Sulfide Minerals as Potential Tracers of Isochemical Processes in Contact Metamorphism: Case Study of the Kochumdek Aureole, East Siberia
Abstract
:1. Introduction
- Major- and trace-element compositions of the marble and gabbro-dolerite samples from the Kochumdek contact aureole;
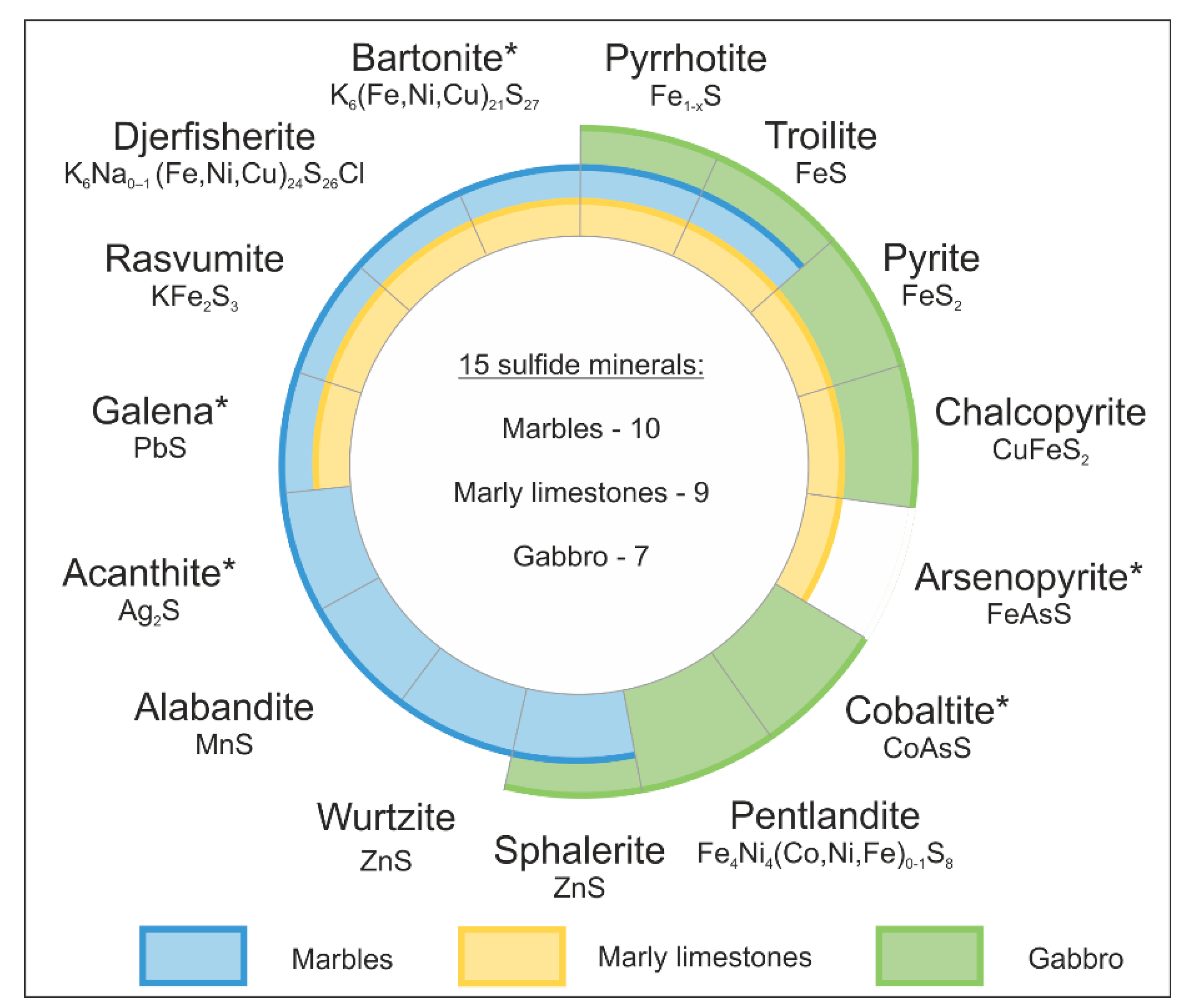
- Sulfide mineralogy in the marble and gabbro-dolerite samples: diversity, distribution patterns, morphology and age relationships;
- Mineral chemistry of sulfides, including trace-element signatures;
- Sulfur isotope composition.
2. Geological Background
2.1. General Information
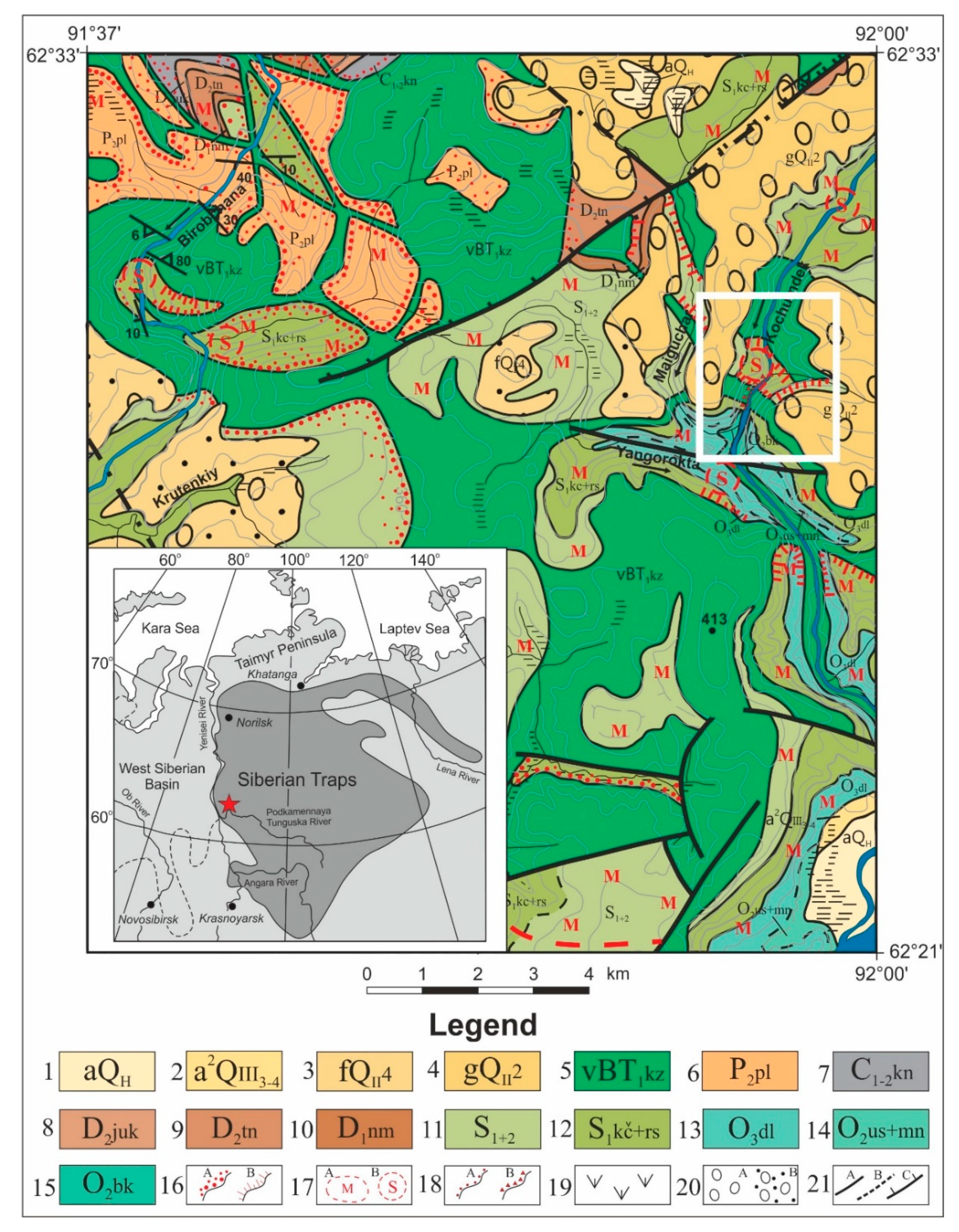
2.2. Site Description
3. Material and Methods
3.1. Sampling
3.2. Analytical Procedures
4. Results
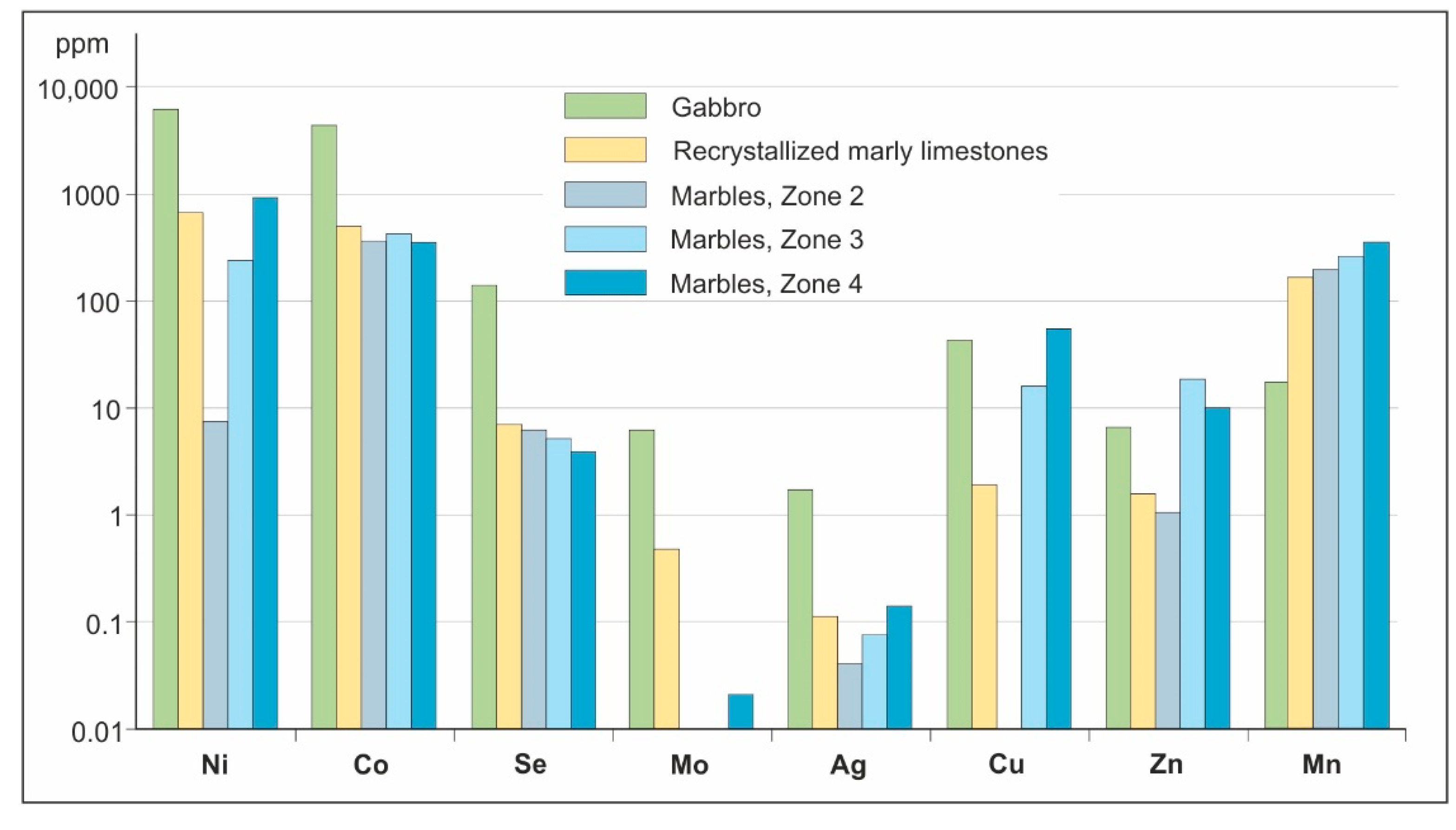
4.1. Bulk Rock Chemistry and Mineralogy
4.1.1. Gabbro
4.1.2. Recrystallized Marly Limestones
4.1.3. Marbles
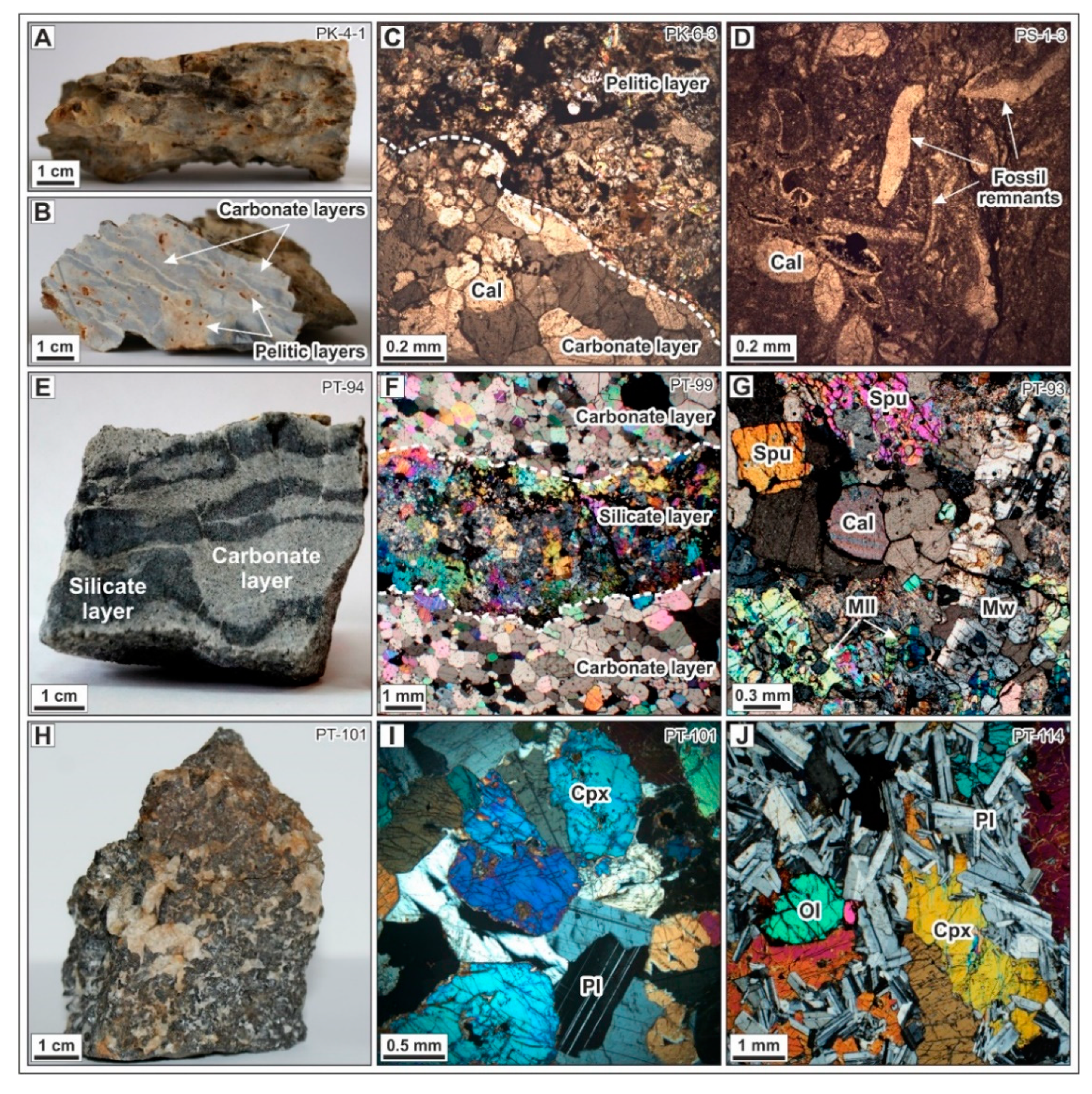
4.2. Mineralogical and Textural Relations of Sulfides
4.2.1. Gabbro
4.2.2. Recrystallized Marly Limestones, Zone 5
4.2.3. Marbles
Sulfides of Merwinite- and Spurrite-Monticellite Zones 2 and 3
Sulfides of Tilleyite-Wollastonite Zone 4
Retrograde Sulfides
4.3. Mineral Chemistry of Sulfides
4.3.1. Gabbro
4.3.2. Recrystallized Marly Limestones
4.3.3. Marbles
4.4. Stable Sulfur Isotopes in Pyrrhotite
5. Discussion
5.1. General Geochemical Features of Adjacent Igneous and Sedimentary Rocks
5.2. Sulfides in High-Grade Contact-Metamorphic Marbles: Applicability to Thermal History Reconstructions
5.2.1. Matrix and Inclusion Sulfides
5.2.2. Crack-Filling (Retrograde) Sulfides
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reverdatto, V.V. Facies of Contact Metamorphism; Nedra: Moscow, Russia, 1970; p. 271. [Google Scholar]
- Pertsev, N.N. High-Temperature Metamorphism and Metasomatism of Carbonate Rocks; Nauka: Moscow, Russia, 1977. [Google Scholar]
- Kerrick, D.M. Contact metamorphism. Mineral. Soc. Am. 1991, 26, 847. [Google Scholar]
- Grapes, R. Pyrometamorphism, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2011; p. 365. [Google Scholar]
- Bucher, K.; Grapes, R. Petrogenesis of Metamorphic Rocks; Springer: Berlin/Heidelberg, Germany, 2011; p. 428. [Google Scholar]
- Gieré, R. Zirconolite, allanite and hoegbomite in a marble skarn from the Bergell contact aureole: Implications for mobility of Ti, Zr and REE. Contrib. Miner. Pet. 1986, 93, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Spear, F.S.; Pyle, J.M. Apatite, monazite, and xenotime in metamorphic rocks. Rev. Mineral. Geochem. 2002, 48, 293–335. [Google Scholar] [CrossRef]
- Valley, J.W. Stable isotope thermometry at high temperatures. Mineral. Soc. Amer. 2001, 43, 365–413. [Google Scholar]
- Khoury, H.; Sokol, E.; Clark, I. Calcium uranium oxides from Central Jordan: Mineral assemblages, chemistry, and alteration products. Can. Min. 2015, 53, 61–82. [Google Scholar] [CrossRef]
- Khoury, H.N.; Sokol, E.V.; Kokh, S.N.; Seryotkin, Y.V.; Nigmatulina, E.N.; Goryainov, S.V.; Belogub, E.V.; Clark, I.D. Tululite, Ca14(Fe3+, Al)(Al, Zn, Fe3+, Si, P, Mn, Mg)15O36: A new Ca zincate-aluminate from combustion metamorphic marbles, Central Jordan. Mineral. Petrol. 2016, 110, 125–140. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gazeev, V.M.; Armbruster, T.; Zadov, A.E.; Galuskina, I.O.; Pertsev, N.N.; Dzierzanovski, P.; Kadiyski, M.; Gurbanov, A.G.; Wrzalik, R.; et al. Lakargiite CaZrO3: A new mineral of the perovskite group from the Northern Caucasus, Kabardino-Balkaria, Russia. Am. Miner. 2008, 93, 1903–1910. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V.; Armbruster, T.; Lazic, B.; Kusz, J.; Dzierzanowski, P.; Gazeev, V.M.; Pertsev, N.N.; Prusik, K.; Zadov, A.E.; et al. Elbrusite-(Zr)—A new uranian garnet from the Upper Chegem caldera, Kabardino-Balkaria, Northern Caucasus, Russia. Am. Mineral. 2010, 95, 1172–1181. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Armbruster, T.; Galuskina, I.O.; Lazic, B.; Winiarski, A.; Gazeev, V.M.; Dzierzanowski, P.; Zadov, A.E.; Pertsev, N.N.; Wrzalik, R.; et al. Vorlanite (CaU6+)O4—A new mineral from the Upper Chegem caldera, Kabardino-Balkaria, Northern Caucasus, Russia. Am. Miner. 2011, 96, 188–196. [Google Scholar] [CrossRef]
- Gazeev, V.M.; Gurbanova, O.A.; Zadov, E.A.; Gurbanov, A.G.; Leksin, A.B. Mineralogy of skarn limy xenoliths of Shadil-hoh volcano (Kel volcanic area of the Great Caucasus). Vestnik Vladikavkazskogo Nauchnogo Tsentra 2012, 2, 23–33. [Google Scholar]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. Nomenclature of the garnet supergroup. Am. Miner. 2013, 98, 785–811. [Google Scholar] [CrossRef]
- Hermann, J.; Rubatto, D.; Korsakov, A.V.; Shatsky, V.S. The age of metamorphism of diamondiferous rocks determined with shrimp dating of zircon. Russ. Geol. Geophys. 2006, 47, 511–518. [Google Scholar]
- Green, T.H.; Hellman, P.L. Fe-Mg partitioning between coexisting garnet and phengite at high pressure, and comments on a garnet-phengite geothermometer. Lithos 1982, 15, 253–266. [Google Scholar] [CrossRef]
- Hermann, J.; Rubatto, D. Relating zircon and monazite domains to garnet growth zones: Age and duration of granulite facies metamorphism in the Val Malenco lower crust. J. Metamorph. Geol. 2003, 21, 833–852. [Google Scholar] [CrossRef]
- Watson, E.B.; Wark, D.A.; Thomas, J.B. Crystallization thermometers for zircon and rutile. Contrib. Miner. Pet. 2006, 151, 413. [Google Scholar] [CrossRef]
- Tomkins, H.S.; Powell, R.; Ellis, D.J. The pressure dependence of the zirconium-in-rutile thermometer. J. Metamorph. Geol. 2007, 25, 703–713. [Google Scholar] [CrossRef]
- Parat, F.; Dungan, M.A.; Streck, M.J. Anhydrite, pyrrhotite, and sulfurrichapatite: Tracing the sulfur evolution of an Oligocene andesite (Eagle Mountain, CO, USA). Lithos 2002, 64, 63–75. [Google Scholar] [CrossRef]
