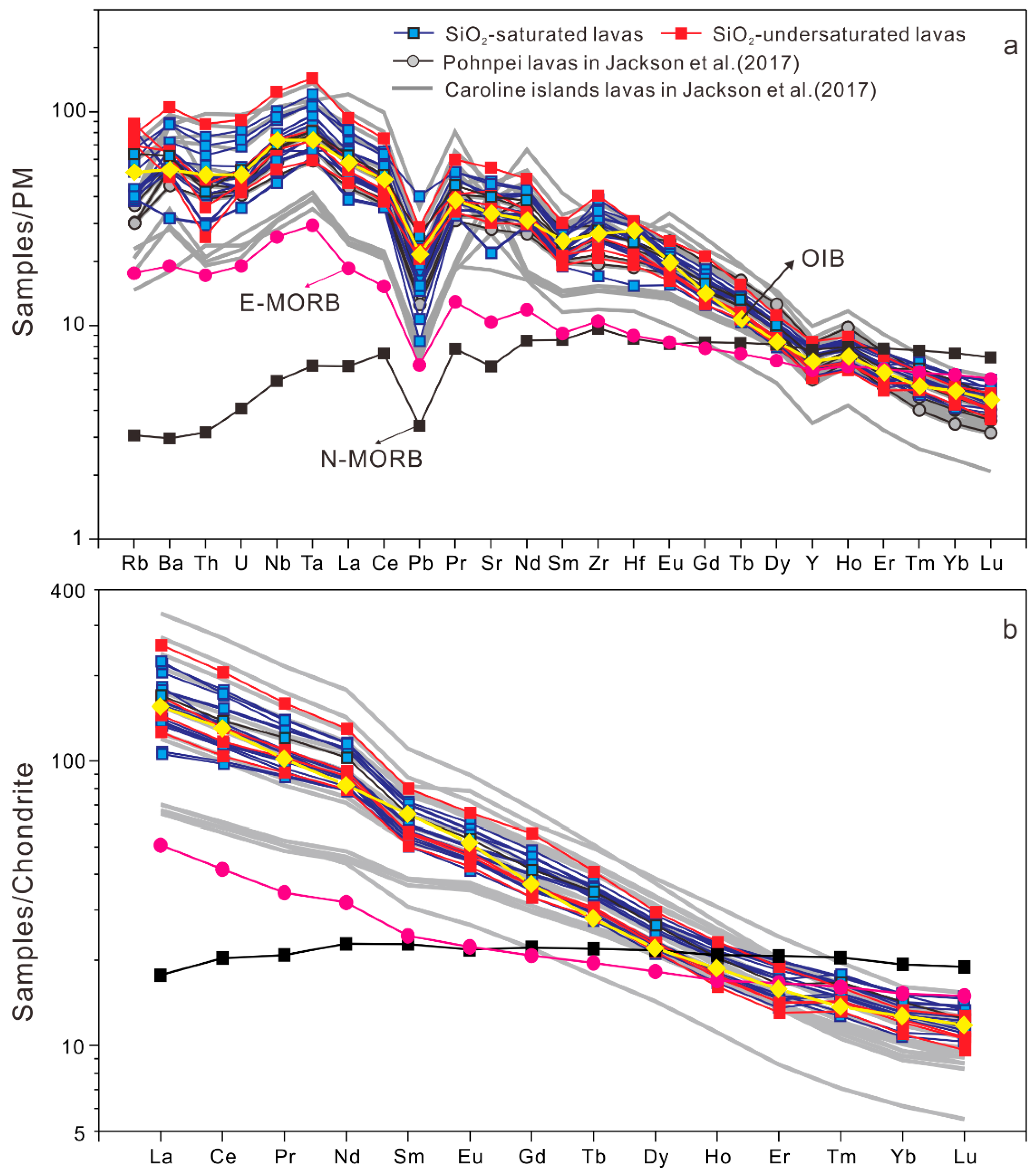
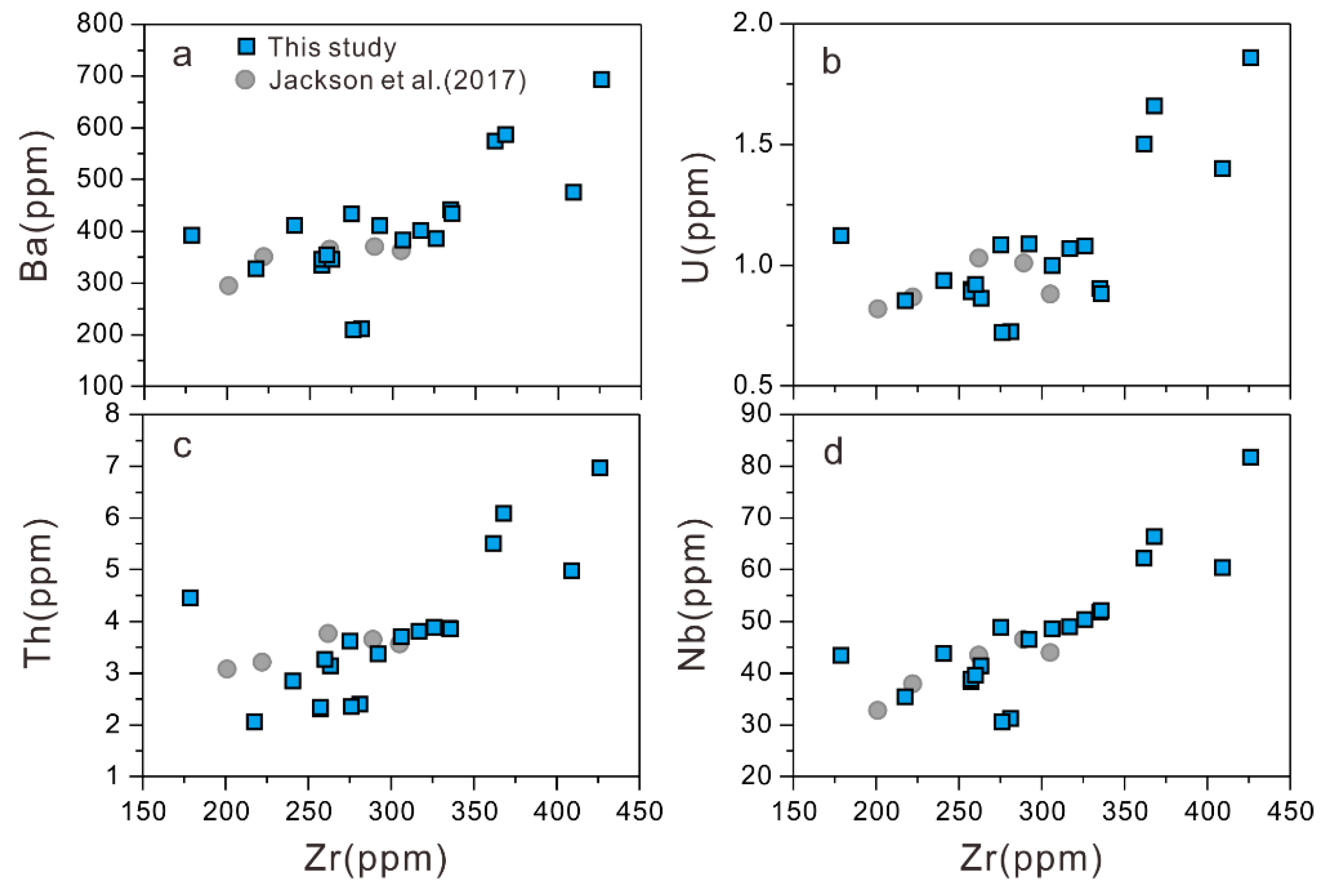
4.1.2. Trace Elements
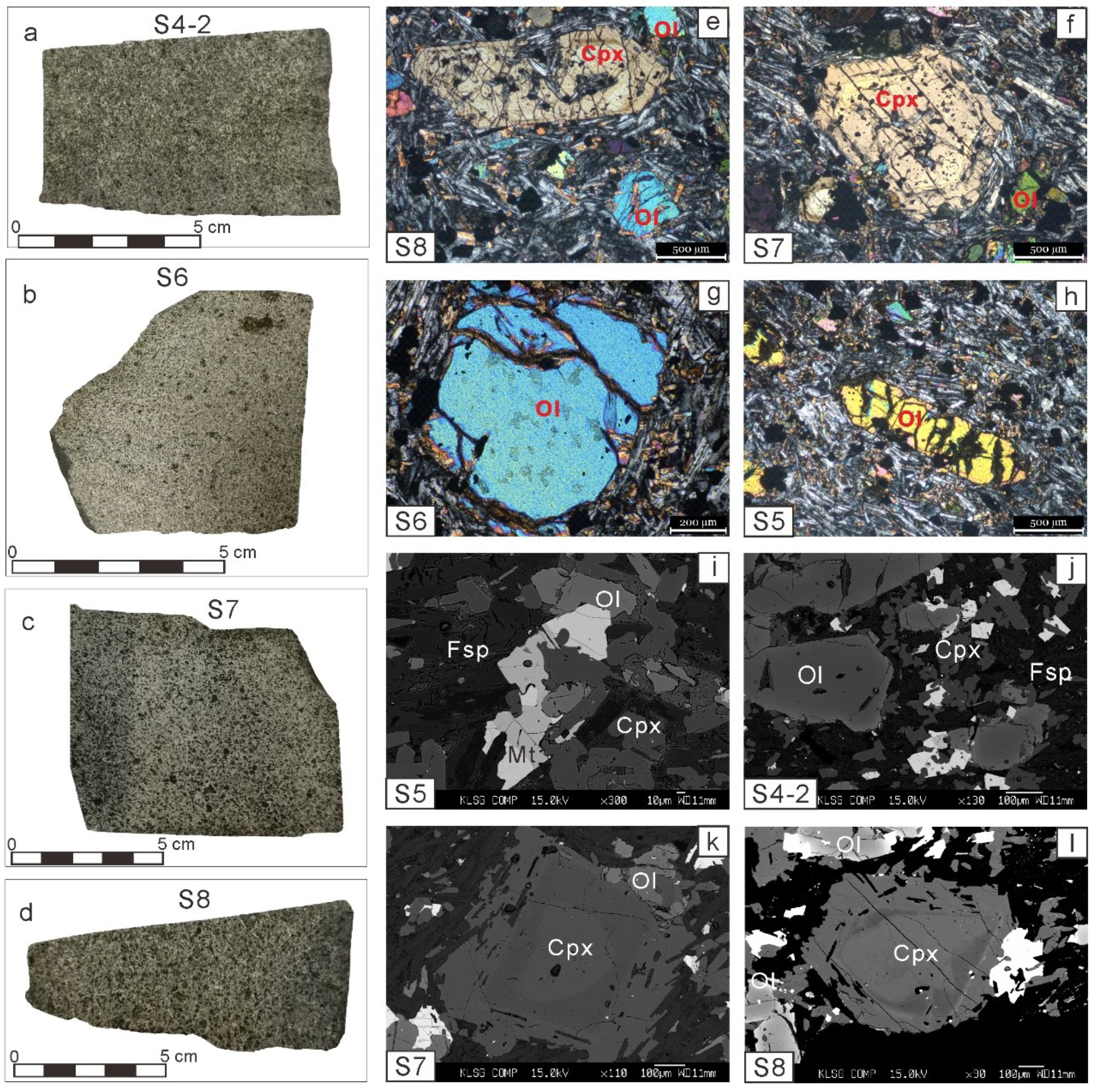
4.2. Major and Minor Elements in Minerals
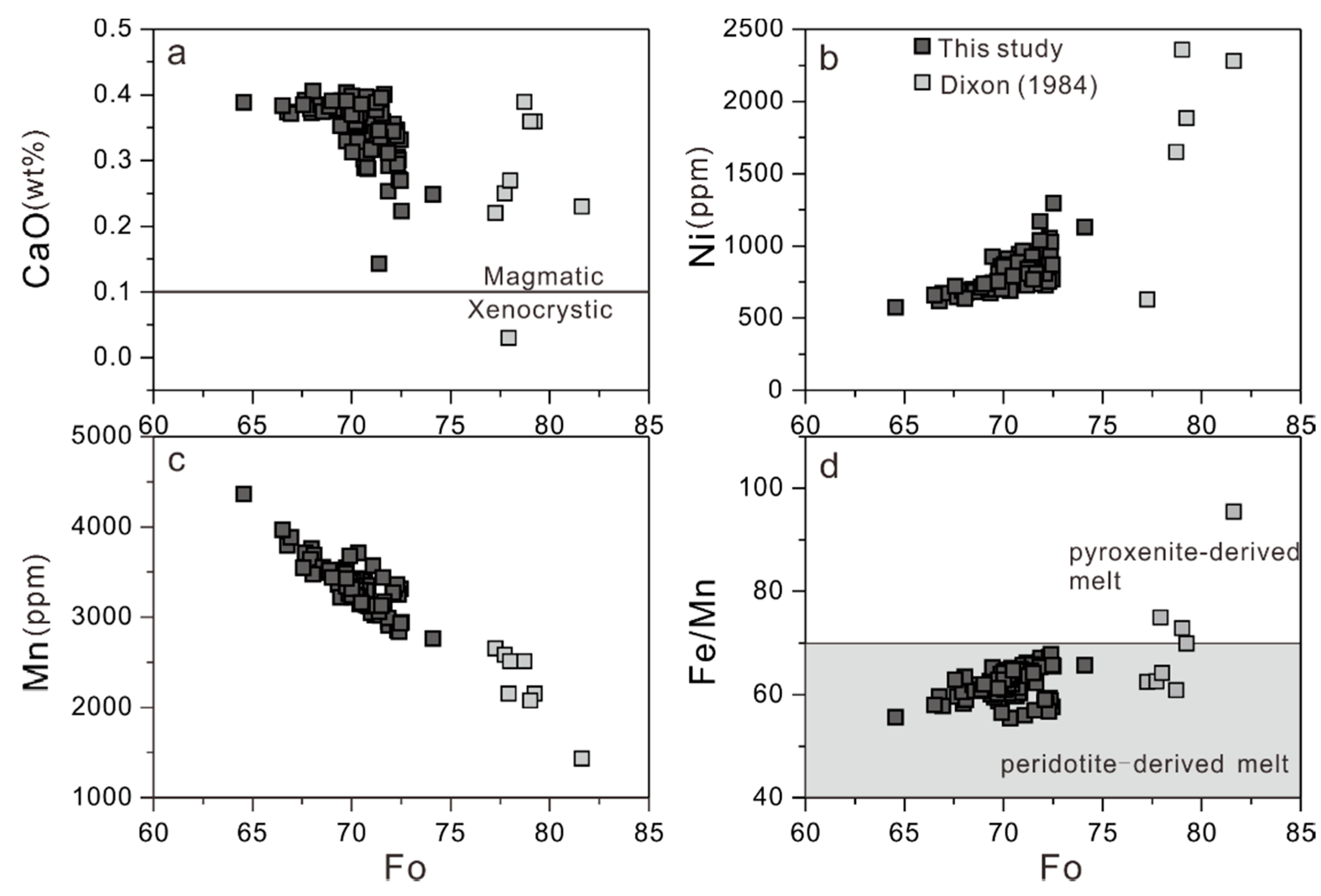
4.2.1. Olivine
4.2.2. Clinopyroxene
4.2.3. Feldspar
5. Discussion
5.1. Rock Alteration
