Complexation of Alkali and Alkaline-Earth Metal Cations at Spodumene-Saltwater Interfaces by Molecular Simulation: Impact on Oleate Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Force Field
2.2. Simulation Details
3. Results
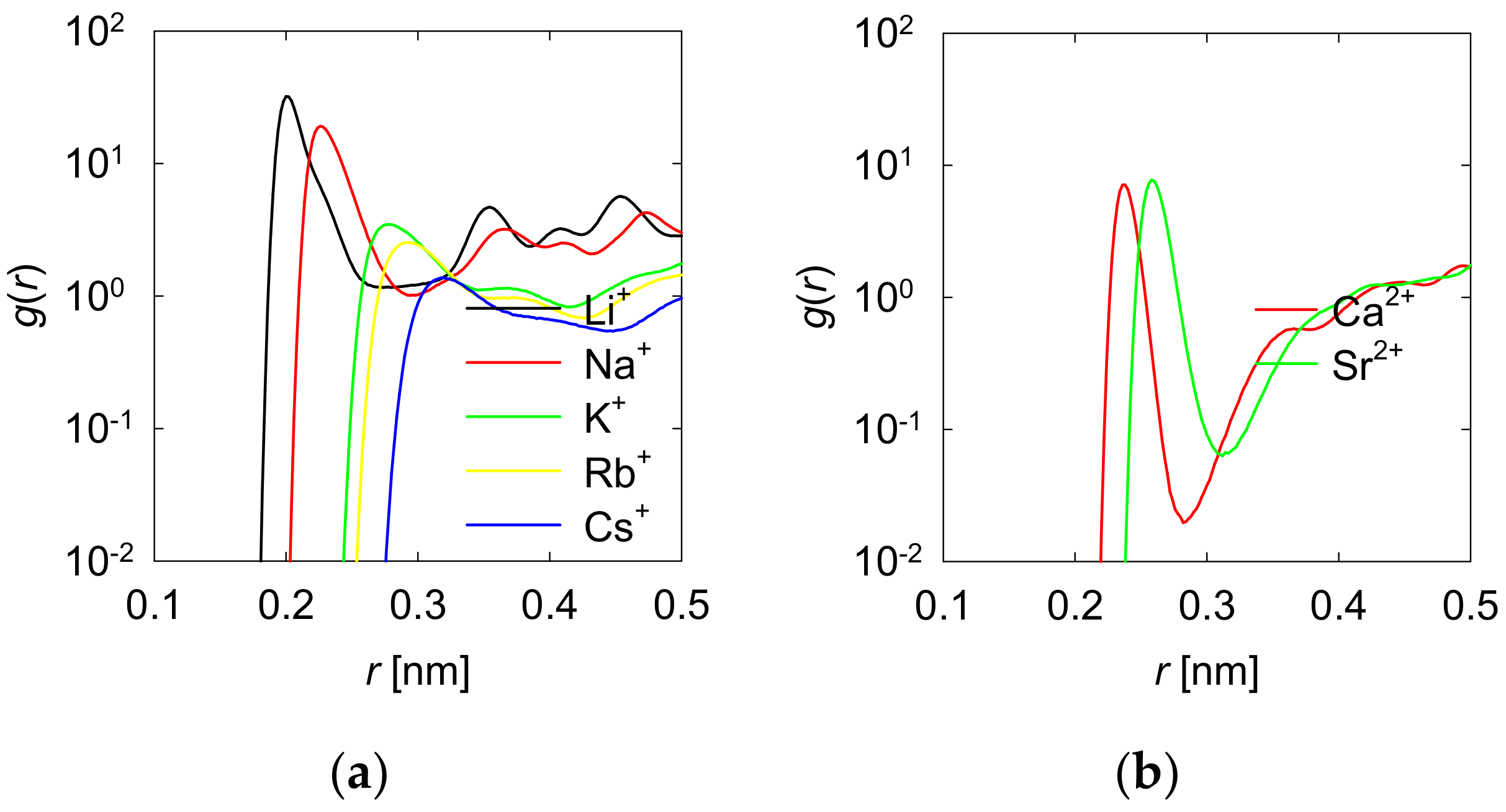
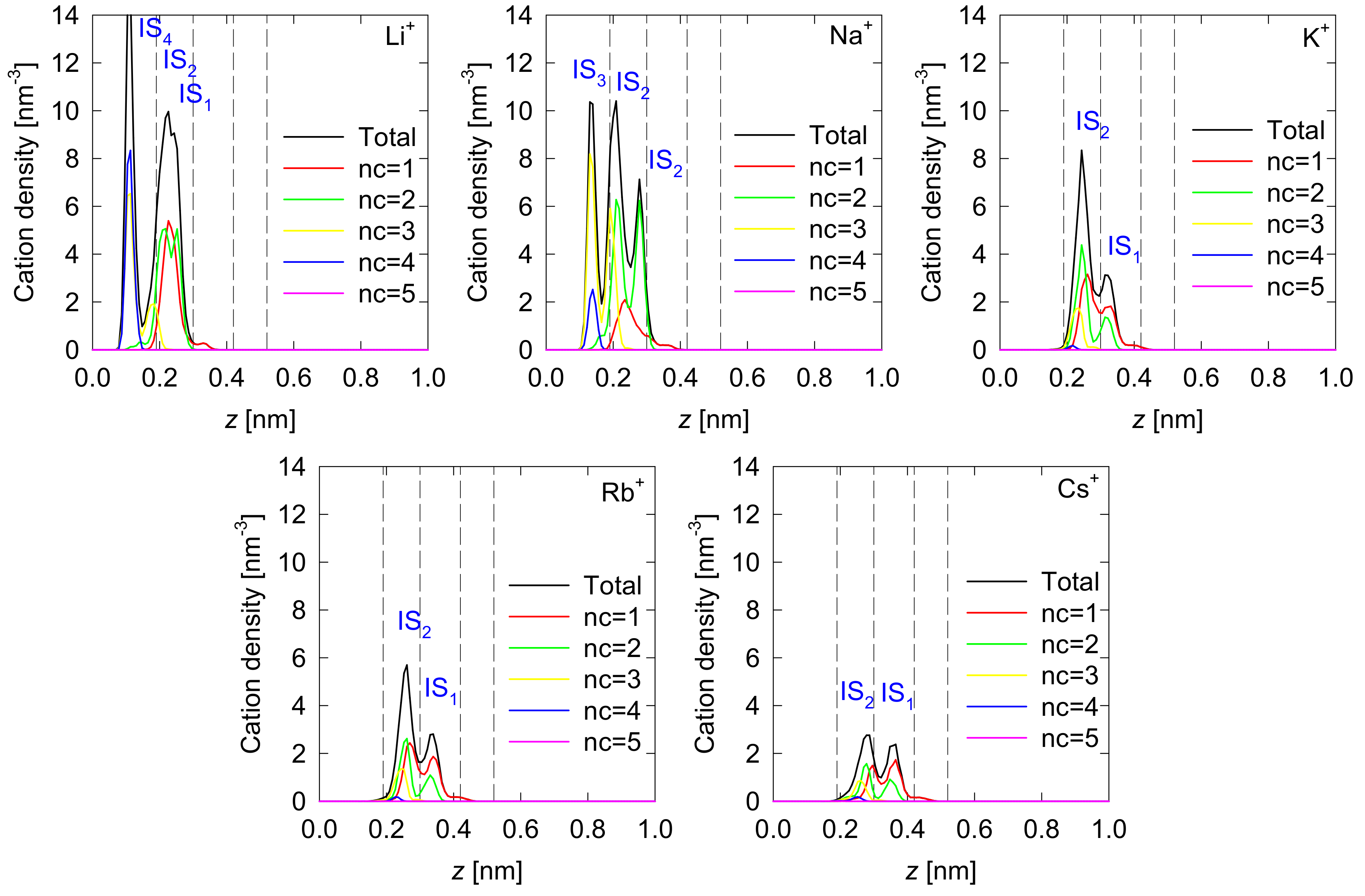
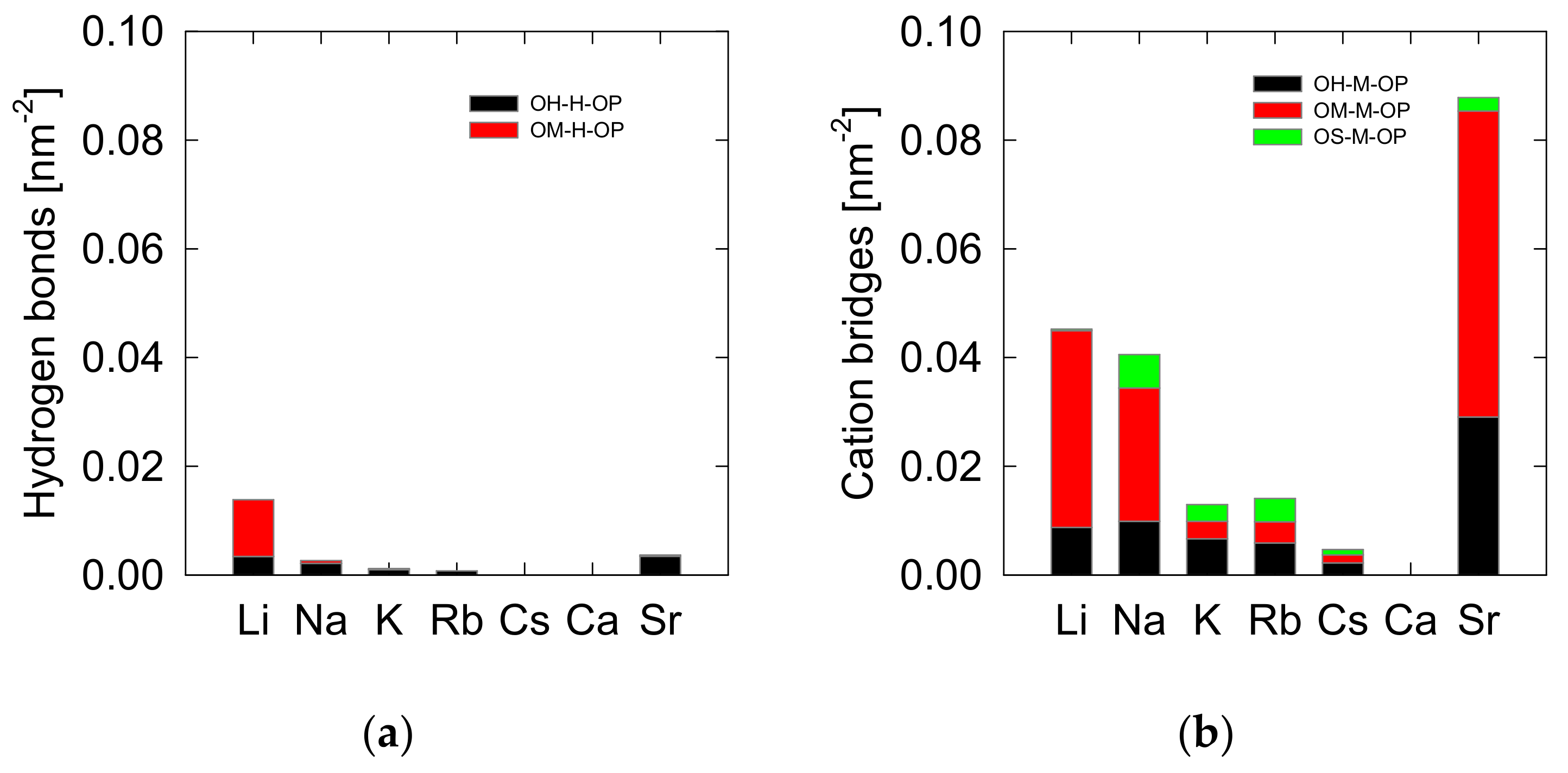
3.1. Density Profiles of Cation Inner-Sphere Complexes
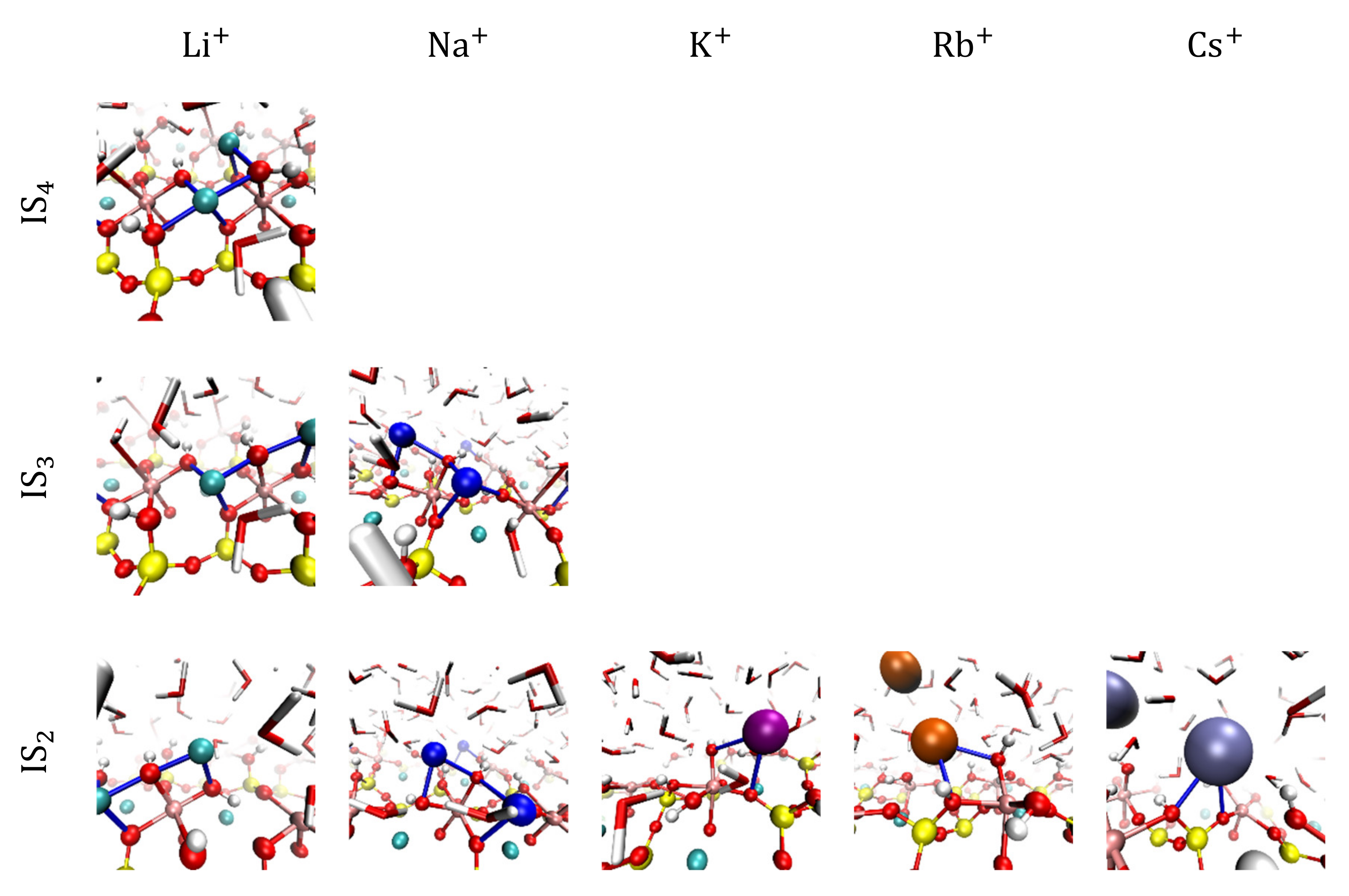
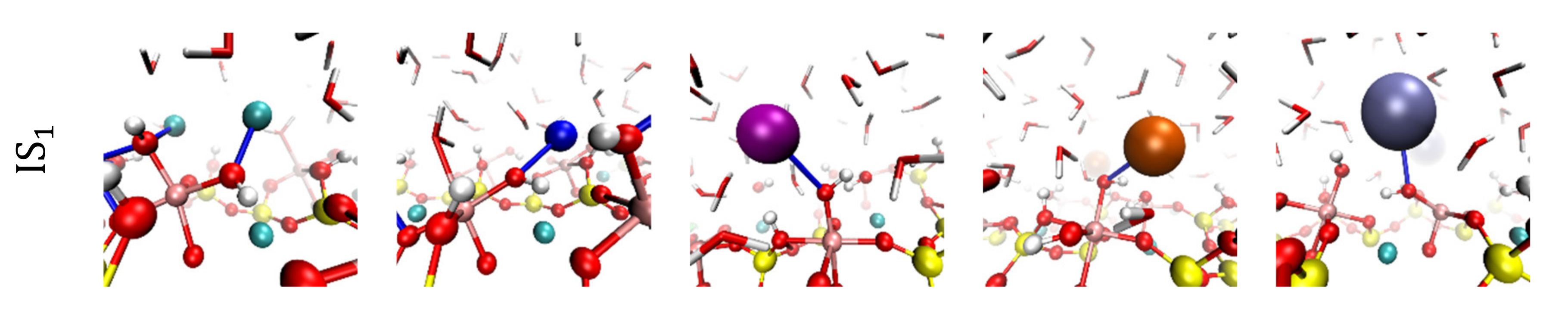
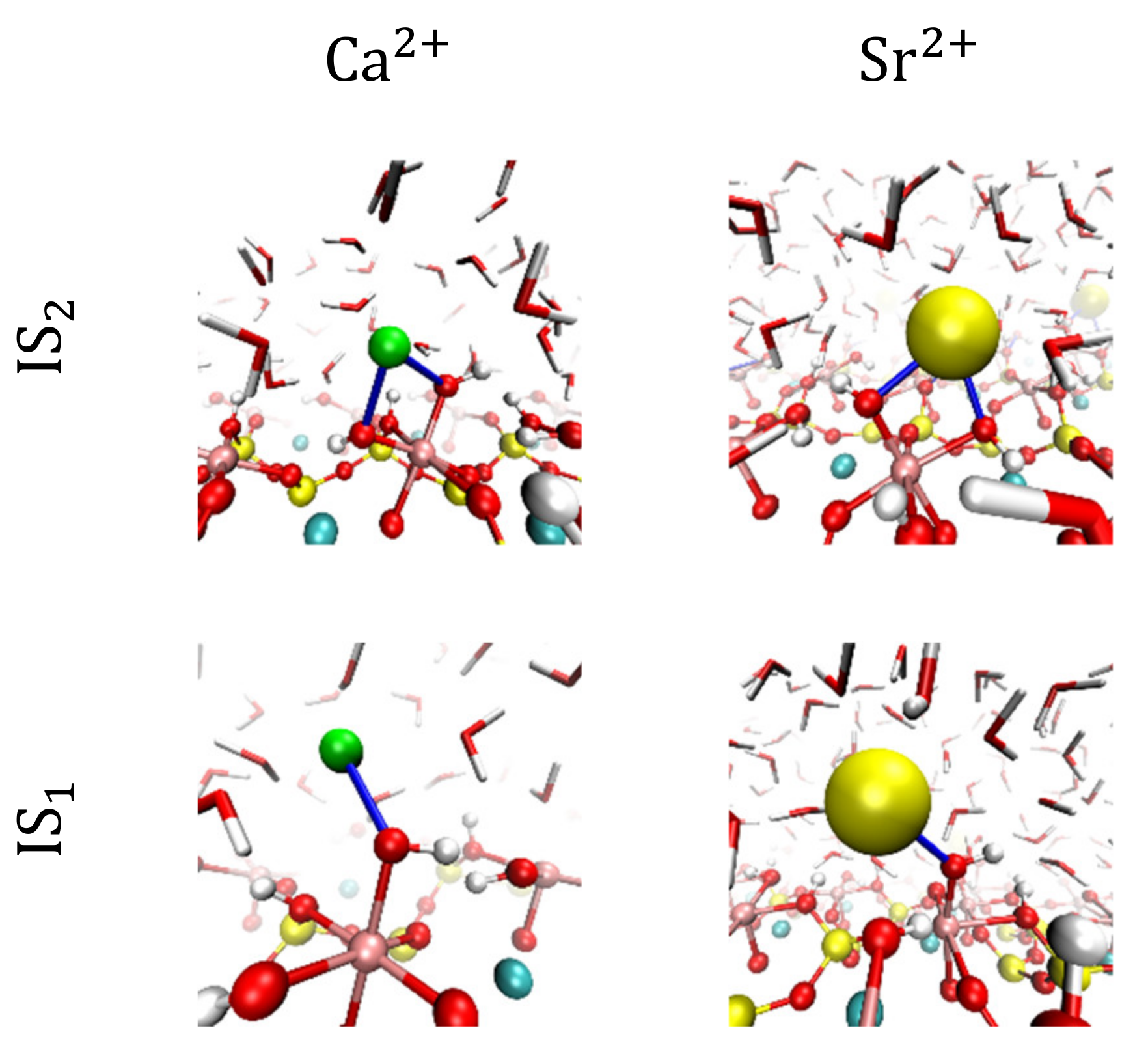
3.2. Visualization of Cation Inner-Sphere Complexes
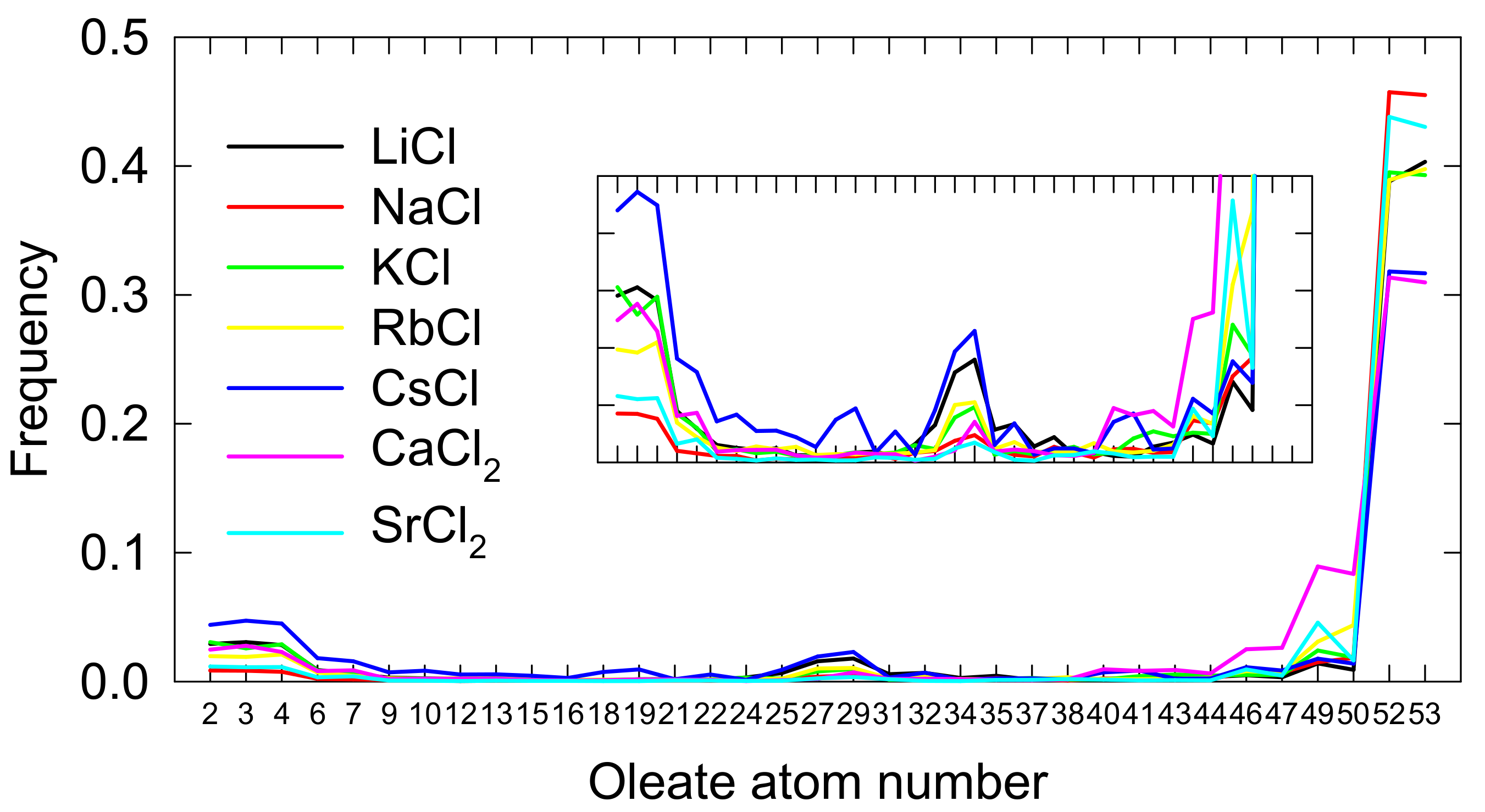
3.3. Spodumene-Oleate Interactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bragg, L.; Claringbull, G.F. Crystal Structures of Minerals; Bragg, S., Ed.; G. Bell & Sons Ltd.: London, UK, 1965. [Google Scholar]
- Filippov, L.; Farrokhpay, S.; Lyo, L.; Filippova, I. Spodumene flotation mechanism. Minerals 2019, 9, 372. [Google Scholar] [CrossRef]
- Sousa, R.; Ramos, V.; Guedes, A.; Botelho de Sousa, A.; Noronha, F.; Leite, M.M. Flotation of Lithium Ores to Obtain High-Grade Li2O Concentrates. Are There Any Mineralogical Limitations? Int. J. Min. Mater. Metall. Eng. 2019, 5, 7–18. [Google Scholar]
