Mobility of Radiogenic Helium in Amphibole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Alkaline Amphiboles and Their Host Rocks
3. Results
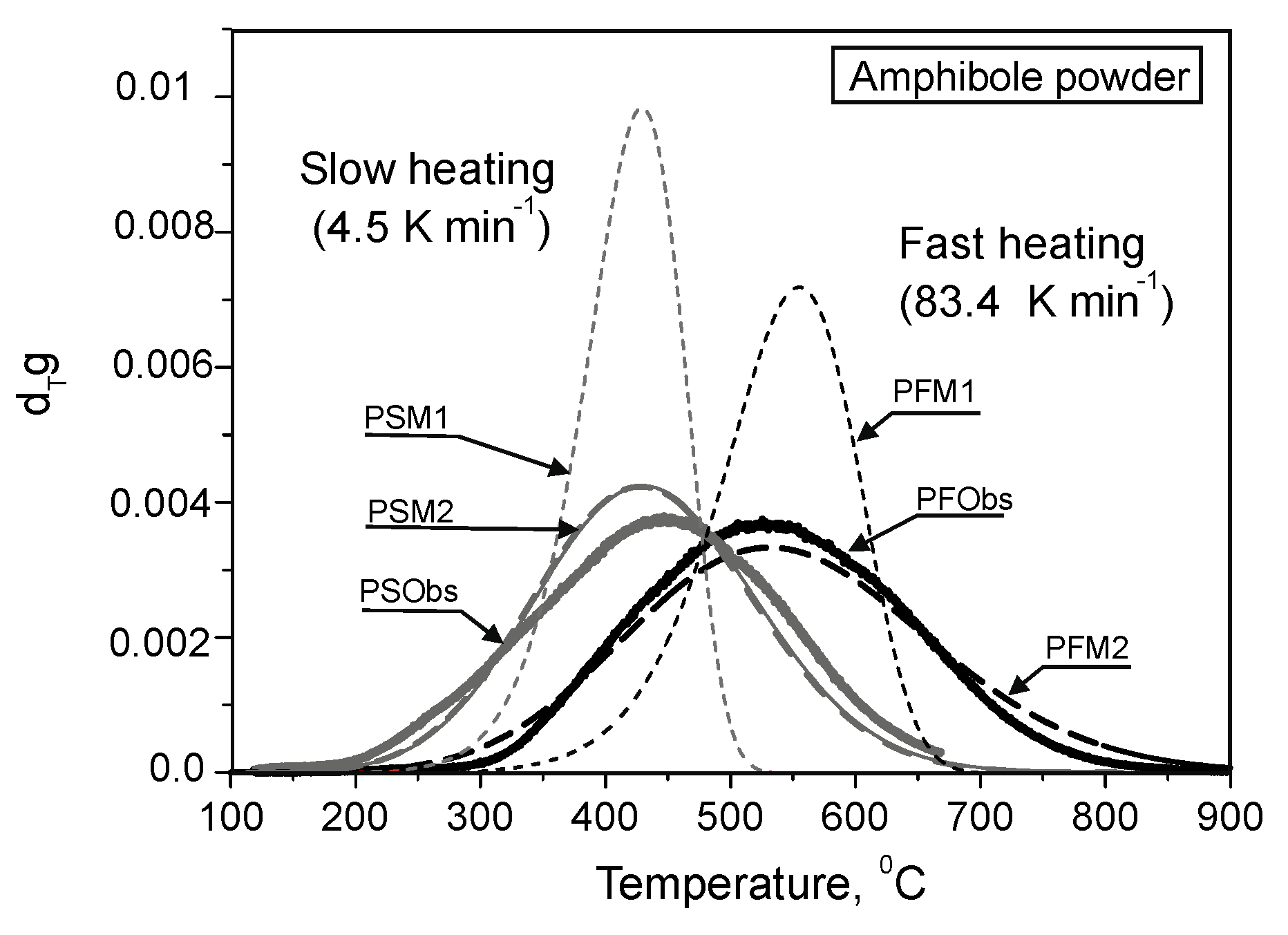
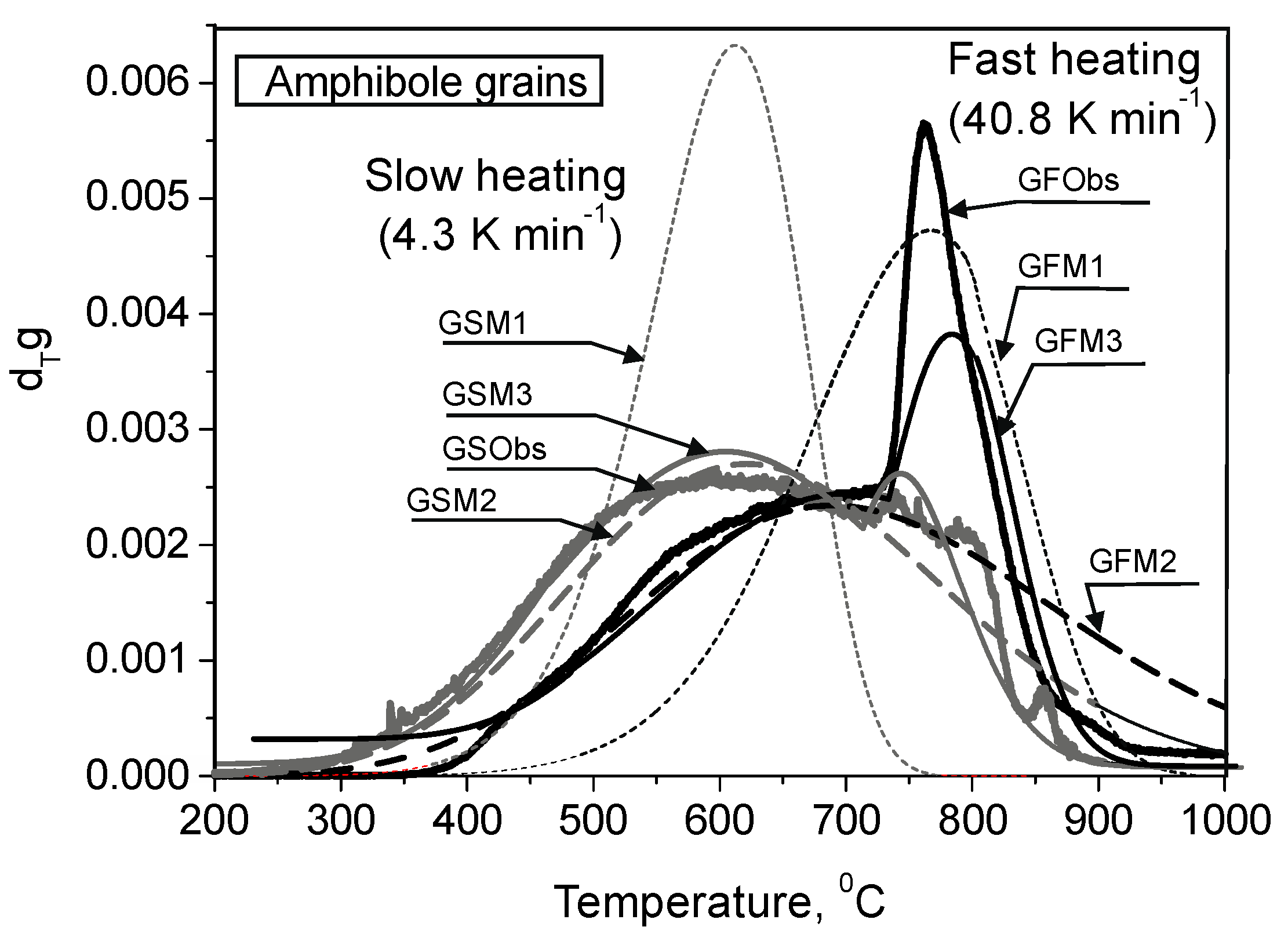
3.1. Experimental Results
- In case of the fast heating rate, He is released from the powder and grains under higher temperatures compared with the slow rate.
- He is released as a smooth peak within a wider temperature interval compared with that expected from He diffusion from the single size diffusion domain.