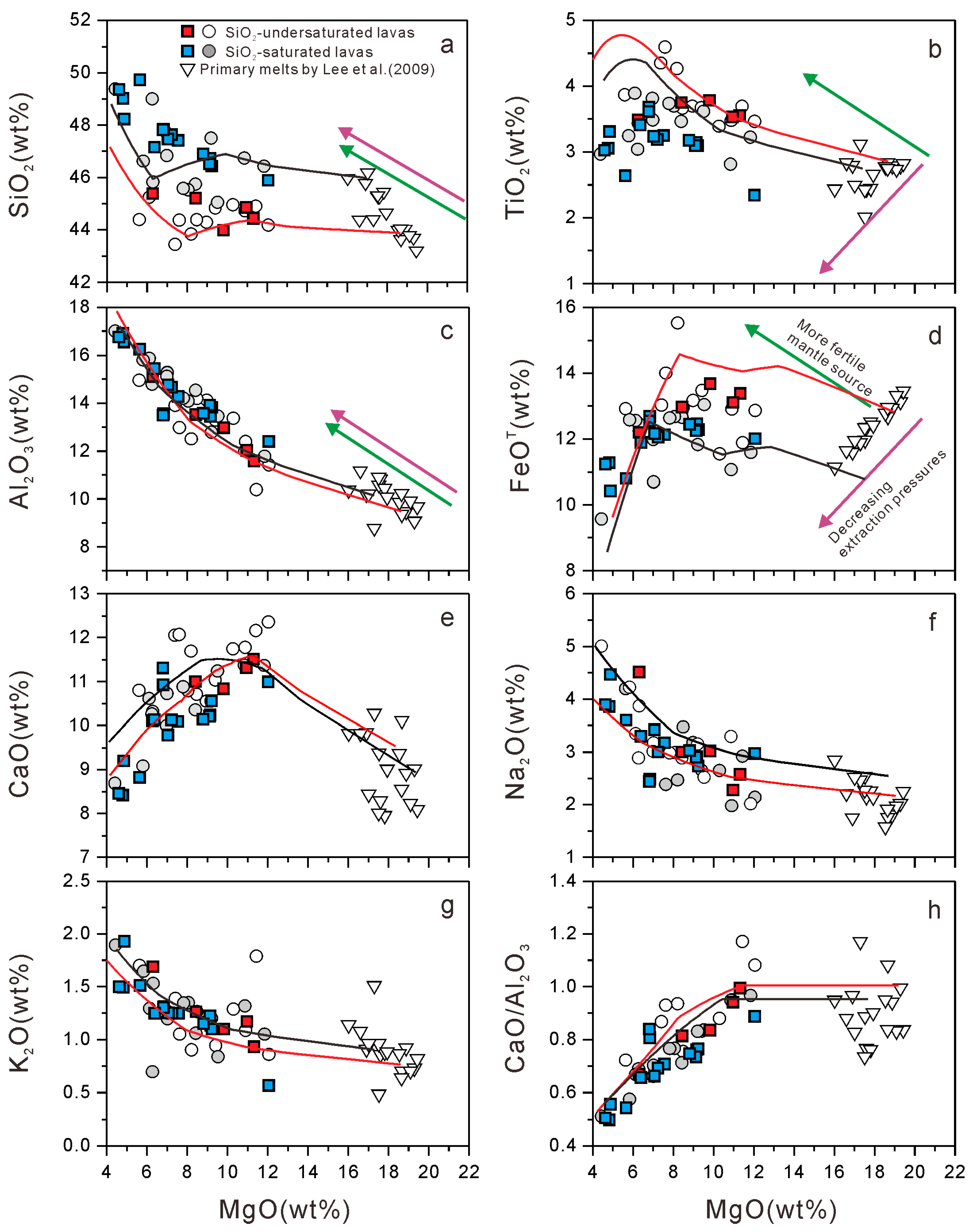
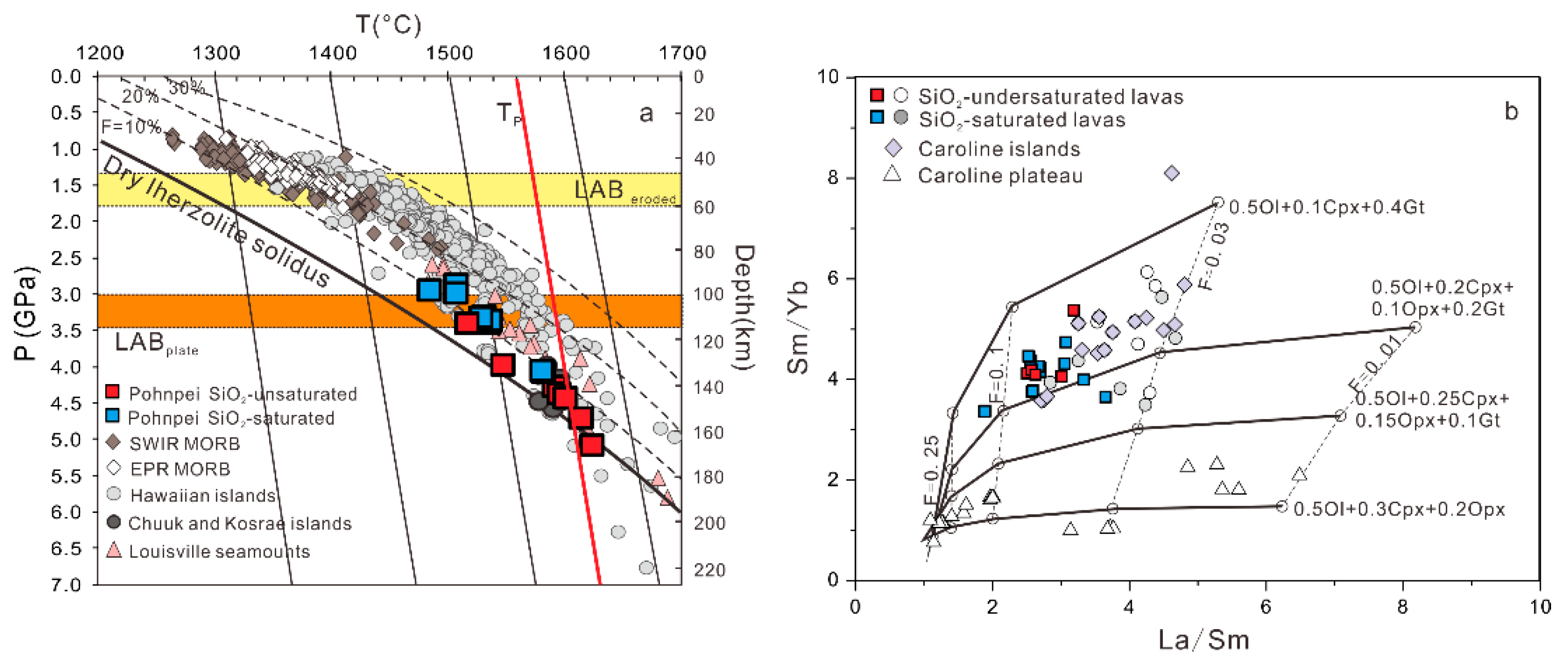
5.2. Primary Magma Composition and Mantle Melting Process
5.3. Modeling of Liquid Lines of Descent and Mineral Crystallization Sequence
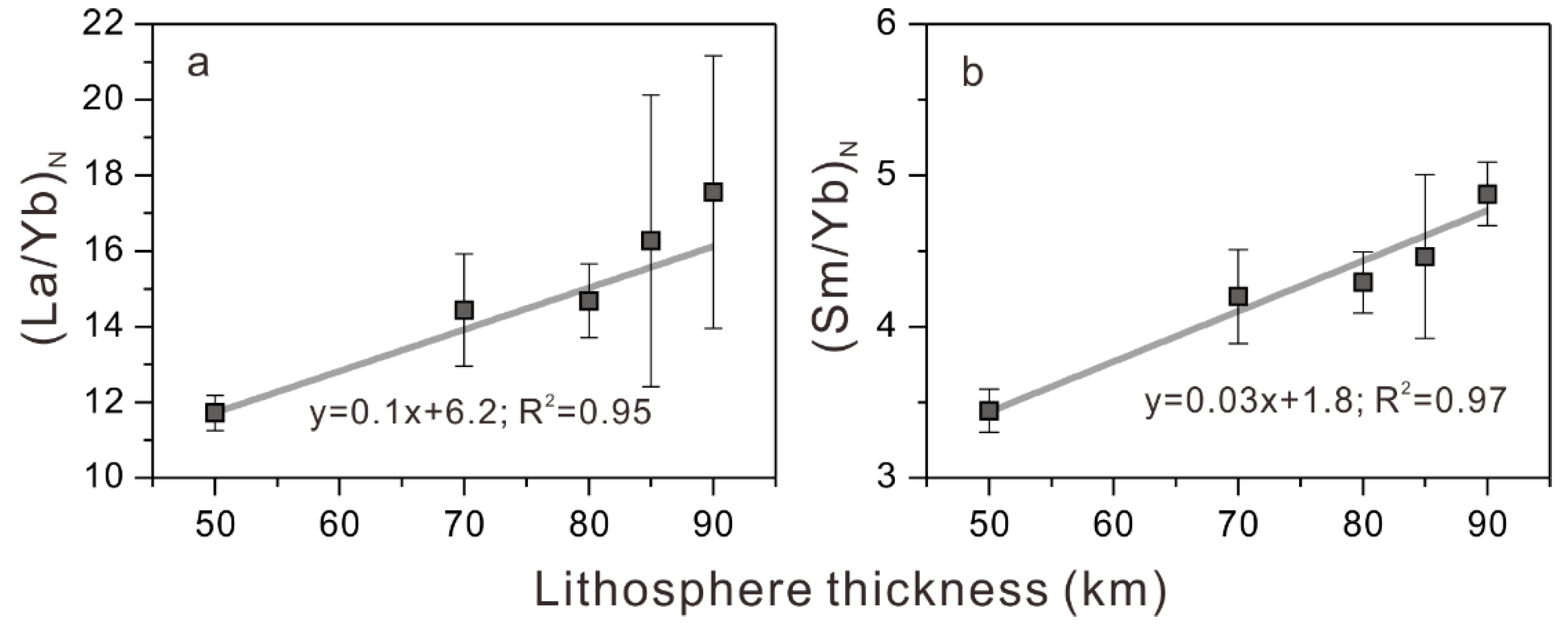
5.4. Lithospheric Thickness Control of Mantle Melting and Magma Evolution
6. Conclusions
- (1)
- Pohnpei Island lavas are all alkalic and can be classified as SiO2-undersaturated or SiO2-saturated, with the former having higher TiO2 and FeOT contents but with no distinct trace-element composition, suggesting they formed by partial melting of a compositionally homogenous mantle source at varying depth.