- Moon, K.S.; Fuerstenau, D.W. Surface crystal chemistry in selective flotation of spodumene (LiAl[SiO3]2) from other aluminosilicates. Int. J. Miner. Process. 2003, 72, 11–24. [Google Scholar] [CrossRef]
- Quezada, G.R.; Toledo, P.G. Structure of the Interface between Lithium-Rich Spodumene and Saltwater by Density Functional Theory Calculations and Molecular Dynamics Simulations. J. Phys. Chem. C 2020, 124, 1446–1457. [Google Scholar] [CrossRef]
- Sposito, G.; Skipper, N.T.; Sutton, R.; Park, S.H.; Soper, A.K.; Greathouse, J.A. Surface geochemistry of the clay minerals. Proc. Natl. Acad. Sci. USA 1999, 96, 3358–3364. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Sathish, P.; Tanwar, J.; Pradip; Moon, K.S.; Fuerstenau, D.W. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Weiqing, W.; Jing, L.; Yang, H.; Qiming, F.; Hong, Z. Fe(III) as an activator for the flotation of spodumene, albite, and quartz minerals. Miner. Eng. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Wang, J.; Xie, Z. Investigation on different behavior and mechanism of Ca(II) and Fe(III) adsorption on spodumene surface. Physicochem. Probl. Miner. Process. 2014, 50, 535–550. [Google Scholar] [CrossRef]