- During fast isothermal heating of the amphibole grains, a sharp second peak appeared with a maximum at ≈ 770 °C; the second peak has never been observed if He is extracted from the powder.
3.2. He Migration in a Diffusion Domain: The Model
3.3. The Model with Variable Sizes of the Diffusion Domains
3.4. He Release from the Powdered Samples: Comparison of the Experimental and Calculated Results
3.5. He Release from the Grains: Comparison of Experimental and Calculated Results
3.6. Diffusion and the Tensile Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tolstikhin, I.N.; Verchovsky, A.B.; Kamensky, I.L.; Skiba, V.I.; Gannibal, M.A.; Vetrin, V.R.; Tarakanov, S.V. Amphibole: A major carrier of helium isotopes in crustal rocks. Chem. Geol. 2016, 444, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Trull, T.W.; Kurz, M.D.; Jenkins, W.J. Diffusion of cosmogenic 3He in olivine and quartz: Implications for surface exposure dating. Earth Planet. Sci. Lett. 1991, 103, 241–256. [Google Scholar] [CrossRef]
- Shuster, D.L.; Farley, K.A.; Sisterson, J.M.; Burnett, D.S. Quantifying the diffusion kinetics and spatial distributions of radiogenic 4He in minerals containing proton-induced 3He. Earth Planet. Sci. Lett. 2003, 217, 19–32. [Google Scholar] [CrossRef]
- Yokochi, R.; Marty, B.; Pik, R.; Burnard, P. High 3He/4He ratios in peridotite xenoliths from SW Japan revisited: Evidence for cosmogenic 3He released by vacuum crushing. Geochem. Geophys. Geosyst. 2005, 6, Q01004. [Google Scholar] [CrossRef]
- Gramlich, J.W.; Naughton, J.J. Nature of source material for ultramafic minerals from Salt Lake crater, Hawaii, from measurement of helium and argon diffusion. J. Geophys. Res. 1972, 77, 3032–3042. [Google Scholar] [CrossRef]
- Shaw, A.M.; Hilton, D.R.; Fischer, T.P.; Walker, J.A.; de Leeuw, G.A.M. Helium isotope variations in mineral separates from Costa Rica and Nicaragua: Assessing crustal contributions, timescale variations and diffusion-related mechanisms. Chem. Geol. 2006, 230, 124–139. [Google Scholar] [CrossRef]
- Tolstikhin, I.; Kamensky, I.L.; Tarakanov, S.; Kramers, J.; Pekala, M.; Skiba, V.; Gannibal, M.; Novikov, D. Noble gas isotope sites and mobility in mafic rocks and olivine. Geochim. Cosmochim. Acta 2010, 74, 1436–1447. [Google Scholar] [CrossRef]
- Tolstikhin, I.; Waber, H.N.; Kamensky, I.; Loosli, H.H.; Skiba, V.; Gannibal, M. Production, redistribution and loss of helium and argon isotopes in a thick sedimentary aquitard-aquifer system (Molasse Basin, Switzerland). Chem. Geol. 2011, 286, 48–58. [Google Scholar] [CrossRef]
- Lee, J.K.W. The argon release mechanisms of hornblende in vacuo. Chem. Geol. 1993, 106, 133–170. [Google Scholar] [CrossRef]
- Shuster, D.L.; Farley, K.A. Diffusion kinetics of proton-induced 21Ne, 3He, and 4He in quartz. Geochim. Cosmochim. Acta 2005, 69, 2349–2359. [Google Scholar] [CrossRef]
- Farley, K.A. He diffusion systematics in minerals: Evidence from synthetic monazite and zircon structure phosphates. Geochim. Cosmochim. Acta 2007, 71, 4015–4024. [Google Scholar] [CrossRef]
- Argunova, T.C.; Sorokin, L.M.; Pevzner, B.Z.; Balitsky, V.C.; Gannibal, M.A.; Je, J.H.; Hwu, Y.; Tsai, W.-L. The Influence of Defects in the Crystal Structure on Helium Diffusion in Quartz. Phys. Solid State 2003, 45, 1910–1917. [Google Scholar] [CrossRef]