- (2)
- The primary magma underwent sequential crystallization of olivine, clinopyroxene, and Fe–Ti oxides (magnetite and ilmenite), as well as a minor plagioclase and alkali feldspar. Early magnetite crystallization (>8 wt.% MgO) and late feldspar crystallization were due to initially high FeOT and low Al2O3 contents in the primary magma, which resulted from a low degree of melting in response to a thick lithosphere (the “lid effect”).
- (3)
- The Pohnpei Island lavas formed at mantle-melting pressures of 2.9–5.1 GPa (average 3.8 ± 0.7 GPa) and temperatures of 1486–1626 °C (average 1557 ± 43 ℃) in a garnet stability field. Our trace-element data for basalt lavas of Pohnpei Island and Louisville seamounts indicate a strong correlation between (La/Yb)N and (Sm/Yb)N ratios and lithospheric thickness, supporting first-order control of lithospheric thickness on chemical compositions of ocean-island lavas.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morgan, W.J. Convection Plumes in the Lower Mantle. Nature 1971, 230, 42–43. [Google Scholar] [CrossRef]
- Mazza, S.E.; Gazel, E.; Bizimis, M.; Moucha, R.; Béguelin, P.; Johnson, E.A.; McAleer, R.J.; Sobolev, A.V. Sampling the volatile-rich transition zone beneath Bermuda. Nature 2019, 569, 398–403. [Google Scholar] [CrossRef]
- Aldanmaz, E.; Köprübaşı, N.; Gürer, Ö.F.; Kaymakçı, N.; Gourgaud, A. Geochemical constraints on the Cenozoic, OIB-type alkaline volcanic rocks of NW Turkey: Implications for mantle sources and melting processes. Lithos 2006, 86, 50–76. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Jackson, M.G.; Hofmann, A.W. Evolution of carbonated melt to alkali basalt in the South China Sea. Nat. Geosci. 2017, 10, 229–235. [Google Scholar] [CrossRef]
- Pilet, S.; Baker, M.B.; Stolper, E.M. Metasomatized Lithosphere and the Origin of Alkaline Lavas. Science 2008, 320, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, S.; Chen, L.; Li, S.; Zeng, G. Compositional transition in natural alkaline lavas through silica-undersaturated melt–lithosphere interaction. Geology (Boulder) 2018, 46, 771–774. [Google Scholar] [CrossRef]
- Maclennan, J.; McKenzie, D.; Grönvold, K.; Shimizu, N.; Eiler, J.M.; Kitchen, N. Melt mixing and crystallization under Theistareykir, northeast Iceland. Geochem. Geophys. Geosyst. 2003, 4. [Google Scholar] [CrossRef]
- Müller, R.D.; Sdrolias, M.; Gaina, C.; Roest, W.R. Age, spreading rates, and spreading asymmetry of the world’s ocean crust. Geochem. Geophys. Geosyst. 2008, 9. [Google Scholar] [CrossRef]
- Dixon, T.H.; Batiza, R.; Futa, K.; Martin, D. Petrochemistry, age and isotopic composition of alkali basalts from Ponape Island, Western Pacific. Chem. Geol. 1984, 43, 1–28. [Google Scholar] [CrossRef]
- Jackson, M.G.; Price, A.A.; Blichert-Toft, J.; Kurz, M.D.; Reinhard, A.A. Geochemistry of lavas from the Caroline hotspot, Micronesia: Evidence for primitive and recycled components in the mantle sources of lavas with moderately elevated 3He/4He. Chem. Geol. 2017, 455, 385–400. [Google Scholar] [CrossRef]
- Keating, B.H.; Mattey, D.P.; Helsley, C.E.; Naughton, J.J.; Epp, D.; Lazarewicz, A.; Schwank, D. Evidence for a hot spot origin of the Caroline Islands. J. Geophys. Res. Solid Earth 1984, 89, 9937–9948. [Google Scholar] [CrossRef]
- Keating, B.H.; Mattey, D.P.; Naughton, J.; Helsley, C.E. Age and origin of Truk Atoll, eastern Caroline Islands: Geochemical, radiometric-age, and paleomagnetic evidence. Geol. Soc. Am. Bull. 1984, 95, 350. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Wang, S.; Zhao, J. Geochemical and chronological constraints on the mantle plume origin of the Caroline Plateau. Chem. Geol. 2020, 540, 119566. [Google Scholar] [CrossRef]
- Spengler, S.; Spencer, K.; Mahoney, J.; Goles, G. Geology and Geochemical Evolution of Lavas on the Island of Pohnpei, Federated States of Micronesia. 1994. Available online: http://www.e2hi.com/wp-content/uploads/2012/10/12_Geology_and_Geochemical_Evolution_of_Lavas_on_the_Island_of_Pohnpei.pdf (accessed on 1 June 2020).