- Zhu, G.; Cao, Y.; Wang, Y.; Wang, X.; Miller, J.D.; Lu, D.; Zheng, X. Surface chemistry features of spodumene with isomorphous substitution. Miner. Eng. 2020, 146, 106139. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Liu, X.; Yu, F.; Lu, D. The cleavage and surface properties of wet and dry ground spodumene and their flotation behavior. Appl. Surf. Sci. 2015, 357, 333–339. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Yang, Y.; Zeng, X.; Wang, Z.; Wang, J. Selective flotation separation of spodumene from feldspar using new mixed anionic/cationic collectors. Miner. Eng. 2016, 89, 84–92. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Wu, H.; Tian, J.; Liu, J.; Gao, Z.; Wang, L. Surface crystal chemistry of spodumene with different size fractions and implications for flotation. Sep. Purif. Technol. 2016, 169, 33–42. [Google Scholar] [CrossRef]
- Xu, L.; Peng, T.; Tian, J.; Lu, Z.; Hu, Y.; Sun, W. Anisotropic surface physicochemical properties of spodumene and albite crystals: Implications for flotation separation. Appl. Surf. Sci. 2017, 426, 1005–1022. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Hu, Y. Adsorption mechanism of new mixed anionic/cationic collectors in a spodumene-feldspar flotation system. