Mechanical Activation of the Ca-Rich Circulating Fluidized Bed Combustion Fly Ash: Development of an Alternative Binder System
Abstract
:1. Introduction
2. Material and Methods
2.1. Circulating Fluidized Bed Combustion Ash
2.2. Mechanical Activation
2.3. Uniaxial Compressive Strength Test
2.4. X-Ray Fluorescence Spectrometry Analysis
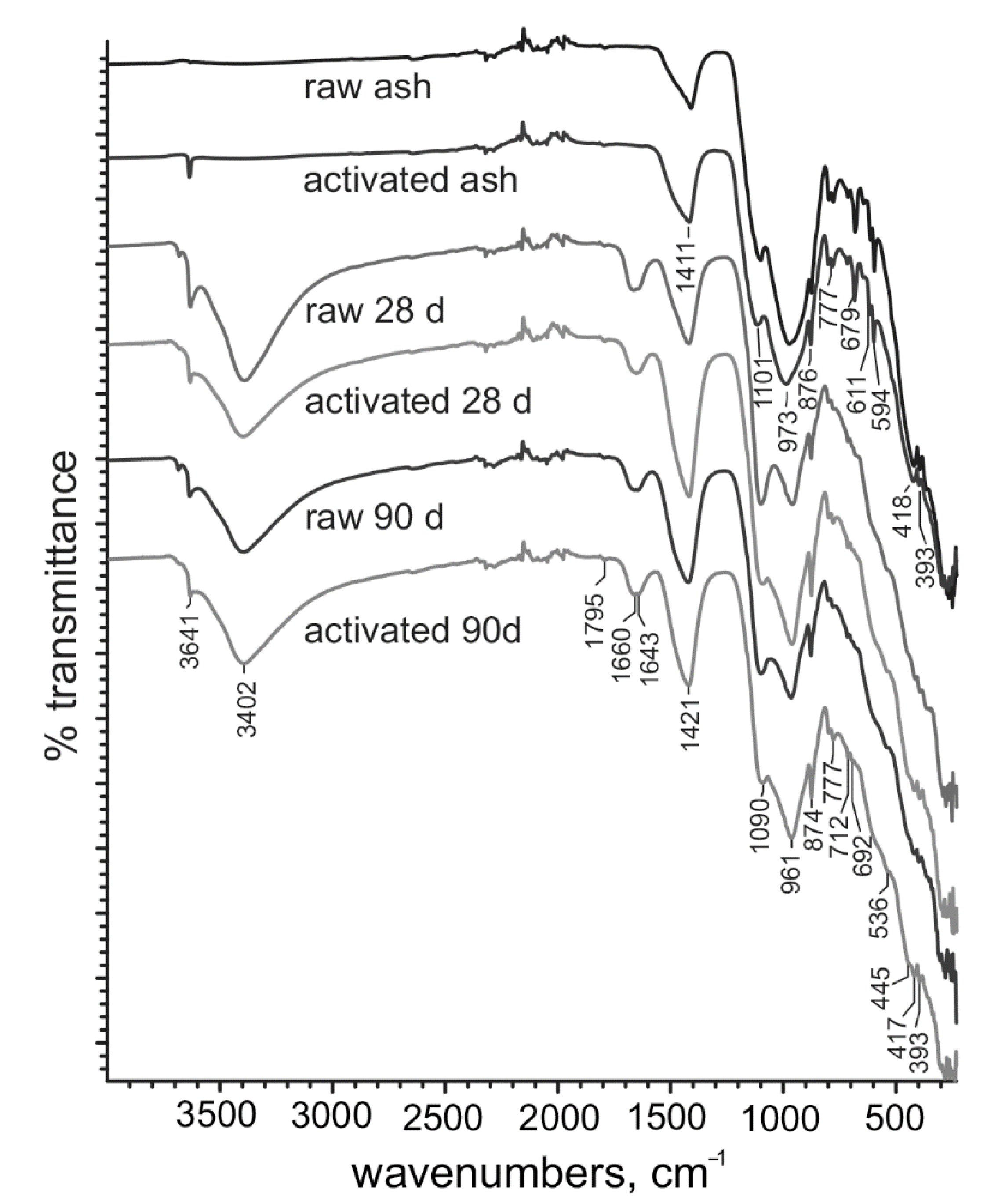
2.5. Infrared Spectroscopy Analysis
2.6. X-Ray Diffraction Analysis
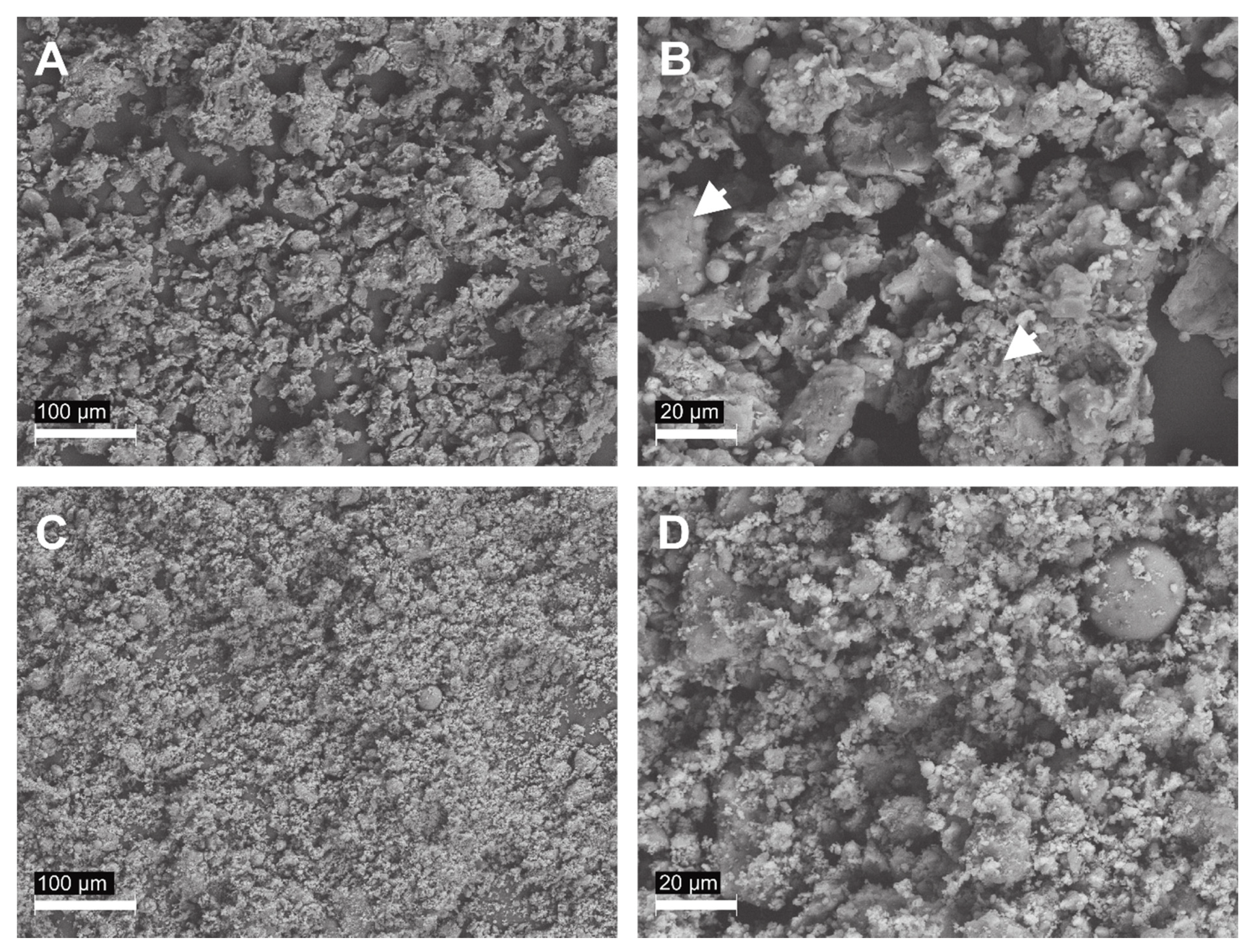
2.7. Electron Microscopy Analysis
3. Results
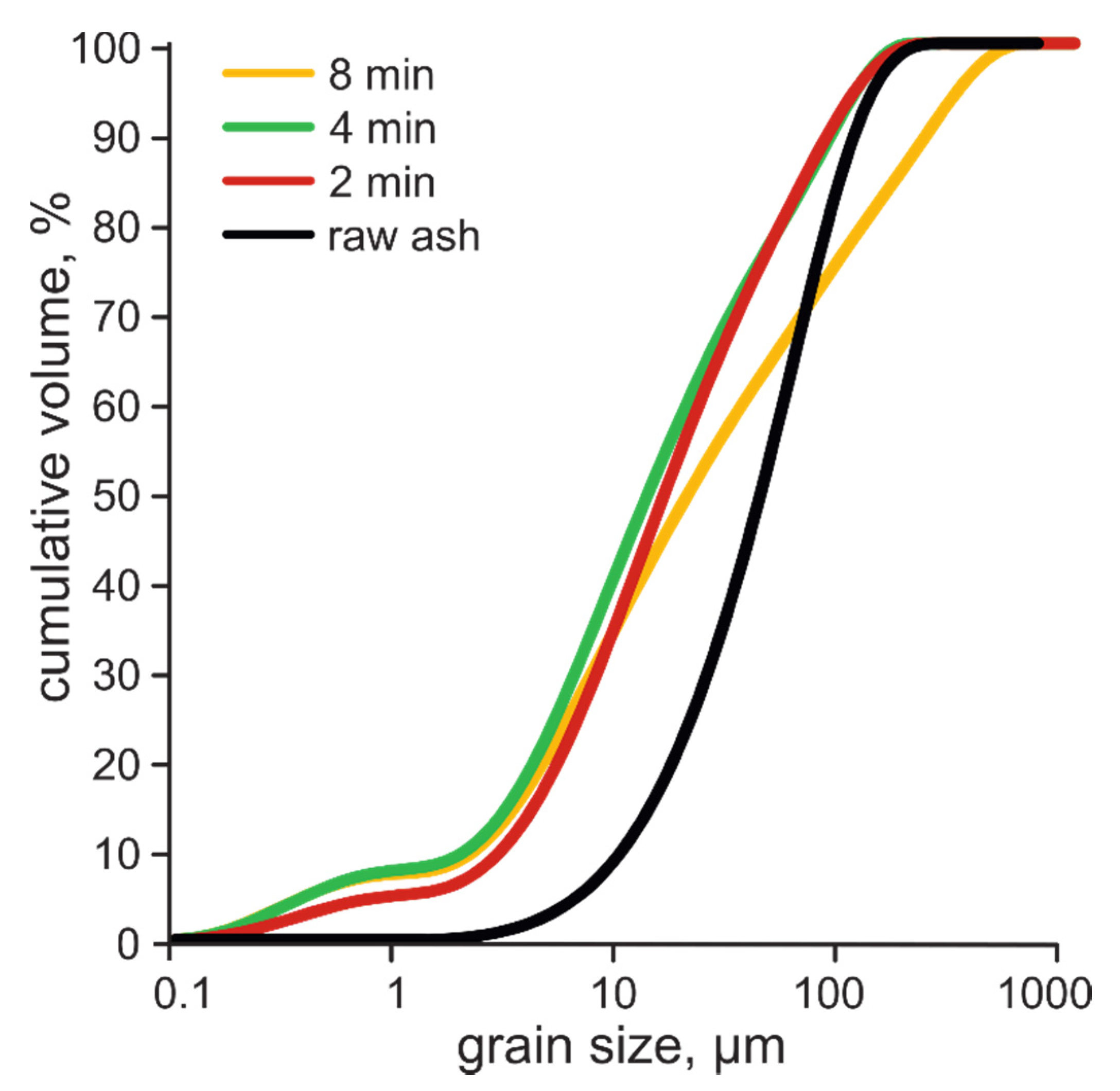
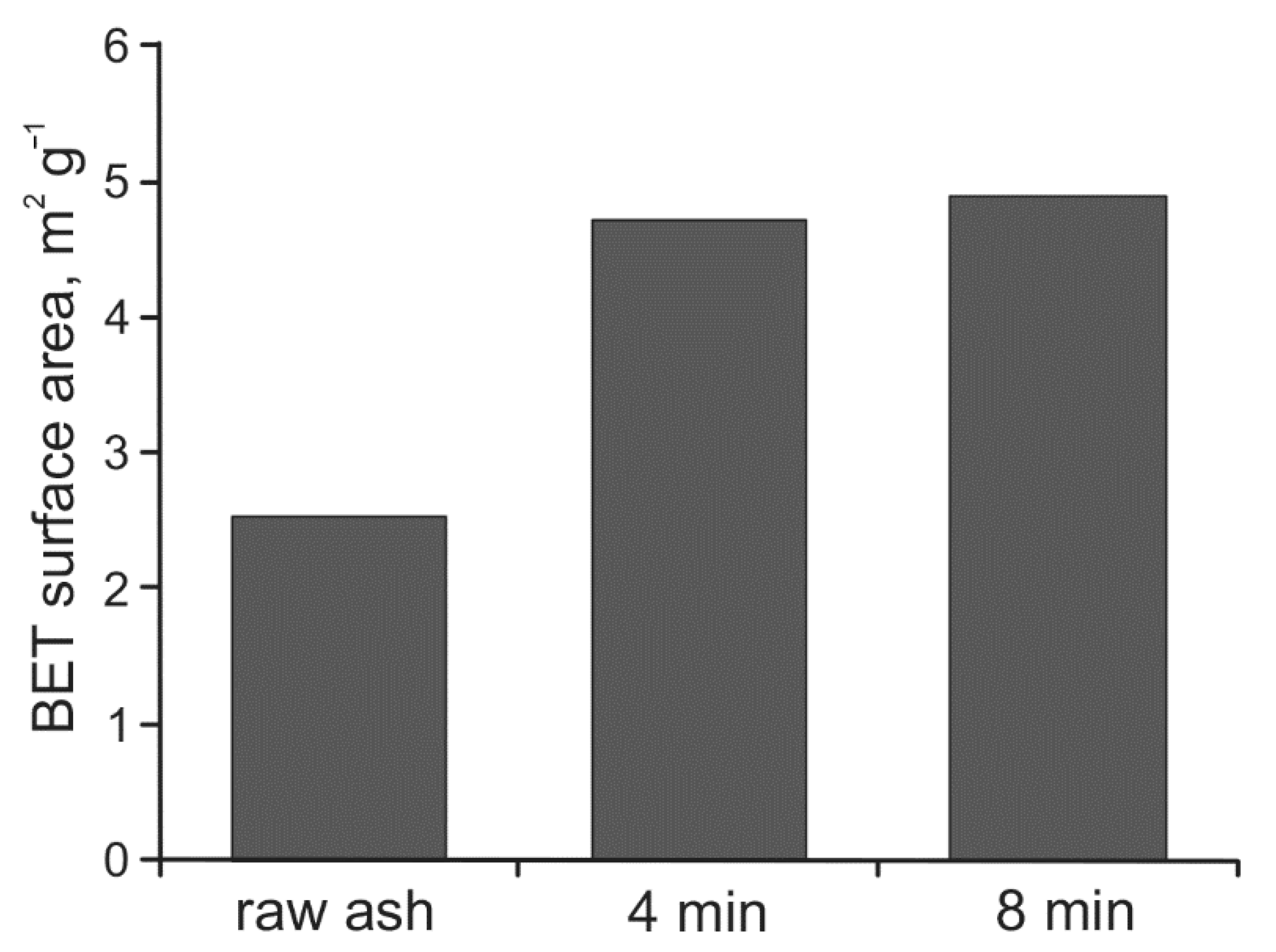
3.1. Grain Size and Specific Surface Area of Raw and Activated Ash
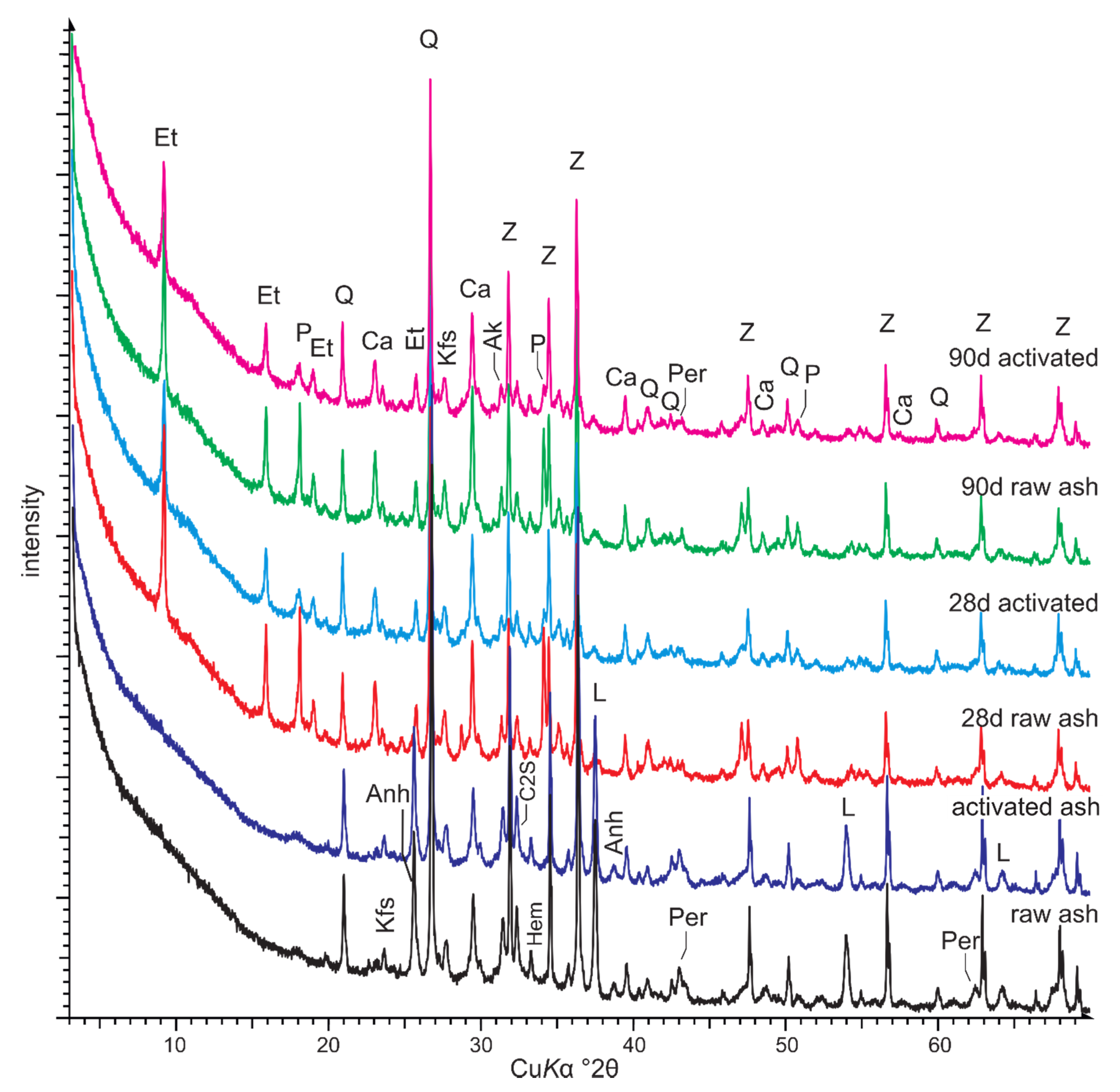
3.2. Chemical and Mineral Composition of the Raw and Activated Ash
3.3. Uniaxial Compressive Strength
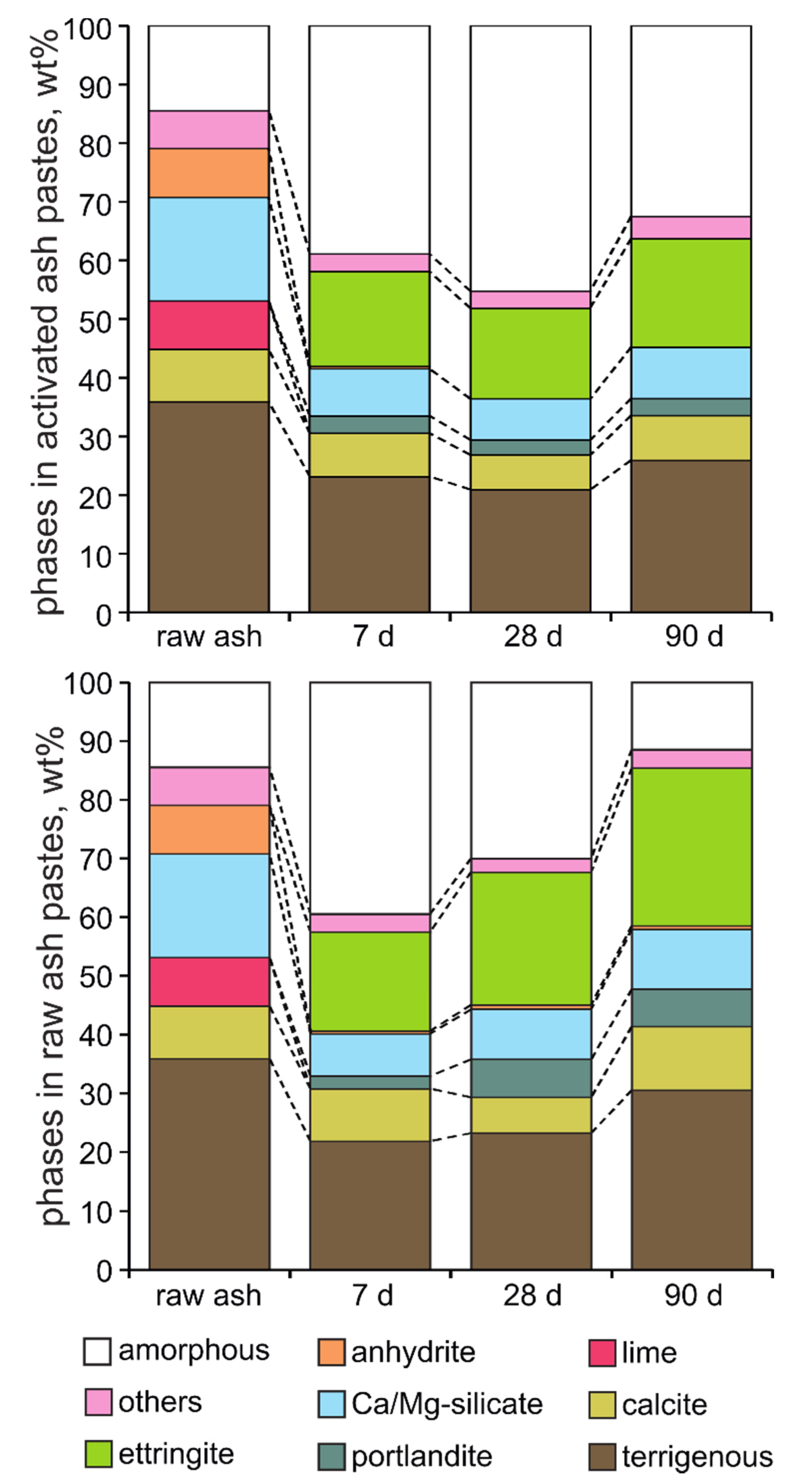
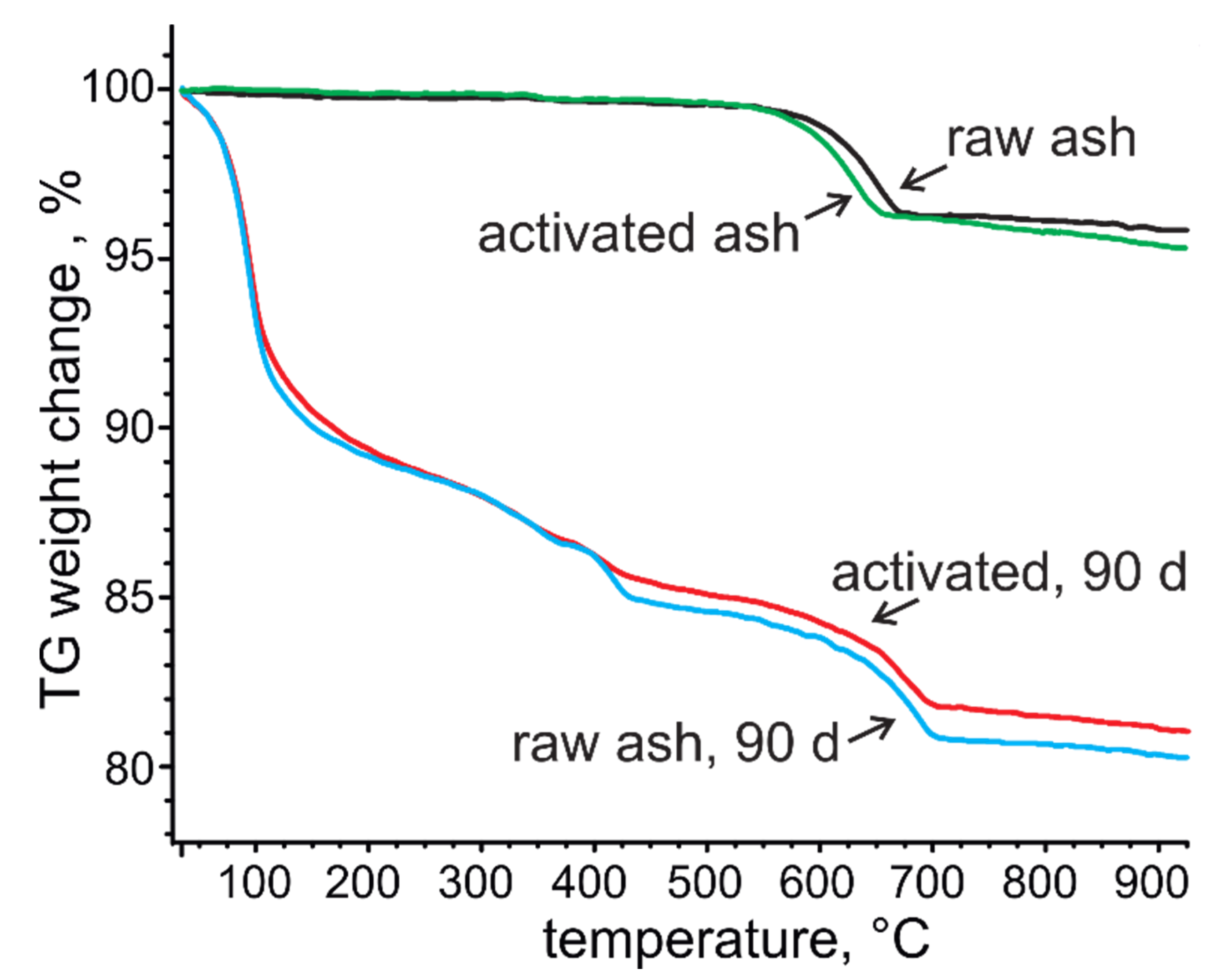
3.4. Phase Composition and Microstructure of Ash—Water Pastes
4. Discussion
5. Conclusions
- Fly-ash-based binders are considered to be viable alternatives for CO2-extensive cement clinker systems. While low calcium fly ashes rich in siliceous glass are mostly used in blends with ordinary cement or in alkali activated binders, the use of high calcium fly ashes, particularly those produced in circulating fluidized bed combustion (CFBC) boilers with high content of free CaO and CaSO4, is hindered, mainly due to their variable composition and poor cementitious properties.
- Results of our study show that even a short period of mechanical activation of the Ca-rich CFBC fly ash yields a nearly 10-fold improvement in the compressive strength of hydrated fly ash pastes, topping at 60 MPa after 90 days without any chemical activation or blending. The mechanical