- Dunai, T.J.; Roselieb, K. Sorption and diffusion of helium in garnet: Implications for volatile tracing and dating. Earth Planet. Sci. Lett. 1996, 139, 411–421. [Google Scholar] [CrossRef]
- Matsumoto, T.; Maruo, K.; Tsuchiyama, A.; Matsuda, J.I. Occlusion of noble gases (He, Ne, Ar, Kr, Xe) into synthetic magnetite at 500–1300 °C. Earth Planet. Sci. Lett. 1996, 141, 315–324. [Google Scholar] [CrossRef]
- Lux, G. The behavior of noble gases in silicate liquids: Solution, diffusion, bubbles and surface effects, with applications to natural samples. Geochim. Cosmochim. Acta 1987, 51, 1549–1560. [Google Scholar] [CrossRef]
- Roselieb, K.; Rammensee, W.; Buttner, H.; Rosenhauer, M. Solubility and diffusion of noble gases in vitreous albite. Chem. Geol. 1992, 96, 241–266. [Google Scholar] [CrossRef]
- Trull, T.W.; Kurz, M.D. Experimental measurements of 3He and 4He mobility in olivine and clinopyroxene at magmatic temperatures. Geochim. Cosmochim. Acta 1993, 57, 1313–1324. [Google Scholar] [CrossRef]
- Parman, S.W.; Kurz, M.D.; Hart, S.R.; Grove, T.L. Helium solubility in olivine and implications for high 3He/4He in ocean island basalts. Nature 2005, 437, 1140–1143. [Google Scholar] [CrossRef]
- Matsuda, J.; Sudo, M.; Ozima, M.; Ito, K.; Ohtaka, O.; Ito, E. Noble gas partitioning between metal and silicate under high pressures. Science 1993, 259, 787–790. [Google Scholar] [CrossRef]
- Heber, V.S.; Brooker, R.A.; Kelley, S.P.; Wood, B.J. Crystal-melt partitioning of noble gases (helium, neon, argon, krypton, and xenon) for olivine and clinopyroxene. Geochim. Cosmochim. Acta 2007, 71, 1041–1061. [Google Scholar] [CrossRef]
- Baxter, E.F. Quantification of the factors controlling the presence of excess 40Ar or 4He. Earth Planet. Sci. Lett. 2003, 216, 619–634. [Google Scholar] [CrossRef]
- Gerling, E.K.; Mamyrin, B.A.; Tolstikhin, I.N.; Yakovleva, S.S. Helium isotopic composition in some rocks. Geochem. Int. 1971, 8, 755–761. [Google Scholar]
- Gerling, E.K.; Tolstikhin, I.N.; Drubetskoy, E.R.; Levkovsky, R.Z.; Sharkov, E.V.; Kozakov, I.K. Helium and argon isotopes in rock-forming minerals. Geochimiya 1976, 11, 1603–1611. (In Russian) [Google Scholar]
- Kamensky, I.L.; Tolstikhin, I.N.; Vetrin, V.R. Juvenile helium in ancient rocks: I. 3He excess in amphiboles from 2.8 Ga charnockite series—Crust-mantle fluid in intracrustal magmatic processes. Geochim. Cosmochim. Acta 1990, 54, 3115–3122. [Google Scholar] [CrossRef]
- Lee, J.K.W.; Onstott, T.C.; Cashman, K.V.; Cumbest, R.J.; Johnson, D.J. Incremental heating of hornblende in vacuo: Implications for 40Ar/39Ar geochronology and the interpretation of thermal histories. Geology 1991, 19, 872–876. [Google Scholar] [CrossRef]
- Vetrin, V.R.; Kamensky, I.L.; Bayanova, T.B.; Timmerman, M.; Belyatsky, B.V.; Levsky, L.K.; Balashov, Y.A. Melanocratic nodules in alkaline granites of the Ponoiskii Massif, Kola Peninsula: A clue to petrogenesis. Geochem. Int. 1999, 37, 1061–1072. [Google Scholar]
- Kendrick, M.A.; Scambelluri, M.; Honda, M.; Phillips, D. High abundances of noble gas and chlorine delivered to the mantle by serpentinite subduction. Nat. Geosci. 2011, 4, 807–812. [Google Scholar] [CrossRef]