- Britvin, S.N.; Bogdanova, A.N.; Boldyreva, M.M.; Aksenova, G.Y. Rudashevskyite, the Fe-dominant analogue of sphalerite, a new mineral: Description and crystal structure. Am. Miner. 2008, 93, 902–909. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Wenzel, T.; Whitehouse, M.J.; Loose, M.; Zack, T.; Barth, M.; Worgard, L.; Krasz, V.; Eby, G.N.; Stosnach, H.; et al. The volatile inventory (F, Cl, Br, S, C) of magmatic apatite: An integrated analytical approach. Chem. Geol. 2012, 291, 241–255. [Google Scholar] [CrossRef]
- Barton, P.B. Sulfide petrology. Mineral. Soc. Am. Rev. Mineral. 1974, 1, B1–B11. [Google Scholar]
- Slotznick, S.P.; Eiler, J.M.; Fischer, W.W. The effects of metamorphism on iron mineralogy and the iron speciation redox proxy. Geochim. Cosmochim. Acta. 2018, 224, 96–115. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, D.J. Sulfide mineralogy and geochemistry. Mineral. Soc. Amer. 2006, 61, 714. [Google Scholar]
- Brown, J.L.; Christy, A.G.; Ellis, D.J.; Arculus, R.J. Prograde sulfide metamorphism in blueschist and eclogite, New Caledonia. J. Pet. 2014, 55, 643–670. [Google Scholar] [CrossRef] [Green Version]
- Robert, R. Seal II. Sulfur isotope geochemistry of sulfide minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar]
- Reverdatto, V.V. High-temperature contact metamorphism of limestones in the Podkamennaya Tunguska basin. Dokl. Earth Sci. USSR. 1964, 155, 104–107. [Google Scholar]
- Sokol, E.V.; Polyansky, O.P.; Semenov, A.N.; Reverdatto, V.V.; Kokh, S.N.; Devyatiyarova, A.S.; Kolobov, V.Y.; Khvorov, P.V.; Babichev, A.V. High-grade contact metamorphism in the Kochumdek River valley (Podkamennaya Tunguska basin, East Siberia): Evidence for magma flow. Russ. Geol. Geophys. 2019, 60, 386–399. [Google Scholar]
- Deviatiiarova, A.S. Potassium Ssulfides in Spurrite Marbles from the Kochumdek River. In Proceedings of the 55 International Scientific Student Conference, Novosibirsk, Russia, 17–20 April 2017; p. 88. [Google Scholar]
- Deviatiiarova, A.S. Specific Sulfide Mineralization in Spurrite Marbles from the Kochumdek Contact Aureole (Podkamennaya Tunguska basin). In Proceedings of the XXIIIrd Scientific Youth School “Metallogeny of Ancient and Modern Oceans–2017. Differentiation and Reasons of Diversity of Ore Deposits”, Miass, Russia, 24–28 April 2017; pp. 229–232. [Google Scholar]
- Golovin, A.V.; Goryainov, S.V.; Kokh, S.N.; Sharygin, I.S.; Rashchenko, S.V.; Kokh, K.A.; Devyatiyarova, A.S.; Sokol, E.V. The application of raman spectroscopy to djerfisherite identification. J. Raman Spectrosc. 2017, 48, 1574–1582. [Google Scholar] [CrossRef]
- Sokol, E.V.; Deviatiiarova, A.S.; Kokh, S.N.; Reverdatto, V.V.; Artemyev, D.A.; Kolobov, V.Y. Sulfide mineralization hosted by spurrite-mervinite marbles (Kochumdek River, East Siberia). Dokl. Earth Sci. 2019, 489, 1326–1329. [Google Scholar] [CrossRef]
- Deviatiiarova, A.S.; Artemyev, D.A.; Abersteiner, A.; Sokol, E.V. Isotope-Geochemical Characteristics of Sulfides in Spurrite Marbles from the Kochumdek River (Podkamennaya Tunguska basin). In Proceedings of the Professor, V.V. Zaykov XXVIth Scientific Youth School “Metallogeny of Ancient and Modern Oceans–2020. Critical Metals in Ore-Forming Systems”, Miass, Russia, 22 April 2020; pp. 205–209. [Google Scholar]
- Malich, N.S.; Grigoriev, V.V. Correlation of Magmatism and Tectonics in the lower Podkamennaya Tunguska and Bakhta River Basins. In Geology and Mineral Resources in the Siberian Craton; VSEGEI: Leningrad, Russia, 1960; pp. 27–36. [Google Scholar]
- Vasil’ev, Y.R.; Zolotukhin, V.V.; Feoktistov, G.D.; Prusskaya, S.N. Evaluation of the volumes and genesis of Permo-Triassic trap magmatism on the Siberian platform. Russ. Geol. Geophys. 2000, 41, 1696–1705. [Google Scholar]
- Dobretsov, N.L.; Kirdyashkin, A.A.; Kirdyashkin, A.G.; Vernikovsky, V.A.; Gladkov, I.N. Modelling of thermochemical plumes and implications for the origin of the Siberian traps. Lithos 2008, 100, 66–92. [Google Scholar] [CrossRef]
- Dobretsov, N.L. 250 Ma large igneous provinces of Asia: Siberian and emeishan traps (plateau basalts) and associated granitoids. Russ. Geol. Geophys. 2005, 9, 847–868. [Google Scholar]
- Zolotukhin, V.V.; Al’mukhamedov, A.I. Basalts of the Siberian platform: Distribution, Mineral Composition, and Mechanism of Formation. In Traps of Siberia and Deccan: Similarities and Differences; Polyakov, G.V., Ed.; Nauka: Novosibirsk, Russia, 1991; pp. 7–39. [Google Scholar]
- Prusskaya, S.N. Petrology of Intrusive Trappean Magmatism in the Western Siberian Craton: Evidence from Petroleum Drilling; Siberian Federal University: Krasnoyarsk, Russia, 2008; p. 248. [Google Scholar]
- Egorova, V.; Latypov, R. Mafic–ultramafic sills: New insights from M-and S-shaped mineral and whole-rock compositional profiles. J. Pet. 2013, 54, 2155–2191. [Google Scholar] [CrossRef] [Green Version]
- Poryadin, V.S.; Strunin, B.M.; Turchin, A.V.; Komarov, V.V.; Fainer, Y.B. State Geological Map of the USSR, Scale 1:200,000, Ser. Turukhansk, Sheet R-46-XIV. Explanatory Note; Krasnoyarskoe Territorialnoe Geologicheskoe Upravlenie: Moscow, Russia, 1977. [Google Scholar]
- Alekseenko, V.D.; Alasev, V.A.; Barmin, V.A.; Belolipetskaya, L.I.; Bozhko, V.V.; Varganov, A.S.; Egorov, V.N.; Egorov, A.S.; Kazhaeva, O.D.; Kachevsky, L.K.; et al. State geological Map of the Russian Federation, scale 1:1,000,000 (third generation). In Ser. Angara-Yenisei. Sheet R-46-North Yenisei. Explanatory Note Kart; VSEGEI: St. Petersburg, Russia, 2010. [Google Scholar]
- Sobolev, V.S. Selected Works. Trap Petrology; Nauka: Novosibirsk, Russia, 1986; p. 210. [Google Scholar]
- Reverdatto, V.V. Metamorphism in the contacts of Anakit trappean massif in the Low Tunguska River. In Materials on Genetik and Experimental Mineralogy; Transactions of the Institute of Geology and Geophysics Siberian Branch Academy of Sciences of USSR: Novosibirsk, Russian, 1964; pp. 97–168. [Google Scholar]