activation facilitated the fragmentation of large irregular flaky porous lumps as well as aggregates characteristic to CFBC ashes, improving their physical properties and reactivity (finer grain size, larger surface area, and disintegration of compact CaSO4 shells on unreacted CaO cores).
- The cementitious properties of Ca-rich CFBC ash are controlled by the formation of crystalline ettringite (Ca6Al2(OH)12(SO4)3·26H2O) and possibly a heterogeneous C-(A)-S-H gel-like phase. In mechanically activated fly ash pastes the enhancement of mechanical strength is achieved by a significantly more compact microstructure where pore space between unreacted particles is completely filled with dense webs of ettringite crystallites and a gel-like Ca-Al-Si phase.
- The phase development in activated Ca-rich CFBC fly ash is similar to that in calcium sulfoaluminate (CSA) cements where the strength development is provided by the hydration of ye’elimite in the presence of gypsum, producing ettringite and nanocrystalline Al-hydroxide. Ettringite forms in Ca- and sulfate-rich CFBC ashes by the reaction of the anhydrite/gypsum and Al-bearing phases (glass, clay minerals, feldspars), being possibly limited by the availability of reactive Al phases.
- Our findings suggest that mechanically activated Ca-rich CFBC fly ashes can be successfully used as an alternative to CSA type binders, providing mechanical strength comparable to common concrete systems. Further studies should be aimed at (1) the influences of additives and/or alkali activation to create a binder with properties exceeding those of one-component systems, and (2) testing durability to reveal the performance of ash pastes in different environments.