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Wu, H.; Fang, S.; Deng, W.; Peng, T.; Sun, W.; Hu, Y. A novel approach for flotation recovery of spodumene, mica and feldspar from a lithium pegmatite ore. J. Clean. Prod. 2018, 174, 625–633. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Peng, L.; Xu, Y.; Luo, L.; Yang, J.; Tang, Z. In situ adsorption of mixed collectors BHA/DDA in spodumene-feldspar flotation system. Sep. Purif. Technol. 2020, 251, 117325. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Xu, Y.; Luo, L.; Yang, J.; Tang, Z.; Wang, Z. In Situ Adsorption of Mixed Anionic/Cationic Collectors in a Spodumene-Feldspar Flotation System: Implications for Collector Design. Langmuir 2020, 36, 8086–8099. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Wang, X.; Yu, F.; Miller, J.D. States of coadsorption for oleate and dodecylamine at selected spodumene surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 313–321. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Yu, F.; Lu, D.; Wang, L.; Zhao, Y.; Zheng, H. Improving spodumene flotation using a mixed cationic and anionic collector. Physicochem. Probl. Miner. Process. 2017, 54, 567–577. [Google Scholar] [CrossRef]
- Wu, H.; Tian, J.; Xu, L.; Fang, S.; Zhang, Z.; Chi, R. Flotation and adsorption of a new mixed anionic/cationic collector in the spodumene-feldspar system. Miner. Eng. 2018, 127, 42–47. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q.; Huang, Y. The effects of Ca(II) and Mg(II) ions on the flotation of spodumene using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Zhang, L.; Zhu, G. Role of oleic acid ionic-molecular complexes in the flotation of spodumene. Miner. Eng. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Pouvreau, M.; Greathouse, J.A.; Cygan, R.T.; Kalinichev, A.G. Structure of Hydrated Gibbsite and Brucite Edge Surfaces: DFT Results and Further Development of the ClayFF Classical Force Field with Metal-O-H Angle Bending Terms. J. Phys. Chem. C 2017, 121, 14757–14771. [Google Scholar] [CrossRef]
- Pouvreau, M.; Greathouse, J.A.; Cygan, R.T.; Kalinichev, A.G. Structure of Hydrated Kaolinite Edge Surfaces: DFT Results and Further Development of the ClayFF Classical Force Field with Metal-O-H Angle Bending Terms. J. Phys. Chem. C 2019, 123, 11628–11638. [Google Scholar] [CrossRef]
- Sorin, E.J.; Pande, V.S. Exploring the helix-coil transition via all-atom equilibrium ensemble simulations. Biophys. J. 2005, 88, 2472–2493. [Google Scholar] [CrossRef]