- Pertsev, N.N.; Shmulovich, K.I. Physicochemical conditions of larnite-merwinite facies contact metamorphism: A case study from the Podkamennaya Tunguska basin. Izvestiya AN SSSR 1972, 6, 39–47. [Google Scholar]
- Tesakov, Y.I. New silurian formations in the southwest of the Siberian platform. Novosti paleontologii i stratigrafii. Suppl. Russ. Geol. Geophys. 2009, 12, 29–41. [Google Scholar]
- Heinrich, W.; Gottschalk, M. Fluid flow patterns and infiltration isograds in melilite marbles from the Bufa del Diente contact metamorphic aureole, north-east Mexico. J. Metamorph. Geol. 1994, 12, 345–359. [Google Scholar] [CrossRef]
- Shatsky, V.; Sitnikova, E.; Kozmenko, O.; Palessky, S.; Nikolaeva, I.; Zayachkovsky, A. Behavior of incompatible elements during ultrahigh-pressure metamorphism (by the example of rocks of the Kokchetav massif). Russ. Geol. Geophys. 2006, 47, 482–496. [Google Scholar]
- Element, C.A.S. Method 3051A-microwave assisted acid digestion of sediments, sludges, soils, and oils. Z. Anal. Chem. 2007, 111, 362–366. [Google Scholar]
- Carvalho, L.; Monteiro, R.; Figueira, P.; Mieiro, C.; Almeida, J.; Pereira, E.; Magalhães, V.; Pinheiro, L.; Vale, C. Vertical distribution of major, minor and trace elements in sediments from mud volcanoes of the Gulf of Cadiz: Evidence of Cd, As and Ba fronts in upper layers. Deep Sea Res. Part. I Oceanogr. Res. Pap. 2018, 131, 133–143. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kokh, S.N.; Seryotkin, Y.V.; Deviatiiarova, A.S.; Goryainov, S.V.; Sharygin, V.V.; Khoury, H.N.; Karmanov, N.S.; Danilovsky, V.A.; Artemyev, D.A.; et al. Ultrahigh-temperature sphalerite from Zn-Cd-Se-rich combustion metamorphic marbles, Daba Complex, Central Jordan: Paragenesis, chemistry, and structure. Minerals 2020, 10, 822. [Google Scholar] [CrossRef]
- Artemyev, D.A.; Ankushev, M.N. Trace elements of Cu-(Fe)-sulfide inclusions in bronze age copper slags from South Urals and Kazakhstan: Ore sources and alloying additions. Minerals 2019, 9, 746. [Google Scholar] [CrossRef] [Green Version]
- Humphries, D.W. The Preparation of thin Sections of Rocks, Minerals and Ceramics. In Royal Microscopical Society Microscopy Handbooks; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Longerich, H.P.; Jackson, S.E.; Günther, D. Inter-laboratory note. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Wilson, S.A.; Ridley, W.I.; Koenig, A.E. Development of sulphide calibration standards for the laser ablation inductively-coupled plasma mass spectrometry technique. J. Anal. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Canberra Industries Inc. Model S506 Interactive Peak Fit. User’s Manual; Canberra Industries Inc.: Canberra, NSW, Australia, 2002. [Google Scholar]
- Gao, S.; Luo, T.C.; Zhang, B.R.; Zhang, H.F.; Han, Y.W.; Hu, Y.K.; Zhao, Z.D. Chemical composition of the continental crust as revealed by studies in east China. Geochim. Cosmochim. Acta. 1998, 62, 1959–1975. [Google Scholar] [CrossRef]
- Deviatiiarova, A.S. Crystal-Chemical Element Fractionation Under HT-LP Metamorphic Conditions: Case Study from Kochumdek Contact Aureole (Podkamennaya Tunguska basin). In Proceedings of the XIX International Meeting on Crystal Chemistry, X-ray Diffraction and Spectroscopy of Minerals, Apatity, Russia, 2–9 July 2019; p. 213. [Google Scholar]
- Gerasimova, Y.V.; Oreshonkov, A.S.; Romanova, O.B.; Ivanenko, A.A.; Krylov, A.S. Raman and infrared characterization of gadolinium-doped manganese sulfide. Spectrosc. Lett. 2017, 50, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Scocioreanu, M.; Baibarac, M.; Baltog, I.; Pasuk, I.; Velula, T. Photoluminescence and raman evidence for mechanico-chemical interaction of polyaniline-emeraldine base with ZnS in cubic and hexagonal phase. J. Solid. State. Chem. 2012, 186, 217–223. [Google Scholar] [CrossRef]
- Osadchii, E.G.; Gorbaty, Y.E. Raman spectra and unit cell parameters of sphalerite solid solutions (FexZn1-xS). Geochim. Cosmochim. Acta. 2010, 74, 568–573. [Google Scholar] [CrossRef]
- Makovicky, E. Crystal structures of sulfides and other chalcogenides. Rev. Mineral. Geochem. 2006, 61, 7–125. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Martin, R.F.; Cabri, L.J. Rare sulfides enriched in K, Tl and Pb from the Noril’sk and Salmagorsky complexes, Russia: New data and implications. Min. Mag. 2015, 79, 799–808. [Google Scholar] [CrossRef]
- Dobrovol’skaya, M.G. Alkaline Metals Sulfides in the Nature; Nauka: Moscow, Russia, 2018. [Google Scholar]
- Li, C.; Ripley, E.M.; Naldrett, A.J. Compositional variations of olivine and sulfur isotopes in the Noril’sk and Talnakh intrusions, Siberia: Implications for ore-forming processes in dynamic magma conduits. Econ. Geol. 2003, 98, 69–86. [Google Scholar] [CrossRef]
- Jamtveit, B.; Dahlgren, S.; Austrheim, H. High-grade contact metamorphism of calcareous rocks from the Oslo Rift, Southern Norway. Am. Miner. 1997, 82, 1241–1254. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H.; Horvath, L. Rb–Cs-enriched rasvumite and sectorial loparite–lueshite intergrowths from the Mont Saint-Hilaire alkaline complex, Quebec, Canada. Geol. Assoc. Can. Mineral. Assoc. Can. Program Abstr. 2001, 26, 24. [Google Scholar]
- Chakhmouradian, A.R.; Halden, N.M.; Mitchell, R.H.; Horváth, L. Rb-Cs-rich rasvumite and sector-zoned “loparite-(Ce)” from Mont Saint-Hilaire (Québec, Canada) and their petrologic significance. Eur. J. Mineral. 2007, 19, 533–546. [Google Scholar] [CrossRef] [Green Version]
- Sharygin, V.V.; Kamenetsky, V.S.; Kamenetsky, M.B. Potassium sulfides in kimberlite-hosted chloride–«nyerereite» and chloride clasts of Udachnaya-East pipe, Yakutia, Russia. Can. Miner. 2008, 46, 1076–1095. [Google Scholar] [CrossRef]
- Dobrovol’skaya, M.G.; Tsepin, A.I.; Ilupin, I.P.; Ponomarenko, A.I. Djerfisherite from Yakutia Kimberlites Minerals and Parageneses of Endogenic Deposits; Nauka: Leningrad, Russia, 1975. [Google Scholar]