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Edelenbosch, O.Y.; Kermeli, K.; Crijns-Graus, W.; Worrell, E.; Bibas, R.; Fais, B.; Fujimori, S.; Kyle, P.; Sano, F.; van Vuuren, D.P. Comparing projections of industrial energy demand and greenhouse gas emissions in long-term energy models. Energy 2017, 122, 701–710. [Google Scholar] [CrossRef]
- Van Damme, H. Concrete material science: Past, present, and future innovations. Cem. Concr. Res. 2018, 112, 5–24. [Google Scholar] [CrossRef]
- Flatt, R.J.; Roussel, N.; Cheeseman, C.R. Concrete: An eco material that needs to be improved. J. Eur. Ceram. Soc. 2012, 32, 2787–2798. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124. [Google Scholar] [CrossRef]
- Hemalatha, T.; Ramaswamy, A. A review on fly ash characteristics—Towards promoting high volume utilization in developing sustainable concrete. J. Clean. Prod. 2017, 147, 546–559. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Giergiczny, Z.; Garbacik, A. Properties of cements with calcareous fly ash addition. Cem. Wapno Beton 2012, 17, 217–224. [Google Scholar]
- Ohenoja, K.; Korkko, M.; Wigren, V.; Osterbacka, J.; Illikainen, M. Increasing the utilization potential of fly ashes from fluidized bed combustion by mechanical treatments. Int. J. Environ. Sci. Technol. 2019, 16, 1839–1846. [Google Scholar] [CrossRef] [Green Version]
- Gollakota, A.R.K.; Volli, V.; Shu, C.M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef] [PubMed]
- Glinicki, M.A.; Jozwiak-Niedzwiedzka, D.; Dabrowski, M. The Influence of Fluidized Bed Combustion Fly Ash on the Phase Composition and Microstructure of Cement Paste. Materials 2019, 12, 2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koornneef, J.; Junginger, M.; Faaij, A. Development of fluidized bed combustion—An overview of trends, performance and cost. Prog. Energy Combust. Sci. 2007, 33, 19–55. [Google Scholar] [CrossRef] [Green Version]
