Morphology, Mineralogy, and Chemistry of Ocherous Precipitate Aggregates Downstream of an Abandoned Mine Site
Abstract
:1. Introduction
2. Materials and Methods
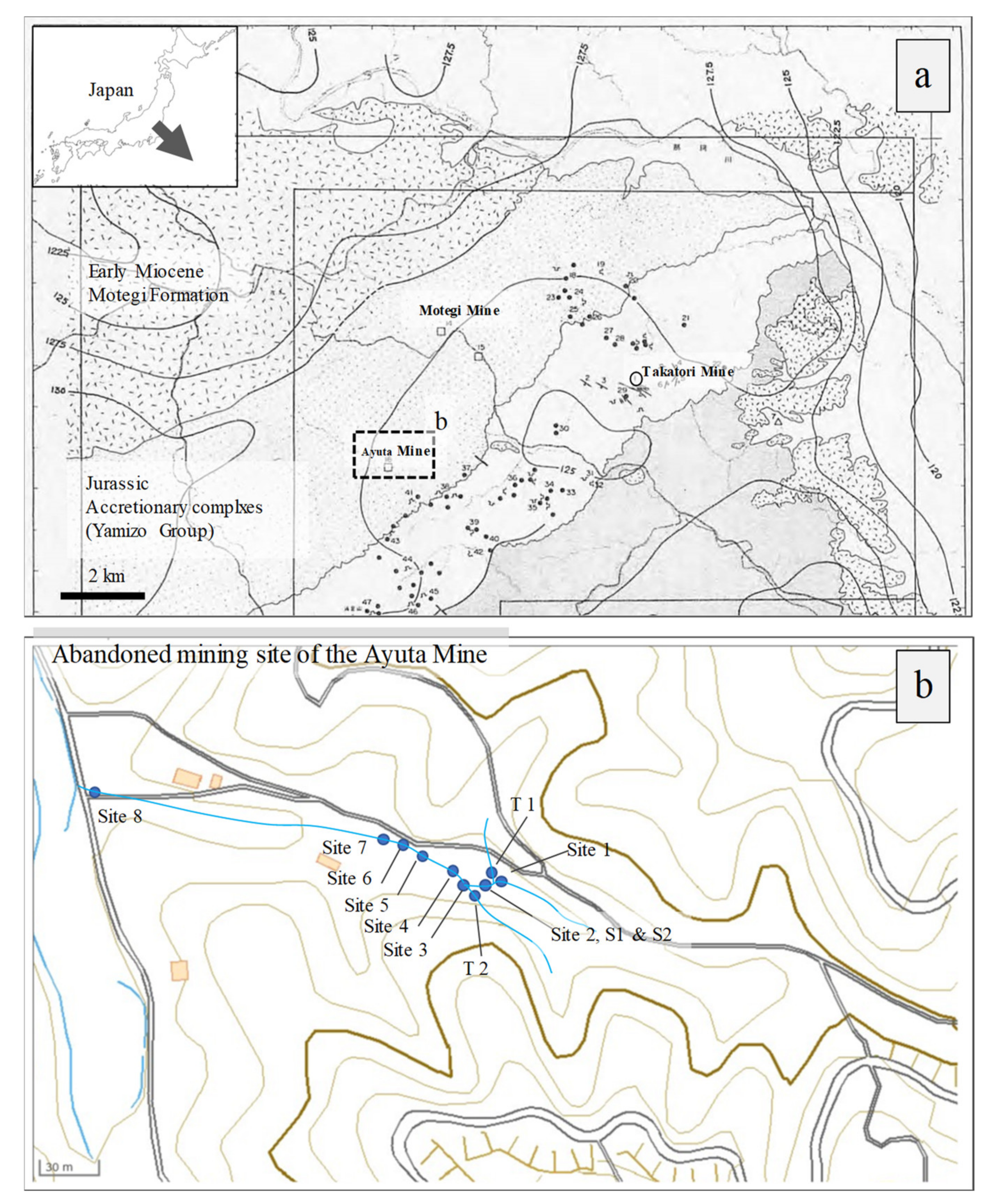
2.1. Site Description
2.2. Analysis of Stream Water
2.3. Analysis of Solid Phases