- Clarke, D.B. Synthesis of Nickeloan Djerfisherites and the Origin of Potassic Sulphides at the Frank Smith mine. In The Mantle Sample: Inclusions in Kimberlites and Other Volcanics; Broyd, F.R., Meyer, H.O.A., Eds.; American Geophysical Union: Washington, DC, USA, 1979; Volume 16, pp. 300–308. [Google Scholar]
- Distler, V.V.; Ilupin, I.P.; Laputina, I.P. Sulfides of deep-seated origin in kimberlites and some aspects of copper-nickel mineralization. Int. Geol. Rev. 1987, 29, 456–464. [Google Scholar] [CrossRef]
- Spetsius, Z.V.; Bulanova, G.P.; Leskova, N.V. Djerfisherite and its genesis in kimberlitic rocks. Dokl Acad. Sci. USSR 1987, 293, 199–202. [Google Scholar]
- Bulanova, G.P.; Spetsius, Z.V.; Leskova, N.V. Sulphides in Diamonds and Xenoliths from Yakutian Kimberlite Pipes; Nauka: Novosibirsk, Russia, 1990; p. 120. [Google Scholar]
- Sharygin, V.V.; Golovin, A.V.; Pokhilenko, N.P.; Kamenetsky, V.S. Djerfisherite in the Udachnaya-East pipe kimberlites (Sakha-Yakutia, Russia): Paragenesis, composition and origin. Eur. J. Mineral. 2007, 19, 51–63. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Pokhilenko, N.P. Djerfisherite in xenoliths of sheared peridotite in the Udachnaya-East pipe (Yakutia): Origin and relationship with kimberlite magmatism. Russ. Geol. Geophys. 2012, 53, 247–261. [Google Scholar] [CrossRef]
- Abersteiner, A.; Kamenetsky, V.S.; Goemann, K.; Golovin, A.V.; Sharygin, I.S.; Giuliani, A.; Rodemann, T.; Spetsius, Z.V.; Kamenetsky, M. Djerfisherite in kimberlites and their xenoliths: Implications for kimberlite melt evolution. Contrib. Mineral. Petrol. 2019, 174, 8. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Pokhilenko, N.P. Djerfisherite in kimberlites of the Kuoikskoe field as an indicator of enrichment of kimberlite melts in chlorine. Dokl. Earth Sci. 2011, 436, 301–307. [Google Scholar] [CrossRef]
- Henderson, C.M.B.; Kogarko, L.N.; Plant, D. Extreme closed system fractionation of volatile-rich, ultrabasic peralkaline melt inclusions and the occurrence of djerfisherite in the Kugda alkaline complex, Siberia. Min. Mag. 1999, 63, 433–438. [Google Scholar] [CrossRef]
- Zaccarini, F.; Thalhammer, O.A.R.; Princivalle, F.; Lenaz, D.; Stanley, C.J.; Garuti, G. Djerfisherite in the guli dunite complex, polar Siberia: A primary or metasomatic phase. Can. Min. 2007, 45, 1201–1211. [Google Scholar] [CrossRef]
- Clay, P.L.; O’Driscoll, B.; Upton, B.G.J.; Busemann, H. Characteristics of djerfisherite from fluid-rich, metasomatized alkaline intrusive environments and anhydrous enstatite chondrites and achondrites. Am. Miner. 2014, 99, 1683–1693. [Google Scholar] [CrossRef]
- Takechi, Y.; Kusachi, I.; Nakamuta, Y.; Kase, K. Nickel-bearing djerfisherite in gehlenite-spurrite skarn at Kushiro, Hiroshima prefecture, Japan. Resour. Geol. 2000, 50, 179–184. [Google Scholar] [CrossRef]
- Fuchs, L.H. Djerfisherite, alkali copper-iron sulfide: A new mineral from enstatite chondrites. Science 1966, 153, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; El Goresy, A. A comparative study of opaque phases in Qingzhen (EH3) and MacAlpine Hills 88136 (EL3): Representatives of EH and EL parent bodies. Meteorit. Planet. Sci. 2002, 37, 577–599. [Google Scholar] [CrossRef]
- Faure, G. Principles of Isotope Geology, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1986; 589p. [Google Scholar]
- Rickard, D. Sulfidic Sediments and Sedimentary Rocks; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]
- Buchko, I.V.; Sorokin, A.A.; Ponomarchuk, V.A.; Izokh, A.E. Geochemical features and geodynamic setting of formation of the Lukinda dunite-troctolite-gabbro massif (southeastern framing of the Siberian Platform). Russ. Geol. Geophys. 2012, 53, 636–648. [Google Scholar] [CrossRef]
- Pokrovsky, B.G.; Zaitsev, A.V.; Dronov, A.V.; Bujakaite, M.I.; Petrov, O.L.; Timokhin, A.V. C, O, S, and Sr isotope geochemistry and chemostratigraphy of Ordovician sediments in the Moyero River section, northern Siberian platform. Lithol. Min. Res. 2018, 53, 283–306. [Google Scholar] [CrossRef]
- Ripley, E.M.; Lightfoot, P.C.; Li, C.; Elswick, E.R. Sulfur isotopic studies of continental flood basalts in the Noril’sk region: Implications for the association between lavas and ore-bearing intrusions. Geochim. Cosmochim. Acta. 2003, 67, 2805–2817. [Google Scholar] [CrossRef]
- Morse, J.W.; Luther Iii, G.W. Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochim. Cosmochim. Acta. 1999, 63, 3373–3378. [Google Scholar] [CrossRef]
- Large, R.R.; Halpin, J.A.; Danyushevsky, L.V.; Maslennikov, V.V.; Bull, S.W.; Long, J.A.; Gregory, D.D.; Lounejeva, E.; Lyons, T.W.; Sack, P.J.; et al. Trace element content of sedimentary pyrite as a new proxy for deep-time ocean–atmosphere evolution. Earth Planet. Sci. Lett. 2014, 389, 209–220. [Google Scholar] [CrossRef]
- Parnell, J.; Perez, M.; Armstrong, J.; Bullock, L.; Feldmann, J.; Boyce, A.J. Geochemistry and metallogeny of neoproterozoic pyrite in oxic and anoxic sediments. Geochem. Perspect. Lett. 2018, 7, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Vernon, R.H.; White, R.W.; Clarke, G.L. False metamorphic events inferred from misinterpretation of microstructural evidence and P-T data. J. Metamorph. Geol. 2008, 26, 437–449. [Google Scholar] [CrossRef]
- Itaya, T.; Brothers, R.N.; Black, P.M. Sulfides, oxides and sphene in high-pressure schists from New Caledonia. Contrib. Miner. Pet. 1985, 91, 151–162. [Google Scholar] [CrossRef]
- Kawakami, T.; Ellis, D.J.; Christy, A.G. Sulfide evolution in high-temperature to ultrahigh-temperature metamorphic rocks from Lutzow-Holm complex, East Antarctica. Lithos 2006, 92, 431–446. [Google Scholar] [CrossRef]
- Seryotkin, Y.V.; Sokol, E.V.; Kokh, S.N. Natural pseudowollastonite: Crystal structure, associated minerals, and geological context. Lithos 2012, 134, 75–90. [Google Scholar] [CrossRef]