- Anthony, E.J. Fluidized-Bed Combustion of Alternative Solid Fuels—Status, Successes and Problems of the Technology. Prog. Energy Combust. Sci. 1995, 21, 239–268. [Google Scholar] [CrossRef]
- Yue, G.X.; Cai, R.X.; Lu, J.F.; Zhang, H. From a CFB reactor to a CFB boiler—The review of R&D progress of CFB coal combustion technology in China. Powder Technol. 2017, 316, 18–28. [Google Scholar] [CrossRef]
- Zahedi, M.; Rajabipour, F. Fluidized Bed Combustion (FBC) Fly Ash and Its Performance in Concrete. Aci Mater. J. 2019, 116, 163–172. [Google Scholar] [CrossRef]
- Leben, K.; Motlep, R.; Paaver, P.; Konist, A.; Pihu, T.; Paiste, P.; Heinmaa, I.; Nurk, G.; Anthony, E.J.; Kirsimae, K. Long-term mineral transformation of Ca-rich oil shale ash waste. Sci. Total Environ. 2019, 658, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.M.; Gao, J.M.; Yan, Y.; Liu, Y.Z. Investigation of expansion properties of cement paste with circulating fluidized bed fly ash. Constr. Build. Mater. 2017, 157, 1154–1162. [Google Scholar] [CrossRef]
- Siler, P.; Bayer, P.; Sehnal, T.; Kolarova, I.; Opravil, T.; Soukal, F. Effects of high-temperature fly ash and fluidized bed combustion ash on the hydration of Portland cement. Constr. Build. Mater. 2015, 78, 181–188. [Google Scholar] [CrossRef]
- Golek, L. Glass powder and high-calcium fly ash based binders—Long term examinations. J. Clean. Prod. 2019, 220, 493–506. [Google Scholar] [CrossRef]