- Ridley, I.; Jakes, P.; Bass, M.; Rhodes, J.; Reid, A.; Shih, C. Basalts from Leg 6 of the Deep-Sea Drilling Project. J. Petrol. 1974, 15, 140–159. [Google Scholar] [CrossRef]
- Rehman, H.U.; Nakaya, H.; Kawai, K. Geological origin of the volcanic Islands of the Caroline Group in the Federated States of Micronesia, Western Pacific. South Pac. Stud. 2013, 33, 101–118. [Google Scholar]
- Dasgupta, R.; Jackson, M.G.; Lee, C.A. Major element chemistry of ocean Island basalts—Conditions of mantle melting and heterogeneity of mantle source. Earth Planet. Sci. Lett. 2010, 289, 377–392. [Google Scholar] [CrossRef]
- Mattey, D.P. The minor and trace element geochemistry of volcanic rocks from Truk, Ponape and Kusaie, Eastern Caroline Islands; the evolution of a young hot spot trace across Old Pacific Ocean Crust. Contrib. Mineral. Petr. 1982, 80, 1–13. [Google Scholar] [CrossRef]
- Eggins, S.M.; Woodhead, J.D.; Kinsley, L.P.J.; Mortimer, G.E.; Sylvester, P.; McCulloch, M.T.; Hergt, J.M.; Handler, M.R. A simple method for the precise determination of ≥40 trace elements in geological samples by ICPMS using enriched isotope internal standardisation. Chem. Geol. 1997, 134, 311–326. [Google Scholar] [CrossRef]
- Kamber, B.; Greig, A.; Schoenberg, R.; Collerson, K.D. A refined solution to Earth’s hidden niobium: Implications for evolution of continental crust and mode of core formation. Precambrian Res. 2003, 126. [Google Scholar] [CrossRef]
- Li, B.P.; Greig, A.; Zhao, J.X.; Collerson, K.D.; Quan, K.S.; Meng, Y.H.; Ma, Z.L. ICP-MS trace element analysis of Song dynasty porcelains from Ding, Jiexiu and Guantai kilns, north China. J. Archaeol. Sci. 2005, 32, 259. [Google Scholar] [CrossRef]
- Sobolev, A.V.; Hofmann, A.W.; Kuzmin, D.V.; Yaxley, G.M.; Arndt, N.T.; Chung, S.L.; Danyushevsky, L.V.; Elliott, T.; Frey, F.A.; Garcia, M.O. The Amount of Recycled Crust in Sources of Mantle-Derived Melts. Science 2007, 316, 412. [Google Scholar] [CrossRef]
- Li, Z.; Chu, F.; Zhu, J.; Ding, Y.; Zhu, Z.; Liu, J.; Wang, H.; Li, X.; Dong, Y.; Zhao, D. Magmatic sulfide formation and oxidative dissolution in the SW Okinawa Trough: A precursor to metal-bearing magmatic fluid. Geochim. Cosmochim. Acta 2019, 258, 138–155. [Google Scholar] [CrossRef]
- Zong, T.; Han, X.; Liu, J.; Wang, Y.; Qiu, Z.; Li, H.; Yu, X. H2O in basaltic glasses from the slow-spreading Carlsberg Ridge: Implications for mantle source and magmatic processes. Lithos 2019, 332–333, 274–286. [Google Scholar] [CrossRef]
- Zong, T.; Han, X.; Liu, J.; Wang, Y.; Qiu, Z.; Yu, X. Fractional crystallization processes of magma beneath the Carlsberg Ridge (57°–65° E). J. Oceanol. Limnol. 2019, 38, 75–92. [Google Scholar] [CrossRef]
- Klein, E.M.; Langmuir, C.H. Global correlations of ocean ridge basalt chemistry with axial depth and crustal thickness. J. Geophys. Res. Solid Earth 1987, 92, 8089–8115. [Google Scholar] [CrossRef]
- Langmuir, C.H.; Klein, E.M.; Plank, T. Petrological systematics of mid-ocean ridge basalts: Constraints on melt generation beneath ocean ridges. Geophys. Monogr. 1992, 71. [Google Scholar] [CrossRef]
- Fitton, J.G.; Godard, M. Origin and evolution of magmas on the Ontong Java Plateau. Geol. Soc. Lond. Spec. Pub. 2004, 229, 151–178. [Google Scholar] [CrossRef]
- Lassiter, J.C.; Hauri, E.H.; Reiners, P.W.; Garcia, M.O. Generation of Hawaiian post-erosional lavas by melting of a mixed lherzolite/pyroxenite source. Earth Planet Sci. Lett. 2000, 178, 284. [Google Scholar] [CrossRef]
- Phillips, E.H.; Sims, K.W.W.; Sherrod, D.R.; Salters, V.J.M.; Blusztajn, J.; Dulai, H. Isotopic constraints on the genesis and evolution of basanitic lavas at Haleakala, Island of Maui, Hawaii. Geochim. Cosmochim. Acta 2016, 195, 201–225. [Google Scholar] [CrossRef]
- Niu, Y. The Meaning of Global Ocean Ridge Basalt Major Element Compositions. J. Petrol. 2016, 57, 2081–2104. [Google Scholar] [CrossRef]
- Danyushevsky, L.V.; Plechov, P. Petrolog3: Integrated software for modeling crystallization processes. Geochem. Geophys. Geosyst. 2011, 12. [Google Scholar] [CrossRef]
- Gaetani, G.A.; Watson, E.B. Modeling the major-element evolution of olivine-hosted melt inclusions. Chem. Geol. 2002, 183, 25–41. [Google Scholar] [CrossRef]
- Ariskin, A.A.; Frenkel, M.Y.; Barmina, G.S.; Nielsen, R.L. Comagmat: A Fortran program to model magma differentiation processes. Comput. Geosci. UK 1993, 19, 1155–1170. [Google Scholar] [CrossRef]
- Ariskin, A.; Barmina, G. An empirical model for the calculation of spinel-melt equilibria in mafic igneous systems at atmospheric pressure: 2. Fe-Ti oxides. Contrib. Mineral. Petr. 1999, 134, 251–263. [Google Scholar] [CrossRef]
- Nielsen, R. EQUIL: A program for the modeling of low-pressure differentiation processes in natural mafic magma bodies. Comput. Geosci. 1985, 11, 531–546. [Google Scholar] [CrossRef]
- Sun, P.; Niu, Y.; Guo, P.; Duan, M.; Wang, X.; Gong, H.; Xiao, Y. The Lithospheric Thickness Control on the Compositional Variation of Continental Intraplate Basalts: A Demonstration Using the Cenozoic Basalts and Clinopyroxene Megacrysts from Eastern China. J. Geophys. Res. Solid Earth 2020, 125. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Gale, A.; Dalton, C.A.; Langmuir, C.H.; Su, Y.; Schilling, J. The mean composition of ocean ridge basalts. Geochem. Geophys. Geosyst. 2013, 14, 489–518. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geo. Soc. Lond. Spec. Pub. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Niu, Y.; O’Hara, M.J. MORB mantle hosts the missing Eu (Sr, Nb, Ta and Ti) in the continental crust: New perspectives on crustal growth, crust-mantle differentiation and chemical structure of oceanic upper mantle. Lithos 2009, 112, 1–17. [Google Scholar] [CrossRef]