- Tomashyk, V.; Feychuk, P.; Scherbak, L. Ternary Alloys Based on II-Vi Semiconductor Compounds; 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; p. 560. [Google Scholar]
- Knitter, S.; Binnewies, M. Chemical transport of MnS/ZnS, FeS/ZnS, and FeS/MnS mixed crystals. J. Inorg. Gen. Chem. 1999, 625, 1582–1588. [Google Scholar]
- Knitter, S.; Binnewies, M. Chemical vapor transport of solid solutions. 7. Chemical vapor transport of FeS/MnS/ZnS mixed crystals. J. Inorg. Gen. Chem. 2000, 626, 2335–2339. [Google Scholar]
- Gorbachev, N.S.; Nekrasov, I.Y. Genesis of synthetic and natural potassium sulfides. Dokl. Acad. Sci. USSR. 1980, 251, 126–129. [Google Scholar]
- Osadchii, V.O.; Voronin, M.V.; Baranov, A.V. Mineralogy of Potassium-Iron Sulfides and Phase Relations in the System K-Fe-S-(Cl). In Proceedings of the XXVII All-Russian Youth Conference with the Participation of Researchers from other Countries, Irkutsk, Russian, 22-28 May 2017; pp. 166–167. [Google Scholar]
- Osadchii, V.O.; Voronin, M.V.; Baranov, A.V. Phase equilibria in the KFeS2-Fe-S system at 300–600 °C and bartonite stability. Contrib. Miner. Pet. 2018, 173, 44. [Google Scholar] [CrossRef]
- Mitchell, R.H. Crystal structures of CsFe2S3 and RbFe2S3: Synthetic analogs of rasvumite KFe2S3. J. Solid. State. Chem. 2004, 177, 1867–1872. [Google Scholar] [CrossRef]
- Amthauer, G.; Bente, K. Mixed-valent iron in synthetic rasvumite, KFe2S3. Naturwissenschaften 1983, 70, 146–147. [Google Scholar] [CrossRef]
- Boller, H. On the synthesis, crystal chemistry and magnetic properties of rasvumite and related compounds. Acta Cryst. 2004, 60, s47. [Google Scholar] [CrossRef] [Green Version]
- Voronin, M.V.; Osadchii, V.O.; Baranov, A.V. Phase Relations Involving Chlorbartonite in the K-Fe-S-Cl System. In Proceedings of the International Conference on Geochemistry and Related Subjects “Goldschmidt”, Barcelona, Spain, 18–23 August 2019; p. 3535. [Google Scholar]
- Buick, I.S.; Cartwright, I. Stable isotope constraints on the mechanism of fluid flow during contact metamorphism around the Marulan Batholith, NSW, Australia. J. Geochem. Explor. 2000, 69, 291–295. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Laajoki, K.V.O.; Gehor, S.A.; Yakovlev, Y.N.; Taikina-Aho, O. Chlorine-poor analogues of djerfisherite-thalfenisite from Noril’sk, Siberia, and Salmagorsky, Kola Peninsula, Russia. Can. Miner. 1997, 35, 1421–1430. [Google Scholar]
- Sluzhenikin, S.F.; Laputina, I.P. Composition of Minerals of the Djerfisherite Group in Copper-Nickel Ores of the Talnakh Deposit. In Proceedings of the “Microprobe and Progress in Geology”, Suzdal, Russia, 21–28 April 1989; pp. 107–110. [Google Scholar]
- Sharygin, V.V.; Starikova, A.Y. Sulfide Associations in Garnet-Melilite-wollastonite Skarns of the Tazheran Alkaline Massif, Baikal Region. In Proceedings of the XXVII International Conference School “Geochemistry of Alkaline Rocks”, Moscow-Koktebel, Russia, 9–16 September 2010; pp. 164–166. [Google Scholar]
- Starikova, A.E. Mineralogy of Metasomatic Rocks of the Tazheran Massif (Western Baikal Area). Ph.D. Thesis, Institute of Geology and Mineralogy SB RAS, Novosibirsk, Russia, 21 May 2013; p. 207. [Google Scholar]
- Heinrich, W.; Churakov, S.S.; Gottschalk, M. Mineral-fluid equilibria in the system CaO–MgO–SiO2–H2O–CO2–NaCl and the record of reactive fluid flow in contact metamorphic aureoles. Contrib. Miner. Pet. 2004, 148, 131–149. [Google Scholar] [CrossRef]















| Locality | Kuz’movsky Sill | Kochumdek Trap | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Mean (n = 8) | S | Min. | Max. | PT-95 | PT-95a | PT-96 | PT-101 | PT-114 | PK-4-8 | PK-7-1 | Mean (n = 7) | S | Min. | Max. |
| SiO2 | 49.90 | 1.00 | 48.74 | 51.86 | 47.43 | 48.83 | 48.74 | 50.06 | 48.90 | 48.11 | 48.35 | 48.63 | 0.81 | 47.43 | 50.06 |
| TiO2 | 1.14 | 0.09 | 1.00 | 1.28 | 2.03 | 1.81 | 1.19 | 1.13 | 1.60 | 1.96 | 1.75 | 1.64 | 0.36 | 1.13 | 2.03 |
| Al2O3 | 14.99 | 0.52 | 14.11 | 15.81 | 9.92 | 10.28 | 9.43 | 8.33 | 15.91 | 16.22 | 14.44 | 12.08 | 3.33 | 8.33 | 16.22 |
| FeO * | 11.22 | 0.20 | 10.89 | 11.47 | 13.25 | 12.30 | 10.13 | 10.93 | 12.22 | 12.64 | 13.45 | 12.13 | 1.21 | 10.13 | 13.45 |
| MnO | 0.18 | 0.01 | 0.17 | 0.20 | 0.27 | 0.26 | 0.22 | 0.22 | 0.20 | 0.20 | 0.23 | 0.23 | 0.03 | 0.20 | 0.27 |
| MgO | 9.54 | 1.22 | 7.86 | 11.17 | 6.13 | 5.86 | 8.90 | 9.97 | 5.04 | 5.07 | 6.30 | 6.75 | 1.92 | 5.04 | 9.97 |
| CaO | 10.11 | 0.22 | 9.75 | 10.40 | 15.54 | 15.06 | 14.83 | 16.37 | 11.48 | 10.26 | 10.20 | 13.39 | 2.65 | 10.20 | 16.37 |
| Na2O | 2.04 | 0.15 | 1.87 | 2.29 | 2.00 | 2.06 | 2.19 | 1.49 | 2.80 | 3.12 | 2.72 | 2.34 | 0.56 | 1.49 | 3.12 |
| K2O | 0.75 | 0.23 | 0.47 | 1.18 | 1.30 | 1.37 | 0.87 | 0.39 | 0.68 | 0.84 | 0.67 | 0.87 | 0.35 | 0.39 | 1.37 |
| P2O5 | 0.16 | 0.03 | 0.11 | 0.20 | 0.24 | 0.26 | 0.10 | 0.10 | 0.19 | 0.25 | 0.19 | 0.19 | 0.07 | 0.10 | 0.26 |
| SO3 | na | 0.13 | 0.10 | 0.14 | <0.03 | <0.03 | 0.14 | <0.03 | 0.13 | 0.02 | <0.03 | 0.14 | |||
| LOI | na | 0.85 | 0.91 | 2.42 | 0.13 | 0.10 | 0.22 | 0.93 | 0.79 | 0.81 | 0.10 | 2.42 | |||
| Total | 100.02 | 99.09 | 99.10 | 99.16 | 99.15 | 99.15 | 99.03 | 99.26 | 99.13 | ||||||
| Co | 54.1 | 3.14 | 50.0 | 60.0 | 46.3 | 45.1 | 40.7 | 49.9 | 43.0 | 42.2 | 57.1 | 46.3 | 5.62 | 40.7 | 57.1 |
| Ni | 184 | 42.11 | 129 | 239 | 43.8 | 40.5 | 86.5 | 85.9 | 51.6 | 76.5 | 107 | 70.2 | 25.23 | 40.5 | 107 |
| Cu | na | 136 | 161 | 120 | 88.6 | 216 | 241 | 206 | 167 | 55.88 | 88.5 | 241 | |||
| Zn | na | 85.3 | 79.0 | 60.4 | 67.8 | 102 | 263 | 128 | 112 | 70.21 | 60.4 | 263 | |||
| Sc | 32.6 | 2.33 | 30.0 | 36.0 | 79.4 | 85.2 | 103 | 117 | 44.4 | 36.2 | 44.7 | 72.9 | 31.69 | 36.2 | 117 |
| V | 246 | 28.75 | 210 | 300 | 415 | 427 | 435 | 474 | 294 | 364 | 336 | 392 | 63.10 | 294 | 474 |
| Cr | 110 | 13.43 | 98.0 | 135 | 38.2 | 36.4 | 119 | 128 | 169 | 124 | 138 | 108 | 50.61 | 36.4 | 169 |