- Hlavacek, P.; Sulc, R.; Smilauer, V.; Rossler, C.; Snop, R. Ternary binder made of CFBC fly ash, conventional fly ash, and calcium hydroxide: Phase and strength evolution. Cem. Concr. Compos. 2018, 90, 100–107. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Robl, T. 8—Ash beneficiation, quality, and standard criteria. In Coal Combustion Products (CCP’s); Robl, T., Oberlink, A., Jones, R., Eds.; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Hela, R.; Orsakova, D. The Mechanical Activation of Fly Ash. Procedia Eng. 2013, 65, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Jimenez, A.; Garcia-Lodeiro, I.; Maltseva, O.; Palomo, A. Mechanical-Chemical Activation of Coal Fly Ashes: An Effective Way for Recycling and Make Cementitious Materials. Front. Mater. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Pihu, T.; Arro, H.; Prikk, A.; Rootamm, R.; Konist, A.; Kirsimae, K.; Liira, M.; Motlep, R. Oil shale CFBC ash cementation properties in ash fields. Fuel 2012, 93, 172–180. [Google Scholar] [CrossRef]
- Uibu, M.; Somelar, P.; Raado, L.M.; Irha, N.; Hain, T.; Koroljova, A.; Kuusik, R. Oil shale ash based backfilling concrete—Strength development, mineral transformations and leachability. Constr. Build. Mater. 2016, 102, 620–630. [Google Scholar] [CrossRef]
- Raado, L.M.; Hain, T.; Liisma, E.; Kuusik, R. Composition and Properties of Oil Shale Ash Concrete. Oil Shale 2014, 31, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Paaver, P.; Paiste, P.; Kirsimae, K. Geopolymeric Potential of the Estonian Oil Shale Solid Residues: Petroter Solid Heat Carrier Retorting Ash. Oil Shale 2016, 33, 373–392. [Google Scholar] [CrossRef] [Green Version]
- Paaver, P.; Paiste, P.; Motlep, R.; Kirsimae, K. Self-Cementing Properties and Alkali Activation of Enefit280 Solid Heat Carrier Retorting Ash. Oil Shale 2017, 34, 263–278. [Google Scholar] [CrossRef]
- Paiste, P.; Liira, M.; Heinmaa, I.; Vahur, S.; Kirsimae, K. Alkali activated construction materials: Assessing the alternative use for oil shale processing solid wastes. Constr. Build. Mater. 2016, 122, 458–464. [Google Scholar] [CrossRef]