- Jackson, C.R.M.; Parman, S.W.; Kelley, S.P.; Cooper, R.F. Noble gas transport into the mantle facilitated by high solubility in amphibole. Nat. Geosci. 2013, 6, 562–565. [Google Scholar] [CrossRef]
- Jackson, C.R.M.; Parman, S.W.; Kelley, S.P.; Cooper, R.F. Light noble gas dissolution into ring structure-bearing materials and lattice influences on noble gas recycling. Geochim. Cosmochim. Acta 2015, 159, 1–15. [Google Scholar] [CrossRef]
- Jackson, C.R.M.; Shuster, D.L.; Parman, S.W.; Smye, A.J. Noble gas diffusivity hindered by low energy sites in amphibole. Geochim. Cosmochim. Acta 2016, 172, 65–75. [Google Scholar] [CrossRef]
- Shelby, J.E. Diffusion of helium isotopes in vitreous silica. Phys. Rev. B 1971, 4, 2681–2686. [Google Scholar] [CrossRef]
- Burnard, P. Diffusive fractionation of noble gases and helium isotopes during mantle melting. Earth Planet. Sci. Lett. 2004, 220, 287–295. [Google Scholar] [CrossRef]
- Gudkov, A.V.; Kolobov, V.V.; Tarakanov, S.V.; Tolstikhin, I.N. Mobility of radiogenic isotopes 4He and 3He and their retention in a mineral (by the example of amphibole). Dokl. Earth Sci. 2019, 488, 1193–1195. [Google Scholar] [CrossRef]
- Vetrin, V.R.; Rodionov, N.V. Geology and geochronology of neoarchean anorogenic magmatism of the Keivy structure, Kola Peninsula. Petrology 2009, 17, 578–600. [Google Scholar] [CrossRef]
- Erlang, A.K. The theory of probabilities and telephone conversations. In The Life and Works of A.K. Erlang: Transactions of the Danish Academy of Technical Sciences; Brockmeyer, E., Halstrøm, H.L., Jensen, A., Eds.; Akademiet for de Tekniske Videnskaber: Copenhagen, Denmark, 1948; Volume 2, pp. 131–137. [Google Scholar]
- Lippolt, H.J.; Weigel, W. 4He diffusion in 40Ar-retantative minerals. Geochim. Cosmochim. Acta 1988, 52, 1449–1458. [Google Scholar] [CrossRef]
- Baxter, E.F. Diffusion of noble gases in minerals. Rev. Miner. Geochem. 2010, 72, 509–997. [Google Scholar] [CrossRef]
- Kalashnikov, E.V.; Pevzner, B.Z. Motion of a helium atom through a comparable-diameter channel in the Frenkel-Kontorova model. Phys. Solid State 2002, 44, 294–299. [Google Scholar] [CrossRef]
- Regel, V.R.; Slucker, A.I.; Tomashevsky, E.E. Kinetic Theory of the Strength of Solid Bodies; Nauka: Moscow, Russia, 1974; 560p. (In Russian) [Google Scholar]
- Kondratiev, V.N. Bond Breaking Energies: Ionization Potentials and Electron Affinity; Nauka: Moscow, Russia, 1974; 351p. (In Russian) [Google Scholar]
- Elliott, B.A. Crystallization conditions of the Wiborg Rapakivi Batholith, SE Finland: An evaluation of amphibole and biotite mineral chemistry. Mineral. Petrol. 2001, 72, 305–324. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolstikhin, I.; Tarakanov, S.; Kolobov, V.; Gannibal, M. Mobility of Radiogenic Helium in Amphibole. Minerals 2021, 11, 27. https://doi.org/10.3390/min11010027
Tolstikhin I, Tarakanov S, Kolobov V, Gannibal M. Mobility of Radiogenic Helium in Amphibole. Minerals. 2021; 11(1):27. https://doi.org/10.3390/min11010027
Chicago/Turabian StyleTolstikhin, Igor, Sergei Tarakanov, Vitalii Kolobov, and Maria Gannibal. 2021. "Mobility of Radiogenic Helium in Amphibole" Minerals 11, no. 1: 27. https://doi.org/10.3390/min11010027