- Niu, Y.; Waggoner, D.G.; Sinton, J.M.; Mahoney, J.J. Mantle source heterogeneity and melting processes beneath seafloor spreading centers: The East Pacific Rise, 18°–19° S. J. Geophys. Res. Solid Earth 1996, 101, 27711–27733. [Google Scholar] [CrossRef]
- Ren, Z.Y. Petrogenesis of Tholeiitic Lavas from the Submarine Hana Ridge, Haleakala Volcano, Hawaii. J. Petrol. 2004, 45, 2067–2099. [Google Scholar] [CrossRef][Green Version]
- Thompson, R.N.; Gibson, S.A. Transient high temperatures in mantle plume heads inferred from magnesian olivines in Phanerozoic picrites. Nature 2000, 407, 502–506. [Google Scholar] [CrossRef]
- Sobolev, A.V.; Hofmann, A.W.; Sobolev, S.V.; Nikogosian, I.K. An olivine-free mantle source of Hawaiian shield basalts. Nature 2005, 434, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Li, X.; Li, J.; Liu, Y.; Long, W.; Zhou, J.; Wang, F. Temperature, Pressure, and Composition of the Mantle Source Region of Late Cenozoic Basalts in Hainan Island, SE Asia: A Consequence of a Young Thermal Mantle Plume close to Subduction Zones? J. Petrol. 2012, 53, 177–233. [Google Scholar] [CrossRef]
- Lee, C.A.; Luffi, P.; Plank, T.; Dalton, H.; Leeman, W.P. Constraints on the depths and temperatures of basaltic magma generation on Earth and other terrestrial planets using new thermobarometers for mafic magmas. Earth Planet Sci. Lett. 2009, 279, 20–33. [Google Scholar] [CrossRef]
- Griffin, W.L.; O’Reilly, S.Y.; Afonso, J.C.; Begg, G.C. The Composition and Evolution of Lithospheric Mantle: A Re-evaluation and its Tectonic Implications. J. Petrol. 2009, 50, 1185–1204. [Google Scholar] [CrossRef]
- Walter, M. Melting of Garnet Peridotite and the Origin of Komatiite and Depleted Lithosphere. J. Petrol. 1998, 39, 29–60. [Google Scholar] [CrossRef]
- Fitton, J.G.; Saunders, A.D.; Norry, M.J.; Hardarson, B.S.; Taylor, R.N. Thermal and chemical structure of the Iceland plume. Earth Planet Sci. Lett. 1997, 153, 197–208. [Google Scholar] [CrossRef]
- Vanderkluysen, L.; Mahoney, J.J.; Koppers, A.A.P.; Beier, C.; Regelous, M.; Gee, J.S.; Lonsdale, P.F. Louisville Seamount Chain: Petrogenetic processes and geochemical evolution of the mantle source. Geochem. Geophys. Geosyst. 2014, 15, 2380–2400. [Google Scholar] [CrossRef]
- Humphreys, E.R.; Niu, Y. On the composition of ocean Island basalts (OIB): The effects of lithospheric thickness variation and mantle metasomatism. Lithos 2009, 112, 118–136. [Google Scholar] [CrossRef]
- Niu, Y.; Wilson, M.; Humphreys, E.R.; O’Hara, M.J. The Origin of Intra-plate Ocean Island Basalts (OIB): The Lid Effect and its Geodynamic Implications. J. Petrol. 2011, 52, 1443–1468. [Google Scholar] [CrossRef]
- Asimow, P.D.; Langmuir, C.H. The importance of water to oceanic mantle melting regimes. Nature 2003, 421, 815–820. [Google Scholar] [CrossRef]
- Katz, R.; Spiegelman, M.; Langmuir, C. A new parameterization of hydrous mantle melting. Geochem. Geophys. Geosyst 2003, 4. [Google Scholar] [CrossRef]
- Beier, C.; Vanderkluysen, L.; Regelous, M.; Mahoney, J.J.; Garbe-Schönberg, D. Lithospheric control on geochemical composition along the Louisville Seamount Chain. Geochem. Geophys. Geosyst. 2011, 12. [Google Scholar] [CrossRef]
- Almeev, R.R.; Ariskin, A.A.; Kimura, J.; Barmina, G.S. The role of polybaric crystallization in genesis of andesitic magmas: Phase equilibria simulations of the Bezymianny volcanic subseries. J. Volcanol. Geoth. Res. 2013, 263, 182–192. [Google Scholar] [CrossRef]
- Danyushevsky, L.V. The effect of small amounts of H2O on crystallisation of mid-ocean ridge and backarc basin magmas. J. Volcanol. Geoth. Res. 2001, 110, 265–280. [Google Scholar] [CrossRef]
- Zimmer, M.M.; Plank, T.; Hauri, E.H.; Yogodzinski, G.M.; Stelling, P.; Larsen, J.; Singer, B.; Jicha, B.; Mandeville, C.; Nye, C.J. The Role of Water in Generating the Calc-alkaline Trend: New Volatile Data for Aleutian Magmas and a New Tholeiitic Index. J. Petrol. 2010, 51, 2411–2444. [Google Scholar] [CrossRef]
- Husen, A.; Almeev, R.R.; Holtz, F. The Effect of H2O and Pressure on Multiple Saturation and Liquid Lines of Descent in Basalt from the Shatsky Rise. J. Petrol. 2016, 57, 309–344. [Google Scholar] [CrossRef]
- Berndt, J.; Koepke, J.; Holtz, F. An experimental investigation of the influence of water and oxygen fugacity on differentiation of MORB at 200 MPa. J. Petrol. 2005, 46, 135–167. [Google Scholar] [CrossRef]
- Li, Z.; Chu, F.; Dong, Y.; Li, X.; Liu, J.; Yang, K.; Tang, L. Origin of selective enrichment of Cu and Au in sulfide deposits formed at immature back-arc ridges: Examples from the Lau and Manus basins. Ore. Geol. Rev. 2016, 74, 52–62. [Google Scholar] [CrossRef]
- Davies, D.R.; Rawlinson, N.; Iaffaldano, G.; Campbell, I.H. Lithospheric controls on magma composition along Earth’s longest continental hotspot track. Nature 2015, 525, 511–514. [Google Scholar] [CrossRef]
- Hole, M.J.; Millett, J.M. Controls of Mantle Potential Temperature and Lithospheric Thickness on Magmatism in the North Atlantic Igneous Province. J. Petrol. 2016, 57, 417–436. [Google Scholar] [CrossRef]
- Koppers, A.; Duncan, R.; Steinberger, B. Implications of a nonlinear 40Ar/39Ar age progression along the Louisville seamount trail for models of fixed and moving hot spots. Geochem. Geophys. Geosyst. 2004, 5. [Google Scholar] [CrossRef]