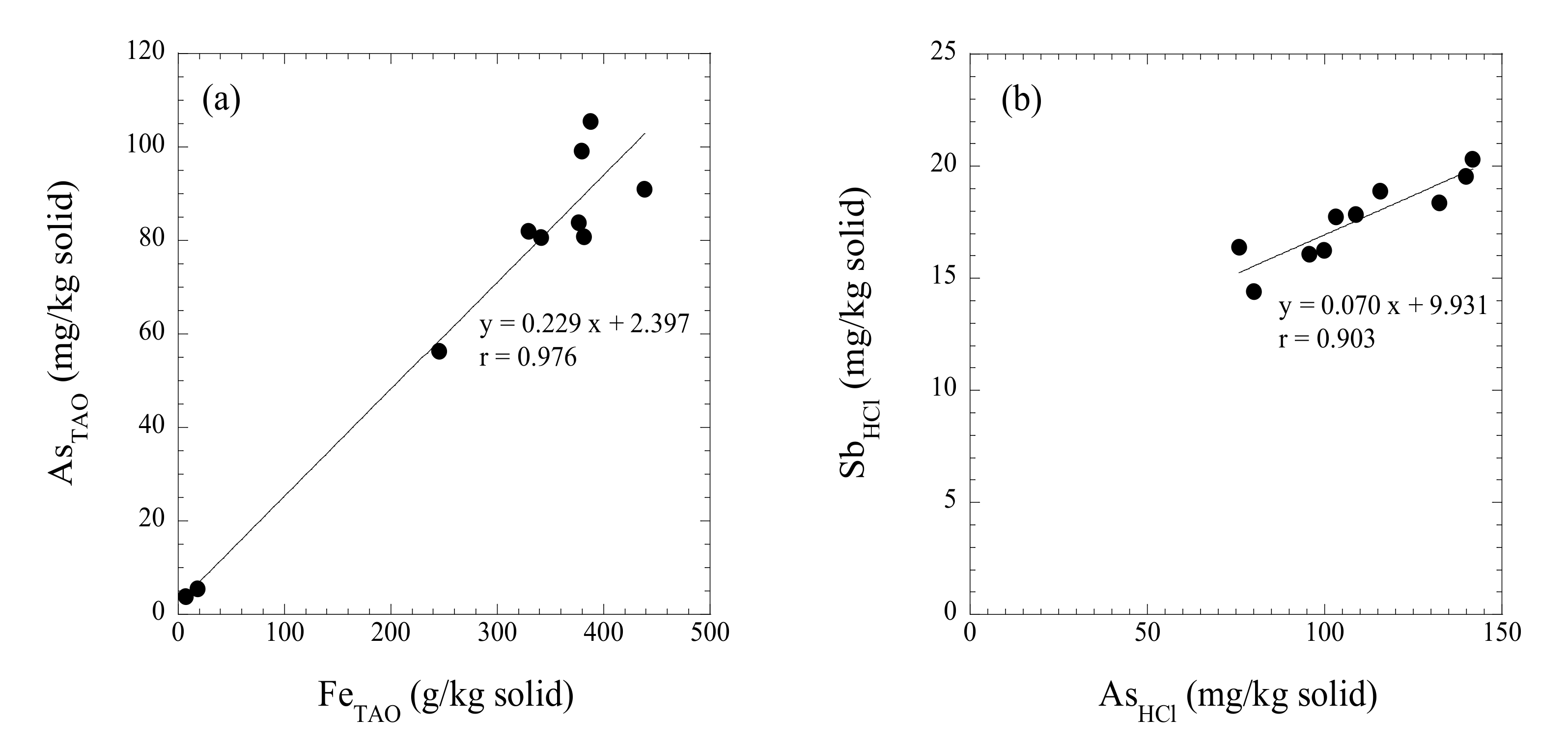
3. Results
3.1. Morphology of Ocherous Aggregate
3.2. Mineralogy of Ocherous Aggregate