- Mintis, D.G.; Alexiou, T.S.; Mavrantzas, V.G. Effect of pH and Molecular Length on the Structure and Dynamics of Linear and Short-Chain Branched Poly (ethylene imine) in Dilute Solution: Scaling Laws from Detailed Molecular Dynamics Simulations. J. Phys. Chem. B 2020, 124, 6154–6169. [Google Scholar] [CrossRef]
- Mintis, D.G.; Mavrantzas, V.G. Effect of pH and Molecular Length on the Structure and Dynamics of Short Poly (acrylic acid) in Dilute Solution: Detailed Molecular Dynamics Study. J. Phys. Chem. B 2019, 123, 4204–4219. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Li, P.; Song, L.F.; Merz, K.M. Systematic parameterization of monovalent ions employing the nonbonded model. J. Chem. Theory Comput. 2015, 11, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Roberts, B.P.; Chakravorty, D.K.; Merz, K.M. Rational design of particle mesh ewald compatible lennard-jones parameters for +2 metal cations in explicit solvent. J. Chem. Theory Comput. 2013, 9, 2733–2748. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Marcus, Y. Ions in Solution and Their Solvation; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9781118892336. [Google Scholar]
- Marcus, Y. Ionic Radii in Aqueous Solutions. Chem. Rev. 1988, 88, 1475–1498. [Google Scholar] [CrossRef]