- Mallik, A.; Lambart, S.; Chin, E.J. Tracking the Evolution of Magmas from Heterogeneous Mantle Sources to Eruption. In Mantle Convection and Surface Expressions (AGU Monograph Series); Konter, J., Ballmer, M., Cottaar, S., Marquardt, H., Eds.; arXiv: Ithaca, NY, USA, 2020. [Google Scholar]
- Pilet, S.; Abe, N.; Rochat, L.; Kaczmarek, M.A.; Hirano, N.; Machida, S.; Buchs, D.M.; Baumgartner, P.O.; Müntener, O. Pre-subduction metasomatic enrichment of the oceanic lithosphere induced by plate flexure. Nat. Geosci. 2016, 9. [Google Scholar] [CrossRef]
| Sample No. | P-1 | P-2 | WF-1 | WF-2 | WF-3 | NAN-1 | NAN-2 | NAN-3 | S1 | S3 |
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 (wt%) | 45.64 | 44.85 | 44.34 | 45.53 | 46.42 | 43.59 | 42.78 | 43.99 | 44.55 | 48.24 |
| TiO2 | 3.10 | 3.02 | 3.50 | 2.33 | 3.19 | 3.50 | 3.68 | 3.66 | 3.43 | 2.56 |
| Al2O3 | 13.21 | 12.92 | 11.90 | 12.31 | 15.93 | 11.36 | 12.62 | 13.17 | 14.84 | 15.77 |
| Fe2O3 | 13.46 | 13.30 | 14.41 | 13.25 | 11.15 | 14.60 | 14.79 | 14.03 | 13.30 | 11.66 |
| MnO | 0.17 | 0.17 | 0.18 | 0.17 | 0.18 | 0.17 | 0.17 | 0.17 | 0.19 | 0.18 |
| MgO | 8.55 | 8.77 | 10.84 | 11.95 | 4.67 | 11.09 | 9.55 | 8.19 | 6.16 | 5.46 |
| CaO | 9.88 | 9.84 | 11.20 | 10.91 | 8.86 | 11.31 | 10.55 | 10.71 | 9.93 | 8.57 |
| Na2O | 2.95 | 2.79 | 2.26 | 2.95 | 4.30 | 2.53 | 2.94 | 2.92 | 4.43 | 3.50 |
| K2O | 1.12 | 1.09 | 1.16 | 0.56 | 1.86 | 0.91 | 1.07 | 1.23 | 1.66 | 1.47 |
| P2O5 | 0.57 | 0.56 | 0.53 | 0.57 | 0.80 | 0.49 | 0.57 | 0.63 | 0.99 | 0.77 |
| LOI | 0.98 | 1.28 | 0.64 | 0.05 | 0.26 | 0.05 | 0.00 | 0.43 | 0.22 | 1.52 |
| Sum | 99.64 | 98.59 | 100.95 | 100.59 | 97.61 | 99.60 | 98.71 | 99.13 | 99.69 | 99.70 |
| Li (ppm) | 5.46 | 5.55 | 5.67 | 5.42 | 7.14 | 5.07 | 5.54 | 6.09 | 8.09 | 6.64 |
| Sc | 24.87 | 25.26 | 29.80 | 27.10 | 12.33 | 31.92 | 25.27 | 27.52 | 17.80 | 16.20 |
| V | 250.7 | 254.5 | 730.0 | 665.3 | 395.5 | 847.9 | 329.3 | 378.1 | 252.0 | 191.0 |
| Cr | 313.3 | 319.9 | 401.8 | 435.2 | 28.6 | 456.5 | 342.0 | 240.2 | 110.0 | 124.0 |
| Co | 65.57 | 53.31 | 66.98 | 64.28 | 71.86 | 65.16 | 63.33 | 56.14 | 70.20 | 57.30 |
| Ni | 162.0 | 166.6 | 128.6 | 131.5 | 25.4 | 108.0 | 171.0 | 139.4 | 74.2 | 74.0 |
| Cu | 62.68 | 58.02 | 53.10 | 69.19 | 39.51 | 90.68 | 56.46 | 76.79 | 44.60 | 34.20 |
| Zn | 137.0 | 133.1 | 136.0 | 121.2 | 126.1 | 130.3 | 142.3 | 143.9 | 156.0 | 137.0 |
| Rb | 49.81 | 49.37 | 41.65 | 24.98 | 40.28 | 42.39 | 52.87 | 38.07 | 46.70 | 31.50 |
| Sr | 674.2 | 683.2 | 847.2 | 612.9 | 913.2 | 602.9 | 729.2 | 796.2 | 1090 | 775.0 |
| Y | 27.42 | 27.00 | 26.21 | 25.07 | 31.51 | 24.49 | 27.21 | 31.04 | 36.20 | 33.30 |
| Zr | 257.3 | 257.3 | 275.0 | 178.6 | 361.6 | 217.4 | 240.5 | 292.0 | 426.0 | 409.0 |
| Nb | 38.37 | 38.90 | 48.89 | 43.38 | 62.22 | 35.42 | 43.78 | 46.49 | 81.80 | 60.40 |
| Cs | 0.36 | 0.43 | 0.48 | 0.27 | 0.70 | 0.36 | 0.40 | 0.42 | 0.45 | 0.20 |
| Ba | 334.7 | 346.0 | 432.9 | 392.6 | 575.2 | 328.0 | 411.5 | 410.9 | 694.0 | 476.0 |
| La | 33.52 | 32.83 | 40.01 | 43.32 | 53.20 | 30.05 | 34.47 | 40.47 | 60.70 | 48.70 |
| Ce | 70.85 | 70.13 | 80.18 | 80.58 | 106.19 | 63.76 | 71.93 | 84.89 | 126.0 | 104.0 |
| Pr | 9.57 | 9.48 | 10.43 | 10.03 | 13.27 | 8.67 | 9.77 | 11.50 | 15.20 | 12.60 |
| Nd | 40.73 | 40.14 | 43.12 | 40.18 | 53.96 | 37.32 | 41.48 | 48.18 | 60.90 | 50.90 |
| Sm | 8.36 | 8.22 | 8.59 | 7.67 | 10.32 | 7.76 | 8.66 | 9.96 | 12.30 | 10.30 |
| Eu | 2.63 | 2.61 | 2.70 | 2.39 | 3.23 | 2.49 | 2.78 | 3.11 | 3.83 | 3.19 |
| Gd | 7.28 | 7.22 | 7.48 | 6.82 | 8.98 | 6.86 | 7.52 | 8.58 | 11.50 | 9.58 |
| Tb | 1.12 | 1.12 | 1.13 | 1.03 | 1.35 | 1.06 | 1.15 | 1.31 | 1.54 | 1.32 |
| Dy | 5.91 | 5.86 | 5.77 | 5.34 | 6.88 | 5.48 | 5.91 | 6.76 | 7.55 | 6.73 |
| Ho | 1.02 | 1.01 | 0.99 | 0.94 | 1.19 | 0.92 | 1.01 | 1.16 | 1.32 | 1.22 |
| Er | 2.44 | 2.43 | 2.36 | 2.25 | 2.85 | 2.18 | 2.36 | 2.74 | 3.16 | 3.04 |
| Tm | 0.39 | 0.38 | 0.37 | 0.36 | 0.46 | 0.34 | 0.37 | 0.43 | 0.41 | 0.42 |
| Yb | 2.21 | 2.18 | 2.12 | 2.10 | 2.58 | 1.88 | 2.07 | 2.43 | 2.29 | 2.39 |
| Lu | 0.29 | 0.29 | 0.27 | 0.28 | 0.34 | 0.25 | 0.27 | 0.32 | 0.33 | 0.35 |
| Hf | 6.15 | 6.24 | 6.42 | 4.35 | 8.03 | 5.43 | 5.86 | 6.94 | 8.71 | 8.65 |
| Ta | 2.42 | 2.46 | 3.03 | 2.42 | 3.93 | 2.19 | 2.70 | 3.01 | 5.32 | 4.04 |
| Pb | 3.53 | 3.43 | 4.09 | 3.22 | 6.06 | 3.08 | 3.38 | 3.94 | 4.34 | 3.12 |
| Th | 2.30 | 2.35 | 3.62 | 4.46 | 5.51 | 2.06 | 2.85 | 3.37 | 6.97 | 4.99 |
| U | 0.90 | 0.89 | 1.09 | 1.12 | 1.50 | 0.85 | 0.94 | 1.09 | 1.86 | 1.40 |
| Sample No. | S4-2 | S5 | S6 | S7 | S8 | AWAK-1 | AWAK-2-1 | AWAK-2-2 | OHWA-1-1 | OHWA-1-2 |
| SiO2 (wt%) | 46.12 | 46.34 | 46.19 | 45.28 | 45.19 | 45.86 | 45.76 | 45.95 | 47.94 | 48.07 |
| TiO2 | 3.09 | 3.17 | 3.18 | 3.03 | 3.01 | 3.32 | 3.46 | 3.54 | 2.94 | 3.01 |
| Al2O3 | 14.20 | 14.42 | 13.91 | 13.48 | 13.52 | 15.01 | 12.91 | 13.02 | 16.28 | 16.58 |
| Fe2O3 | 12.97 | 13.20 | 13.15 | 13.31 | 13.24 | 12.86 | 13.35 | 13.55 | 12.14 | 12.30 |
| MnO | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.19 | 0.18 | 0.18 | 0.17 | 0.18 |
| MgO | 6.99 | 6.87 | 7.35 | 8.98 | 8.86 | 6.19 | 6.50 | 6.55 | 4.46 | 4.70 |
| CaO | 9.82 | 9.56 | 9.84 | 10.31 | 9.93 | 9.86 | 10.83 | 10.50 | 8.22 | 8.26 |
| Na2O | 2.91 | 3.34 | 3.10 | 2.67 | 2.83 | 3.21 | 2.34 | 2.39 | 3.78 | 3.79 |
| K2O | 1.21 | 1.22 | 1.22 | 1.07 | 1.19 | 1.22 | 1.25 | 1.26 | 1.46 | 1.46 |
| P2O5 | 0.64 | 0.64 | 0.64 | 0.54 | 0.55 | 0.79 | 0.44 | 0.43 | 0.93 | 0.94 |
| LOI | 1.58 | 0.97 | 1.46 | 0.76 | 1.01 | 1.53 | 2.43 | 2.29 | 0.87 | 0.75 |