| Rb | na | 17.3 | 25.6 | 20.2 | 8.48 | 12.7 | 12.2 | 10.7 | 15.3 | 6.03 | 8.48 | 25.6 | |||
| Sr | 234 | 20.01 | 209 | 271 | 482 | 647 | 513 | 181 | 297 | 290 | 268 | 383 | 166.50 | 181 | 647 |
| Cs | na | 0.87 | 0.61 | 6.68 | 0.29 | 0.21 | 0.47 | 1.00 | 1.45 | 2.33 | 0.21 | 6.68 | |||
| Ba | 173 | 15.82 | 157 | 201 | 215 | 241 | 147 | 80.4 | 150 | 596 | 126 | 222 | 173.46 | 80.4 | 596 |
| Mg# | 60.05 | 3.03 | 55.8 | 63.9 | 45.22 | 45.94 | 61.05 | 61.93 | 42.39 | 41.71 | 45.51 | 49.11 | 8.61 | 41.71 | 61.93 |
| Rock Type | Main Phases | Minor Phases | Accessory Phases | |||
|---|---|---|---|---|---|---|
| Oxides, Silicates and Phosphates | Sulfides | |||||
| Matrix | Inclusions | Rims/Cracks | ||||
| Recrystallized marly limestones n = 25 | Calcite | Clinopyroxene (En28–55), Plagioclase (An72–78), K-feldspar (Or80–98), Amphibole, Grossular, Biotite, Chlorite, Quartz | Fluorapatite (F—2.97.4 wt%, Cl—0.1–0.9 wt%), Zircon, Titanite | Pyrrhotite *, Troilite *, Chalcopyrite, Galena, Pyrite, Arsenopyrite | Pyrrhotite | Rasvumite, Djerfisherite, Bartonite |
| Marbles, Zone 4 n = 10 | Calcite, Melilite, Tilleyite, Wollastonite | Kalsilite, Cuspidine | Perovskite Hydroxy-Fluorapatite (F—0.8–5.3 wt%, Cl—0.2 wt%) | Pyrrhotite *, Troilite *, Alabandite, Galena, Acanthite | Pyrrhotite | Rasvumite, Djerfisherite, Bartonite |
| Marbles, Zone 3 n = 5 | Calcite, Melilite, Spurrite, Monticellite | Merwinite, Bredigite | Perovskite, Magnetite | Pyrrhotite *, Troilite *,Alabandite, Sphalerite | Pyrrhotite, Galena, Acanthite, Rasvumite, Alabandite, Sphalerite, Wurtzite | Rasvumite, Djerfisherite |
| Marbles, Zone 2 n = 14 | Calcite, Melilite, Spurrite, Merwinite | Monticellite, Rankinite, Bredigite, Cuspidine | Perovskite, Magnetite, Baghdadite, Hydroxyl-apatite (SiO2—5.6–6.1 wt%, SO3—2.5–2.6 wt%) Sc-garnet (Sc2O3—7.7-9.4 wt%) | Pyrrhotite *, Troilite *, Rasvumite, Alabandite, Sphalerite | Pyrrhotite, Galena, Acanthite, Rasvumite, Alabandite, Sphalerite, Wurtzite | Rasvumite, Djerfisherite |
| Gabbro n = 12 | Plagioclase (An1–82), Clinopyroxene (En23–43) | Olivine (Fo31–34), Ortopyroxene (En47), K-feldspar (Or75–100), Biotite (Cl—0.1–4.5 wt%), Ferro-hornblende (Cl—0.4–1.6 wt%), Chlorite (Cl—0.1–0.5 wt%) | Ti-Magnetite, Ilmenite, Fluor-Chlorapatite (F—1.3–3.8 wt%, Cl—0.5–2.8 wt%), Titanite, Baddeleyite, Zircon, Allanite-(Y), Zirconolite | Pyrrhotite, Chalcopyrite, Troilite, Pentlandite, Sphalerite, Cobaltite, Pyrite | ||
| Rock | Recrystallized Marly Limestones | Marbles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone 2 | Zone 3 | Zone 4 | |||||||||
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| wt% | |||||||||||
| SiO2 | 13.66 | 10.79 | 14.34 | 12.92 | 22.79 | 10.58 | 21.04 | 9.84 | 13.53 | 22.75 | 10.45 |
| TiO2 | 0.10 | 0.19 | 0.30 | 0.26 | 0.41 | 0.20 | 0.45 | 0.19 | 0.33 | 0.40 | 0.14 |
| Al2O3 | 1.76 | 3.68 | 4.64 | 4.08 | 7.61 | 3.27 | 5.79 | 2.97 | 4.56 | 7.19 | 2.53 |
| Fe2O3* | 0.86 | 1.54 | 1.75 | 1.59 | 3.14 | 1.80 | 3.34 | 1.83 | 2.50 | 2.92 | 1.31 |
| MnO | 0.06 | 0.07 | 0.09 | 0.11 | 0.14 | 0.10 | 0.17 | 0.12 | 0.13 | 0.09 | 0.05 |
| MgO | 10.58 | 1.37 | 1.81 | 1.59 | 2.54 | 1.45 | 2.63 | 1.43 | 1.79 | 2.39 | 1.38 |
| CaO | 34.45 | 46.94 | 44.10 | 45.46 | 36.99 | 52.74 | 51.49 | 53.60 | 51.90 | 44.97 | 50.76 |
| Na2O | 0.17 | 0.17 | 0.24 | 0.30 | 0.42 | 0.07 | 0.14 | 0.08 | 0.16 | 0.70 | 0.12 |
| K2O | 0.62 | 0.85 | 1.07 | 0.90 | 1.58 | 0.04 | 0.05 | 0.03 | 0.12 | 0.50 | 0.44 |
| P2O5 | 0.05 | 0.09 | 0.15 | 0.10 | 0.18 | 0.11 | 0.14 | 0.09 | 0.11 | 0.17 | 0.05 |
| SO3 | na | 0.77 | 0.55 | 1.26 | 1.31 | 1.27 | 1.65 | 1.54 | 1.51 | 1.57 | 0.69 |
| LOI | 37.30 | 33.60 | 30.82 | 31.47 | 23.01 | 27.98 | 12.85 | 28.28 | 23.35 | 16.00 | 31.83 |
| Total | 99.61 | 100.06 | 99.86 | 100.04 | 100.12 | 99.61 | 99.74 | 100.00 | 99.99 | 99.65 | 99.75 |
| ppm | |||||||||||
| Co | 3.00 | 4.89 | 5.91 | 10.1 | 12.6 | 8.03 | 11.8 | 6.29 | 8.21 | 9.11 | 2.40 |
| Ni | 6.00 | 11.4 | 13.4 | 15.3 | 23.9 | 18.1 | 23.4 | 17.2 | 19.1 | 21.2 | 6.06 |
| Cu | 8.00 | 6.82 | 9.14 | 3.95 | 27.7 | 16.7 | 33.4 | 11.5 | 17.3 | 22.5 | 7.20 |
| Zn | 22.0 | 415 | 19.2 | 19.4 | 112 | 38.1 | 56.2 | 805 | 25.0 | 63.3 | 7.66 |
| Sc | na | 13.0 | 4.47 | 3.81 | 11.6 | 4.08 | 9.48 | 16.1 | 5.57 | 10.1 | 1.85 |
| V | 20.0 | 5.97 | 21.3 | 14.8 | 45.3 | 6.57 | 16.8 | 9.00 | 15.2 | 19.6 | 12.2 |
| Cr | 9.00 | 18.3 | 16.5 | 16.6 | 32.3 | 19.4 | 37.7 | 20.0 | 23.8 | 37.9 | 11.2 |
| Rb | 17.0 | 6.46 | 17.6 | 16.4 | 30.3 | 1.54 | 1.77 | 2.86 | 4.22 | 5.67 | 5.95 |
| Sr | 245 | 427 | 372 | 373 | 306 | 587 | 385 | 412 | 387 | 495 | 226 |
| Cs | na | 0.69 | 0.37 | 0.77 | 0.63 | <0.01 | <0.01 | 0.05 | <0.01 | <0.01 | 0.25 |
| Ba | 178 | 450 | 61.8 | 45.1 | 500 | 60.2 | 46.8 | 61.2 | 66.3 | 60.0 | 249 |
| Backscattered Electron Image | Gabbro | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral | Pyrrhotite (n = 82) | Troilite (n = 14) | |||||||||||
 | Sample | PK-4-8 | Mean | S | Min. | Max. | PT-95 | Mean | S | Min. | Max. | ||
| Fe | 59.59 | 61.15 | 59.79 | 0.57 | 58.70 | 61.15 | 62.59 | 61.89 | 62.95 | 0.58 | 61.89 | 63.82 | |
| Ni | 0.43 | 0.15 | 0.33 | 0.23 | 0.07 | 0.81 | 1.17 | 0.47 | 0.19 | 0.34 | 0.08 | 1.17 | |
| Co | 0.48 | 0.15 | 0.23 | 0.20 | 0.04 | 0.66 | <0.03 | <0.03 | |||||
| Cu | 0.16 | 0.05 | 0.04 | 0.04 | <0.04 | 0.16 | <0.04 | <0.04 | |||||
| S | 40.11 | 39.10 | 39.61 | 0.29 | 38.75 | 40.26 | 36.76 | 37.47 | 36.80 | 0.49 | 35.99 | 37.73 | |
| Total | 100.96 | 100.60 | 99.97 | 100.52 | 99.94 | 99.94 | |||||||
| (Fe + Ni + Co + Cu)/S * | 0.87 | 0.90 | 0.87 | 0.99 | 0.96 | 0.98 | |||||||
 | Recrystallized marly limestones | ||||||||||||
| Mineral | Pyrrhotite (n = 11) | Troilite (n = 8) | |||||||||||
| Sample | PK-3-3 | PK-4-1 | Mean | S | Min. | Max. | PK-3-3 | Mean | S | Min. | Max. | ||
| Fe | 61.03 | 61.53 | 61.48 | 0.28 | 61.03 | 61.89 | 62.36 | 63.80 | 63.18 | 0.78 | 62.24 | 64.28 | |