- Larentzos, J.P.; Criscenti, L.J. A molecular dynamics study of alkaline earth metal-chloride complexation in aqueous solution. J. Phys. Chem. B 2008, 112, 14243–14250. [Google Scholar] [CrossRef]
- Quezada, G.R.; Rozas, R.E.; Toledo, P.G. Molecular Dynamics Simulations of Quartz (101)-Water and Corundum (001)-Water Interfaces: Effect of Surface Charge and Ions on Cation Adsorption, Water Orientation, and Surface Charge Reversal. J. Phys. Chem. C 2017, 121, 25271–25282. [Google Scholar] [CrossRef]
- Quezada, G.R.; Rozas, R.E.; Toledo, P.G. Ab Initio Calculations of Partial Charges at Kaolinite Edge Sites and Molecular Dynamics Simulations of Cation Adsorption in Saline Solutions at and above the pH of Zero Charge. J. Phys. Chem. C 2019, 123, 22971–22980. [Google Scholar] [CrossRef]
- Li, Y.; Narayanan, N.A.K.; Kadoura, A.; Yang, Y.; Sun, S. Molecular Simulation Study of Montmorillonite in Contact with Water. Ind. Eng. Chem. Res. 2019, 58, 1396–1403. [Google Scholar] [CrossRef]
- Yang, Y.; Narayanan, N.A.K.; Sun, S. Layer Charge Effects on Adsorption and Diffusion of Water and Ions in Interlayers and on External Surfaces of Montmorillonite. ACS Earth Space Chem. 2019. [Google Scholar] [CrossRef]