| Sum | 99.69 | 99.92 | 100.20 | 99.59 | 99.49 | 100.04 | 99.45 | 99.65 | 99.19 | 100.04 |
| Li (ppm) | 6.08 | 6.12 | 6.25 | 4.99 | 5.34 | 6.32 | 2.70 | 2.74 | 5.22 | 5.91 |
| Sc | 20.90 | 21.10 | 22.00 | 23.40 | 22.10 | 17.50 | 29.70 | 29.60 | 13.60 | 13.70 |
| V | 257.0 | 256.0 | 265.0 | 271.0 | 255.0 | 227.0 | 311.0 | 312.0 | 173.0 | 173.0 |
| Cr | 197.0 | 179.0 | 222.0 | 336.0 | 316.0 | 97.8 | 129.0 | 126.0 | 40.8 | 40.7 |
| Co | 62.70 | 63.70 | 72.50 | 76.20 | 64.50 | 55.20 | 49.60 | 54.20 | 38.90 | 37.50 |
| Ni | 110.0 | 106.0 | 124.0 | 172.0 | 160.0 | 69.6 | 78.7 | 77.6 | 22.8 | 23.3 |
| Cu | 49.30 | 50.70 | 49.90 | 51.80 | 49.80 | 44.30 | 88.60 | 90.40 | 21.90 | 24.50 |
| Zn | 144.0 | 133.0 | 131.0 | 123.0 | 119.0 | 139.0 | 117.0 | 115.0 | 133.0 | 133.0 |
| Rb | 24.20 | 26.00 | 23.00 | 24.10 | 26.20 | 35.10 | 23.70 | 23.50 | 23.60 | 24.20 |
| Sr | 866.0 | 634.0 | 725.0 | 707.0 | 665.0 | 942.0 | 438.0 | 435.0 | 819.0 | 793.0 |
| Y | 31.20 | 31.70 | 30.90 | 26.80 | 26.70 | 34.00 | 33.60 | 32.70 | 32.60 | 33.20 |
| Zr | 317.0 | 326.0 | 306.0 | 263.0 | 260.0 | 368.0 | 281.0 | 276.0 | 335.0 | 336.0 |
| Nb | 49.00 | 50.40 | 48.60 | 41.40 | 39.70 | 66.40 | 31.20 | 30.60 | 51.90 | 52.10 |
| Cs | 0.31 | 0.35 | 0.33 | 0.28 | 0.30 | 0.64 | 0.06 | 0.06 | 0.13 | 0.15 |
| Ba | 401.0 | 386.0 | 383.0 | 346.0 | 355.0 | 587.0 | 211.0 | 210.0 | 442.0 | 435.0 |
| La | 38.10 | 38.40 | 37.40 | 31.60 | 32.20 | 52.30 | 25.60 | 25.10 | 42.00 | 42.20 |
| Ce | 83.10 | 83.00 | 81.30 | 68.40 | 69.40 | 109.0 | 61.10 | 59.90 | 92.90 | 93.90 |
| Pr | 10.30 | 10.40 | 10.10 | 8.70 | 8.94 | 13.30 | 8.44 | 8.34 | 12.20 | 12.30 |
| Nd | 42.80 | 43.20 | 42.20 | 36.60 | 37.90 | 54.40 | 37.20 | 36.70 | 51.80 | 51.90 |
| Sm | 9.12 | 9.21 | 9.03 | 7.97 | 8.15 | 11.00 | 8.74 | 8.62 | 10.70 | 10.80 |
| Eu | 2.87 | 2.91 | 2.88 | 2.57 | 2.63 | 3.51 | 2.75 | 2.71 | 3.32 | 3.35 |
| Gd | 8.78 | 8.88 | 8.77 | 7.67 | 7.51 | 9.97 | 8.28 | 8.17 | 9.41 | 9.47 |
| Tb | 1.24 | 1.26 | 1.23 | 1.08 | 1.09 | 1.42 | 1.29 | 1.26 | 1.35 | 1.36 |
| Dy | 6.36 | 6.49 | 6.34 | 5.52 | 5.69 | 7.30 | 6.91 | 6.90 | 7.00 | 7.01 |
| Ho | 1.15 | 1.17 | 1.14 | 0.99 | 1.02 | 1.29 | 1.29 | 1.28 | 1.25 | 1.27 |
| Er | 2.82 | 2.90 | 2.81 | 2.42 | 2.50 | 3.15 | 3.29 | 3.26 | 3.12 | 3.15 |
| Tm | 0.38 | 0.39 | 0.38 | 0.32 | 0.33 | 0.42 | 0.45 | 0.44 | 0.42 | 0.42 |
| Yb | 2.16 | 2.22 | 2.12 | 1.82 | 1.88 | 2.32 | 2.59 | 2.56 | 2.40 | 2.42 |
| Lu | 0.31 | 0.32 | 0.30 | 0.26 | 0.28 | 0.34 | 0.38 | 0.37 | 0.35 | 0.35 |
| Hf | 7.06 | 7.22 | 6.84 | 5.95 | 6.42 | 8.66 | 7.16 | 7.04 | 8.18 | 8.31 |
| Ta | 3.22 | 3.40 | 3.25 | 2.76 | 2.83 | 4.47 | 2.29 | 2.30 | 3.60 | 3.56 |
| Pb | 2.59 | 2.71 | 2.45 | 2.19 | 2.30 | 3.73 | 1.61 | 1.27 | 2.91 | 2.90 |
| Th | 3.81 | 3.89 | 3.71 | 3.14 | 3.27 | 6.09 | 2.40 | 2.36 | 3.87 | 3.86 |
| U | 1.07 | 1.08 | 0.99 | 0.86 | 0.92 | 1.66 | 0.73 | 0.72 | 0.90 | 0.88 |
| Samples | SiO2 | TiO2 | Al2O3 | FeOT | MnO | MgO | CaO | Na2O | K2O | T (°C) | P (GPa) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WF-2 | 45.26 | 2.00 | 10.57 | 11.97 | 0.15 | 17.55 | 9.37 | 2.54 | 0.48 | 1537 | 3.4 |
| NAN-1 | 43.68 | 2.79 | 9.06 | 13.14 | 0.14 | 19.33 | 9.01 | 2.02 | 0.73 | 1616 | 4.7 |
| WF-1 | 44.03 | 2.79 | 9.46 | 12.93 | 0.14 | 18.91 | 8.91 | 1.79 | 0.92 | 1593 | 4.3 |
| NAN-2 | 43.19 | 2.82 | 9.67 | 13.46 | 0.13 | 19.47 | 8.08 | 2.25 | 0.82 | 1626 | 5.1 |
| S7 | 45.26 | 2.43 | 10.82 | 12.36 | 0.13 | 17.62 | 8.27 | 2.14 | 0.86 | 1533 | 3.3 |
| P-02 | 45.44 | 2.44 | 10.43 | 12.52 | 0.14 | 17.85 | 7.94 | 2.26 | 0.88 | 1538 | 3.4 |
| S8 | 45.31 | 2.43 | 10.89 | 12.35 | 0.14 | 17.55 | 8.00 | 2.28 | 0.96 | 1530 | 3.3 |
| PON13-26 a | 43.62 | 2.84 | 9.33 | 12.68 | 0.14 | 18.70 | 10.11 | 1.75 | 0.70 | 1597 | 4.4 |
| PON13-06 a | 43.97 | 2.77 | 9.85 | 12.78 | 0.15 | 18.57 | 9.36 | 1.57 | 0.86 | 1583 | 4.1 |
| PON13-13 a | 43.78 | 2.73 | 9.88 | 13.33 | 0.15 | 19.13 | 8.21 | 1.97 | 0.70 | 1602 | 4.5 |
| P-2 b | 45.77 | 2.79 | 10.18 | 11.60 | 0.13 | 16.93 | 9.83 | 1.74 | 0.91 | 1509 | 2.9 |
| P18 b | 44.39 | 3.12 | 8.76 | 11.88 | 0.15 | 17.32 | 10.28 | 2.47 | 1.51 | 1549 | 4.0 |
| P23 b | 45.97 | 2.43 | 10.36 | 11.14 | 0.14 | 16.05 | 9.83 | 2.85 | 1.14 | 1486 | 3.0 |
| P-3B c | 44.36 | 2.83 | 11.17 | 11.64 | 0.15 | 16.63 | 9.82 | 2.21 | 1.08 | 1518 | 3.4 |
| P-9 c | 44.04 | 2.75 | 10.22 | 12.97 | 0.13 | 18.69 | 8.54 | 1.91 | 0.64 | 1584 | 4.1 |
| P27 c | 46.17 | 2.49 | 10.20 | 11.96 | 0.13 | 17.04 | 8.43 | 2.52 | 0.96 | 1508 | 3.0 |
| Average | 44.64 | 2.65 | 10.05 | 12.42 | 0.14 | 17.96 | 9.00 | 2.14 | 0.88 | 1557 | 3.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, T.; Li, Z.-G.; Dong, Y.-H.; Li, X.-P.; Zhu, J.-H.; Chen, L.; Liu, J.-Q. Geochemical Constraints on Mantle Melting and Magma Genesis at Pohnpei Island, Micronesia. Minerals 2020, 10, 816. https://doi.org/10.3390/min10090816
Zong T, Li Z-G, Dong Y-H, Li X-P, Zhu J-H, Chen L, Liu J-Q. Geochemical Constraints on Mantle Melting and Magma Genesis at Pohnpei Island, Micronesia. Minerals. 2020; 10(9):816. https://doi.org/10.3390/min10090816
Chicago/Turabian StyleZong, Tong, Zheng-Gang Li, Yan-Hui Dong, Xu-Ping Li, Ji-Hao Zhu, Ling Chen, and Ji-Qiang Liu. 2020. "Geochemical Constraints on Mantle Melting and Magma Genesis at Pohnpei Island, Micronesia" Minerals 10, no. 9: 816. https://doi.org/10.3390/min10090816
APA StyleZong, T., Li, Z.-G., Dong, Y.-H., Li, X.-P., Zhu, J.-H., Chen, L., & Liu, J.-Q. (2020). Geochemical Constraints on Mantle Melting and Magma Genesis at Pohnpei Island, Micronesia. Minerals, 10(9), 816. https://doi.org/10.3390/min10090816