- Paiste, P.; Külaviir, M.; Paaver, P.; Heinmaa, I.; Vahur, S.; Kirsimäe, K. Beneficiation of Oil Shale Processing Waste: Secondary Binder Phases in Alkali Activated Composites. Waste Biomass Valorization 2019, 10, 1407–1417. [Google Scholar] [CrossRef]
- Paaver, P.; Paiste, P.; Liira, M.; Kirsimae, K. Alkali Activation of Estonian Ca-Rich Oil Shale Ashes: A Synthesis. Oil Shale 2019, 36, 214–225. [Google Scholar] [CrossRef]
- Tole, I.; Habermehl-Cwirzen, K.; Cwirzen, A. Mechanochemical activation of natural clay minerals: An alternative to produce sustainable cementitious binders—Review. Mineral. Petrol. 2019, 113, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Bityukova, L.; Mõtlep, R.; Kirsimäe, K. Composition of Oil Shale Ashes from Pulverized Firing and Circulating Fluidized-Bed Boiler in Narva Thermal Power Plants, Estonia. Oil Shale 2010, 27, 339–353. [Google Scholar] [CrossRef] [Green Version]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Infrared Spectroscopy in the Analysis of Building and Construction Materials. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: Durham, AL, USA, 2012. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X.D. Structure of calcium silicate hydrate (C-S-H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. In situ ATR-FTIR study of the early stages of fly ash geopolymer gel formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S.J. One-Part Geopolymer Mixes from Geothermal Silica and Sodium Aluminate. Ind. Eng. Chem. Res. 2008, 47, 9396–9405. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Ltd.: London, UK, 1997; p. 480. [Google Scholar]