| S | 38.83 | 39.07 | 38.69 | 0.25 | 38.33 | 39.07 | 37.80 | 36.24 | 36.89 | 0.87 | 36.05 | 38.27 | |
| Total | 99.86 | 100.60 | 100.17 | 100.16 | 100.04 | 100.07 | |||||||
| Fe/S * | 0.90 | 0.90 | 0.91 | 0.95 | 1.01 | 0.98 | |||||||
 | Marbles | ||||||||||||
| Mineral | Pyrrhotite (n = 83) | Troilite (n = 53) | |||||||||||
| Sample | PT-97 | PT-122 | Mean | S | Min. | Max. | PT-93 | PK-16-3 | Mean | S | Min. | Max. | |
| Fe | 61.27 | 60.87 | 61.27 | 0.51 | 60.07 | 62.43 | 63.09 | 62.91 | 63.11 | 0.51 | 62.06 | 64.35 | |
| Co | 0.12 | 0.14 | 0.13 | 0.01 | 0.12 | 0.15 | 0.09 | 0.11 | 0.10 | 0.01 | 0.09 | 0.11 | |
| S | 37.95 | 39.11 | 38.56 | 0.49 | 37.53 | 39.51 | 36.80 | 36.51 | 36.78 | 0.38 | 36.00 | 37.82 | |
| Total | 99.34 | 100.12 | 99.96 | 99.98 | 99.53 | 99.99 | |||||||
| (Fe + Co)/S * | 0.93 | 0.89 | 0.91 | 0.98 | 0.99 | 0.99 | |||||||
| Mineral | Rock Type | Merwinite Marbles | Spurrite-Monticellite Marbles | Tilleyite-Wollastonite Marbles | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zone | Zone 2 | Zone 3 | Zone 4 | |||||||
| Formula | Matrix | Inclusions | Rims/Cracks | Matrix | Inclusions | Rims/Cracks | Matrix | Inclusions | Rims/Cracks | |
| Pyrrhotite * | Fe1-xS | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | |||
| Troilite * | FeS | ▲ | ▲ | ▲ | ||||||
| Alabandite | (Mn,Fe)S | ● | □ | ● | □ | ● | ||||
| Sphalerite | (Zn,Fe,Mn)S | ● | □ | ● | □ | |||||
| Wurtzite | (Zn,Mn,Fe)S | □ | □ | |||||||
| Galena | PbS | ● | ● | □ | ||||||
| Acanthite | Ag2S | ● | ● | □ | ||||||
| Rasvumite | KFe2S3 | ● | □ | ▲ | □ | ▲ | ▲ | |||
| Djerfisherite | K6(Fe,Ni,Co,Cu)25S26Cl | ● | ● | ● | ||||||
| Bartonite | K6Fe21S27 | □ | ||||||||
| Mineral/Backscattered Electron Image | Element | Fe | Mn | Zn | Cd | S | Total | Fe | Mn | Zn | Cd | ∑Me | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | wt% | a.p.f.u. | |||||||||||
| Sphalerite |  | PT-97 | 17.92 | 7.43 | 38.91 | 0.50 | 34.44 | 99.20 | 0.299 | 0.126 | 0.554 | 0.004 | 0.983 |
| 18.26 | 4.50 | 41.68 | 0.54 | 34.17 | 99.15 | 0.307 | 0.077 | 0.598 | 0.005 | 0.986 | |||
| 21.87 | 5.26 | 37.18 | 0.47 | 34.34 | 99.12 | 0.366 | 0.089 | 0.531 | 0.004 | 0.990 | |||
| 19.07 | 5.87 | 39.40 | 0.64 | 34.52 | 99.50 | 0.317 | 0.099 | 0.560 | 0.005 | 0.981 | |||
| 20.65 | 4.01 | 40.03 | 0.38 | 34.27 | 99.34 | 0.346 | 0.068 | 0.573 | 0.003 | 0.990 | |||
| Mean (n = 17) | 19.82 | 5.06 | 39.47 | 0.44 | 34.46 | 99.25 | 0.330 | 0.086 | 0.562 | 0.004 | 0.981 | ||
| S | 1.11 | 0.90 | 1.12 | 0.08 | 0.38 | ||||||||
| Min | 17.92 | 4.01 | 37.18 | 0.31 | 34.11 | ||||||||
| Max | 21.87 | 7.43 | 41.68 | 0.64 | 35.74 | ||||||||
| Wurtzite |  | PT-104 | 8.86 | 16.30 | 39.35 | <0.30 | 35.32 | 99.83 | 0.144 | 0.279 | 0.566 | 0.000 | 0.989 |
| PT-93 | 10.52 | 17.74 | 36.68 | <0.30 | 34.69 | 99.13 | 0.174 | 0.290 | 0.519 | 0.000 | 0.983 | ||
| PT-97 | 19.06 | 16.84 | 28.48 | <0.30 | 35.48 | 99.86 | 0.308 | 0.277 | 0.394 | 0.000 | 0.979 | ||
| PT-97 | 12.03 | 18.25 | 33.33 | <0.30 | 35.54 | 99.15 | 0.194 | 0.300 | 0.500 | 0.000 | 0.994 | ||
| Mean (n = 7) | 12.37 | 16.96 | 34.66 | <0.30 | 35.07 | 99.06 | 0.202 | 0.282 | 0.500 | 0.000 | 0.984 | ||
| S | 4.78 | 0.94 | 4.69 | 0.51 | |||||||||
| Min | 7.86 | 16.00 | 28.48 | 34.57 | |||||||||
| Max | 19.06 | 18.25 | 39.35 | 35.54 | |||||||||
| Alabandite |  | PT-102 | 10.54 | 52.95 | <0.30 | <0.30 | 35.80 | 99.29 | 0.169 | 0.863 | 0.000 | 0.000 | 1.032 |
| PT-97 | 7.49 | 56.22 | <0.30 | <0.30 | 36.78 | 100.49 | 0.117 | 0.892 | 0.000 | 0.000 | 1.009 | ||
| PT-88 | 12.19 | 50.45 | <0.30 | <0.30 | 36.97 | 99.61 | 0.189 | 0.796 | 0.000 | 0.000 | 0.986 | ||
| PK-16-1 | 15.68 | 47.18 | <0.30 | <0.30 | 36.31 | 99.17 | 0.248 | 0.758 | 0.000 | 0.000 | 1.006 | ||
| Mean (n = 25) | 10.24 | 53.03 | <0.30 | <0.30 | 36.60 | 99.87 | 0.161 | 0.849 | 0.000 | 0.000 | 1.010 | ||
| S | 2.28 | 2.51 | 0.28 | ||||||||||
| Min | 7.49 | 47.18 | 35.80 | ||||||||||
| Max | 15.68 | 56.22 | 36.97 | ||||||||||
| Mineral | Element | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Ni | Co | Cu | Zn | S | Total | ||
| Sphalerite n = 10 | Mean | 11.76 | 0.13 | 0.23 | 0.49 | 53.15 | 34.02 | 99.78 |
| S | 0.54 | 0.20 | 0.06 | 0.37 | 0.66 | 0.13 | ||
| Min | 10.63 | 0.05 | 0.16 | 0.15 | 52.23 | 33.87 | ||
| Max | 12.40 | 0.57 | 0.33 | 1.18 | 54.40 | 34.25 | ||
| Chalcopyrite n = 35 | Mean | 30.79 | <0.03 | <0.04 | 34.01 | 0.06 | 34.97 | 99.83 |
| S | 0.29 | 0.26 | 0.09 | 0.21 | ||||
| Min | 30.24 | 33.38 | <0.05 | 34.50 | ||||
| Max | 31.42 | 34.43 | 0.41 | 35.43 | ||||
| Pyrite n = 10 | Mean | 46.13 | 0.34 | 0.13 | <0.04 | <0.05 | 52.88 | 99.49 |
| S | 0.67 | 0.42 | 0.03 | 0.35 | ||||
| Min | 45.01 | 0.05 | 0.07 | 52.55 | ||||
| Max | 46.78 | 1.14 | 0.20 | 53.53 | ||||
| Pentlandite n = 12 | Mean | 31.54 | 28.58 | 5.60 | <0.04 | <0.05 | 33.85 | 99.57 |
| S | 3.25 | 3.64 | 2.51 | 0.52 | ||||
| Min. | 27.10 | 21.92 | 3.78 | 33.14 | ||||
| Max. | 37.32 | 32.84 | 13.16 | 35.01 | ||||
| Rock Type | Element | Mn | Co | Ni | Cu | Zn | Se | Mo | Ag |
|---|---|---|---|---|---|---|---|---|---|
| Recrystallized marly limestones n = 17 | Mean | 151 | 485 | 655 | 1.72 | 1.56 | 6.81 | 0.49 | 0.12 |
| S | 65.6 | 14.5 | 159 | 1.00 | 0.63 | 5.47 | 0.18 | 0.18 | |
| Min | 61.0 | 465 | 343 | 1.02 | 0.99 | 2.10 | 0.23 | <0.01 | |
| Max | 263 | 517 | 808 | 4.10 | 3.09 | 23.1 | 0.97 | 0.75 | |
| Tly-Wo marbles, Zone 4 n = 10 | Mean | 342 | 345 | 909 | 50.9 | 9.60 | 3.35 | 0.02 | 0.14 |
| S | 235 | 101 | 523 | 41.9 | 11.1 | 1.61 | 0.02 | 0.14 | |
| Min | 72.4 | 186 | 180 | 2.35 | 1.38 | 3.10 | 0.01 | 0.02 | |
| Max | 733 | 569 | 1751 | 116 | 34.6 | 4.40 | 0.05 | 0.39 | |
| Spu-Mtc marbles, Zone 3 n = 18 | Mean | 258 | 410 | 242 | 15.3 | 17.2 | 4.96 | <0.01 | 0.08 |
| S | 99.4 | 72.6 | 276 | 10.9 | 12.5 | 2.63 | 0.07 | ||
| Min | 135 | 278 | 16.7 | 1.67 | 0.98 | 3.70 | <0.01 | ||
| Max | 509 | 515 | 913 | 32.1 | 35.9 | 6.50 | 0.22 | ||