- Quezada, G.R.; Jeldres, M.; Toro, N.; Robles, P.; Toledo, P.G.; Jeldres, R.I. Understanding the flocculation mechanism of quartz and kaolinite with polyacrylamide in seawater: A molecular dynamics approach. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125576. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quezada, G.R.; Toledo, P.G. Complexation of Alkali and Alkaline-Earth Metal Cations at Spodumene-Saltwater Interfaces by Molecular Simulation: Impact on Oleate Adsorption. Minerals 2021, 11, 12. https://doi.org/10.3390/min11010012
Quezada GR, Toledo PG. Complexation of Alkali and Alkaline-Earth Metal Cations at Spodumene-Saltwater Interfaces by Molecular Simulation: Impact on Oleate Adsorption. Minerals. 2021; 11(1):12. https://doi.org/10.3390/min11010012
Chicago/Turabian StyleQuezada, Gonzalo R., and Pedro G. Toledo. 2021. "Complexation of Alkali and Alkaline-Earth Metal Cations at Spodumene-Saltwater Interfaces by Molecular Simulation: Impact on Oleate Adsorption" Minerals 11, no. 1: 12. https://doi.org/10.3390/min11010012
APA StyleQuezada, G. R., & Toledo, P. G. (2021). Complexation of Alkali and Alkaline-Earth Metal Cations at Spodumene-Saltwater Interfaces by Molecular Simulation: Impact on Oleate Adsorption. Minerals, 11(1), 12. https://doi.org/10.3390/min11010012