- Scrivener, K.L.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2018; p. 540. [Google Scholar]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, G. Dehydration kinetics of Portland cement paste at high temperature. J. Therm. Anal. Calorim. 2012, 110, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Mucsi, G.; Kristaly, F.; Pekker, P. Mechanical activation of fly ash and its influence on micro and nano-structural behaviour of resulting geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
- Marjanovic, N.; Komljenovic, M.; Bascarevic, Z.; Nikolic, V. Improving reactivity of fly ash and properties of ensuing geopolymers through mechanical activation. Constr Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Sadique, M.; Al-Nageim, H.; Atherton, W.; Seton, L.; Dempster, N. Mechano-chemical activation of high-Ca fly ash by cement free blending and gypsum aided grinding. Constr. Build. Mater. 2013, 43, 480–489. [Google Scholar] [CrossRef]
- Anthony, E.J.; Granatstein, D.L. Sulfation phenomena in fluidized bed combustion systems. Prog. Energy Combust. Sci. 2001, 27, 215–236. [Google Scholar] [CrossRef]
- Wu, Y.H.; Anthony, E.J. Investigation of sulphation behavior of two fly ash samples produced from combustion of different fuels in a 165 MWe CFB boiler. Powder Technol. 2011, 208, 237–241. [Google Scholar] [CrossRef]
- Ben Haha, M.; Winnefeld, F.; Pisch, A. Advances in understanding ye’elimite-rich cements. Cem. Concr. Res. 2019, 123. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Winnefeld, F.; Tschopp, E.; Muller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Bernardo, G.; Telesca, A.; Valenti, G.L.; Montagnaro, F. Role of ettringite in the reuse of hydrated fly ash from fluidized-bed combustion as a sulfur sorbent: A hydration study. Ind. Eng. Chem. Res. 2004, 43, 4054–4059. [Google Scholar] [CrossRef]