| Spu-Mw marbles, Zone 2 n = 4 | Mean | 182 | 349 | 7.63 | <0.02 | 1.02 | 6.00 | <0.01 | 0.04 |
| S | 67.2 | 61.4 | 2.68 | 0.54 | 2.86 | ||||
| Min | 112 | 297 | 4.57 | 0.83 | 3.20 | 0.04 | |||
| Max | 246 | 417 | 9.56 | 1.22 | 9.90 | 0.04 | |||
| Gabbro n = 16 | Mean | 15.8 | 4422 | 6051 | 41.7 | 6.44 | 136 | 6.39 | 1.71 |
| S | 15.2 | 447 | 1850 | 49.6 | 5.15 | 16.4 | 5.20 | 1.93 | |
| Min | 3.20 | 3643 | 3027 | 1.67 | 2.19 | 121 | 1.57 | 0.21 | |
| Max | 64.9 | 5260 | 9230 | 159 | 17.9 | 192 | 20.7 | 6.40 |
| Sample | Zone 2 | Zone 3 | Zone 4 | Zones 2–4 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT-104 | PT-91 | PT-93 | PT-94 | PT-97 | PK-16-3 | Mean (n = 30) | S | Min. | Max. | ||||||
| wt% | |||||||||||||||
| K | 15.22 | 16.04 | 15.76 | 15.27 | 16.36 | 16.14 | 15.42 | 15.31 | 15.63 | 15.52 | 15.54 | 0.32 | 15.15 | 16.36 | |
| Fe | 45.11 | 45.46 | 44.92 | 45.20 | 45.16 | 45.11 | 45.57 | 45.32 | 44.84 | 45.08 | 44.98 | 0.37 | 44.34 | 45.62 | |
| S | 38.88 | 37.50 | 38.80 | 38.90 | 38.26 | 37.95 | 38.86 | 38.97 | 38.76 | 38.68 | 38.84 | 0.56 | 37.50 | 39.71 | |
| Total | 99.21 | 99.01 | 99.48 | 99.37 | 99.78 | 99.20 | 99.85 | 99.60 | 99.23 | 99.28 | 99.36 | ||||
| a.p.f.u. | |||||||||||||||
| K | 0.963 | 1.052 | 0.999 | 0.966 | 1.052 | 1.047 | 0.976 | 0.966 | 0.992 | 0.987 | 0.984 | ||||
| Fe | 1.998 | 2.088 | 1.994 | 2.001 | 2.033 | 2.047 | 2.020 | 2.003 | 1.992 | 2.007 | 1.994 | ||||
| Sample | Zone 2 | Zone 3 | Zone 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PT-104 | PT-107 | PT-97 | PT-89 | PK-16-3 | |||||
| wt% | |||||||||
| K | 9.52 | 9.57 | 8.93 | 9.39 | 9.62 | 9.45 | 9.50 | 9.39 | 9.58 |
| Fe | 51.84 | 42.85 | 44.67 | 46.42 | 47.04 | 51.42 | 51.35 | 52.59 | 51.47 |
| Ni | 0.99 | 11.01 | 9.19 | 6.37 | 2.54 | 1.13 | 1.92 | 1.77 | 1.97 |
| Co | <0.03 | 0.66 | 1.00 | 0.35 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 |
| Cu | 0.94 | 0.94 | 1.60 | 1.67 | 5.22 | 1.04 | 1.32 | <0.04 | <0.04 |
| Mn | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | 1.39 | 1.55 |
| S | 34.27 | 34.30 | 32.75 | 34.14 | 34.00 | 34.58 | 33.53 | 33.08 | 33.80 |
| Cl | 1.55 | 1.47 | 1.33 | 1.43 | 1.47 | 1.43 | 1.55 | 1.22 | 1.30 |
| Total | 99.11 | 100.80 | 99.47 | 99.77 | 99.89 | 99.05 | 99.17 | 99.44 | 99.67 |
| a.p.f.u. | |||||||||
| K | 5.923 | 5.948 | 5.813 | 5.864 | 6.032 | 5.826 | 6.040 | 6.052 | 6.043 |
| Fe | 22.578 | 18.646 | 20.358 | 20.295 | 20.650 | 22.194 | 22.858 | 23.729 | 22.729 |
| Ni | 0.410 | 4.558 | 3.985 | 2.650 | 1.061 | 0.464 | 0.813 | 0.760 | 0.828 |
| Co | 0.000 | 0.272 | 0.432 | 0.145 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Cu | 0.360 | 0.359 | 0.641 | 0.642 | 2.014 | 0.395 | 0.516 | 0.000 | 0.000 |
| Mn | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.638 | 0.698 |
| Cl | 1.063 | 1.008 | 0.955 | 0.985 | 1.017 | 0.972 | 1.087 | 0.867 | 0.904 |
| ∑ Me | 23.348 | 23.836 | 25.416 | 23.731 | 23.725 | 23.053 | 24.188 | 25.126 | 24.252 |
| Element | Sample | ||||||
|---|---|---|---|---|---|---|---|
| PT-88 | PT-91 | PK-16-1 | |||||
| wt% | |||||||
| K | 10.10 | 10.38 | 10.19 | 10.33 | 10.26 | 10.14 | 10.28 |
| Fe | 47.04 | 47.12 | 46.31 | 45.30 | 51.45 | 50.80 | 50.04 |
| Ni | 2.45 | 2.48 | 3.03 | 3.33 | <0.30 | <0.30 | 0.48 |
| Co | 0.53 | 0.52 | 0.50 | 0.49 | <0.30 | <0.30 | <0.30 |
| Cu | 1.43 | 1.68 | 1.68 | 1.69 | <0.30 | 0.46 | 0.71 |
| S | 37.56 | 38.03 | 37.57 | 38.11 | 37.35 | 37.64 | 38.12 |
| Total | 99.11 | 100.21 | 99.28 | 99.25 | 99.06 | 99.04 | 99.63 |
| a.p.f.u. | |||||||
| K | 5.953 | 6.043 | 6.005 | 6.001 | 6.082 | 5.964 | 5.971 |
| Fe | 19.414 | 19.205 | 19.106 | 18.424 | 21.351 | 20.919 | 20.347 |
| Ni | 0.962 | 0.962 | 1.188 | 1.289 | 0.000 | 0.000 | 0.186 |
| Co | 0.207 | 0.201 | 0.195 | 0.189 | 0.000 | 0.000 | 0.000 |
| Cu | 0.519 | 0.602 | 0.609 | 0.604 | 0.000 | 0.166 | 0.254 |
| ∑ Me | 21.100 | 20.969 | 21.100 | 20.506 | 21.351 | 21.086 | 20.787 |
| Sample | Rock Type | δ34 S (CDT)‰ |
|---|---|---|
| PK-16-5 * | Marly limestone | –28.25 ± 0.20 |
| PK-2 | Recrystallized marly limestone | –26.75 ± 0.20 |
| PK-3-2 | Recrystallized marly limestone | –27.62 ± 0.20 |
| PK-3-3 | Recrystallized marly limestone | –25.84 ± 0.20 |
| PK-4-7 | Recrystallized marly limestone | –28.68 ± 0.20 |
| PK-6-1 | Recrystallized marly limestone | –23.32 ± 0.20 |
| PK-12-1 | Tly-Wo marble (Zone 4) | –15.07 ± 0.20 |
| PT-117 | Mw-Spu marble (Zone 2) | –25.18 ± 0.20 |
| PT-122 | Mw-Spu marble (Zone 2) | –25.36 ± 0.20 |
| PT-95 | Gabbro | +13.10 ± 0.20 |
| PK-9-2 | Gabbro | +2.68 ± 0.20 |
| PK-17-4 | Gabbro | +3.31 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokol, E.V.; Deviatiiarova, A.S.; Kokh, S.N.; Reutsky, V.N.; Abersteiner, A.; Philippova, K.A.; Artemyev, D.A. Sulfide Minerals as Potential Tracers of Isochemical Processes in Contact Metamorphism: Case Study of the Kochumdek Aureole, East Siberia. Minerals 2021, 11, 17. https://doi.org/10.3390/min11010017
Sokol EV, Deviatiiarova AS, Kokh SN, Reutsky VN, Abersteiner A, Philippova KA, Artemyev DA. Sulfide Minerals as Potential Tracers of Isochemical Processes in Contact Metamorphism: Case Study of the Kochumdek Aureole, East Siberia. Minerals. 2021; 11(1):17. https://doi.org/10.3390/min11010017
Chicago/Turabian StyleSokol, Ella V., Anna S. Deviatiiarova, Svetlana N. Kokh, Vadim N. Reutsky, Adam Abersteiner, Kseniya A. Philippova, and Dmitry A. Artemyev. 2021. "Sulfide Minerals as Potential Tracers of Isochemical Processes in Contact Metamorphism: Case Study of the Kochumdek Aureole, East Siberia" Minerals 11, no. 1: 17. https://doi.org/10.3390/min11010017
APA StyleSokol, E. V., Deviatiiarova, A. S., Kokh, S. N., Reutsky, V. N., Abersteiner, A., Philippova, K. A., & Artemyev, D. A. (2021). Sulfide Minerals as Potential Tracers of Isochemical Processes in Contact Metamorphism: Case Study of the Kochumdek Aureole, East Siberia. Minerals, 11(1), 17. https://doi.org/10.3390/min11010017