- Liira, M.; Kirsimae, K.; Kuusik, R.; Motlep, R. Transformation of calcareous oil-shale circulating fluidized-bed combustion boiler ashes under wet conditions. Fuel 2009, 88, 712–718. [Google Scholar] [CrossRef]
- Min, D.; Mingshu, T. Formation and Expansion of Ettringite Crystals. Cem. Concr. Res. 1994, 24, 119–126. [Google Scholar] [CrossRef]



| SiO2 | Al2O3 | TiO2 | Fe2O3 | MnO | CaO | MgO | Na2O | K2O | P2O5 | SO3 | L.O.I. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | 35.19 | 8.47 | 0.49 | 3.98 | 0.06 | 29.81 | 5.07 | 0.11 | 4.25 | 0.15 | 6.74 | 4.71 |
| Activated | 35.12 | 8.49 | 0.44 | 3.93 | 0.06 | 29.87 | 5.04 | 0.10 | 4.25 | 0.14 | 6.77 | 4.94 |
| Activated Ash | Raw Ash | |||||
|---|---|---|---|---|---|---|
| M-0.3 | M-0.35 | M-0.4 | R-0.4 | R-0.45 | R-0.5 | |
| 7 days | 11.4 | 29.2 | 17.9 | 1.8 | 2.1 | 1.7 |
| 28 days | 20.5 | 54.2 | 44.4 | 4.8 | 4.9 | 4.6 |
| 90 days | 17.5 | 62.2 | 48.0 | 7.1 | 8.4 | 9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paaver, P.; Paiste, P.; Liira, M.; Kirsimäe, K. Mechanical Activation of the Ca-Rich Circulating Fluidized Bed Combustion Fly Ash: Development of an Alternative Binder System. Minerals 2021, 11, 3. https://doi.org/10.3390/min11010003
Paaver P, Paiste P, Liira M, Kirsimäe K. Mechanical Activation of the Ca-Rich Circulating Fluidized Bed Combustion Fly Ash: Development of an Alternative Binder System. Minerals. 2021; 11(1):3. https://doi.org/10.3390/min11010003
Chicago/Turabian StylePaaver, Peeter, Päärn Paiste, Martin Liira, and Kalle Kirsimäe. 2021. "Mechanical Activation of the Ca-Rich Circulating Fluidized Bed Combustion Fly Ash: Development of an Alternative Binder System" Minerals 11, no. 1: 3. https://doi.org/10.3390/min11010003