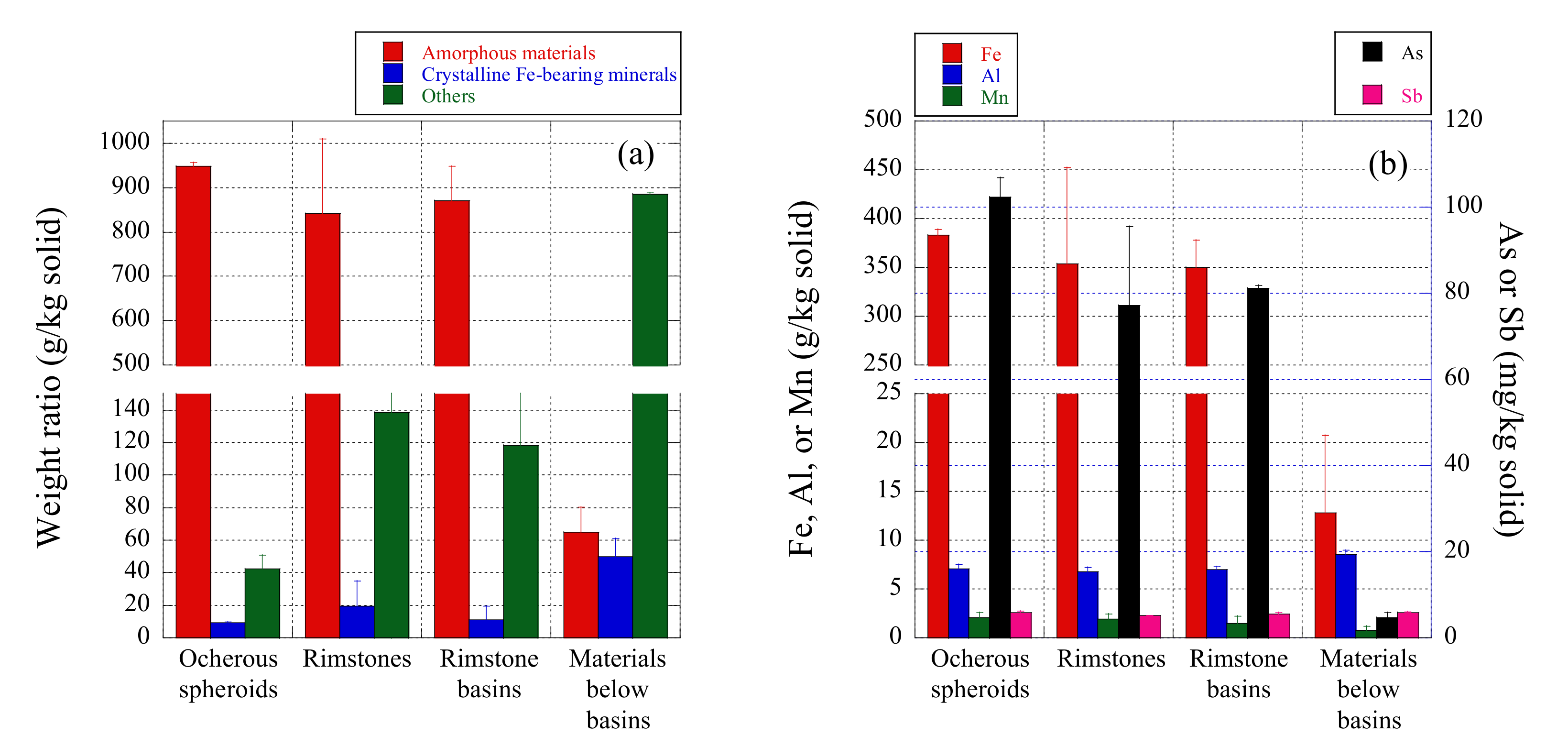
3.3. Chemistry of Ocherous Aggregate
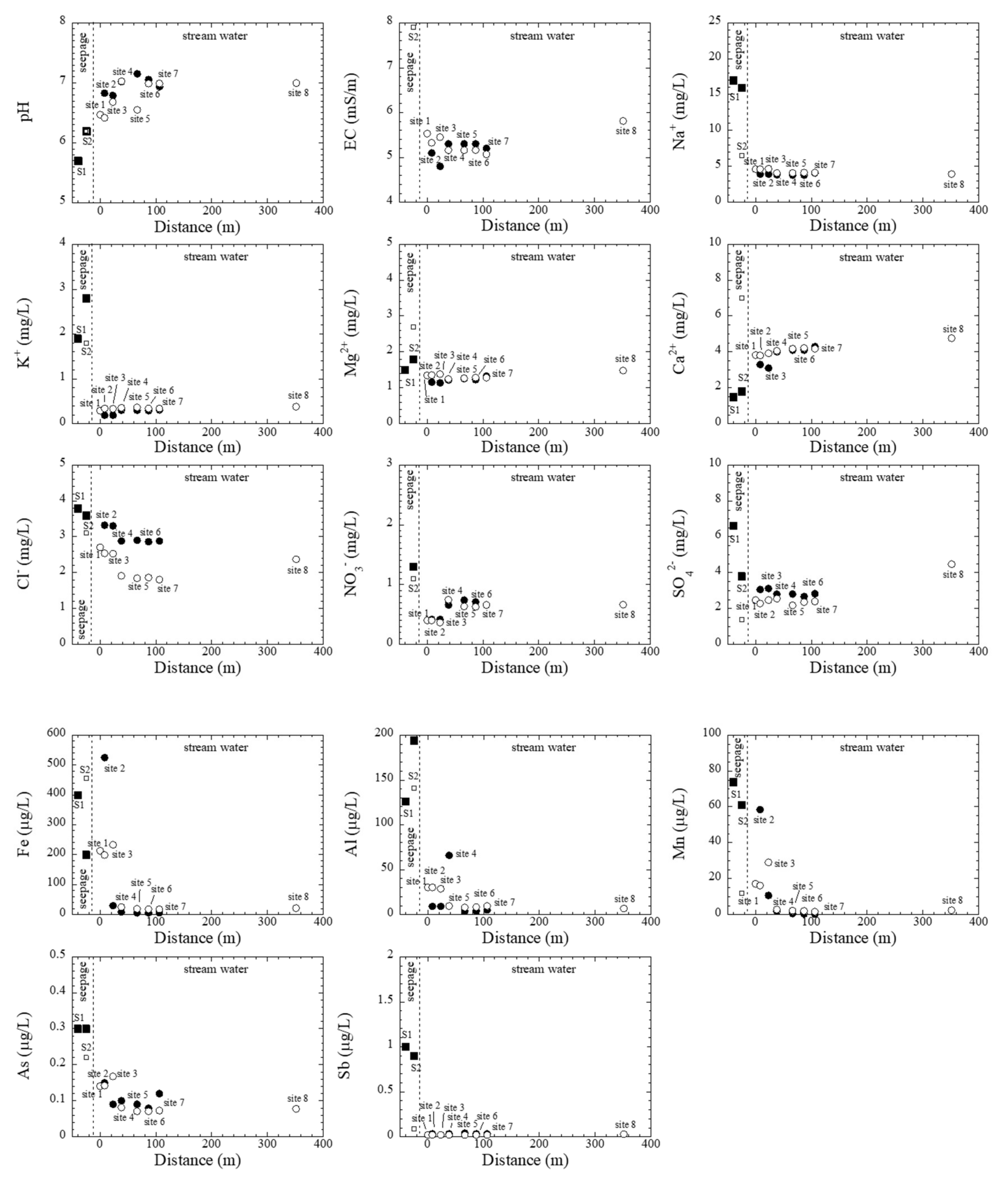
3.4. Chemistry of Stream Water
4. Discussion
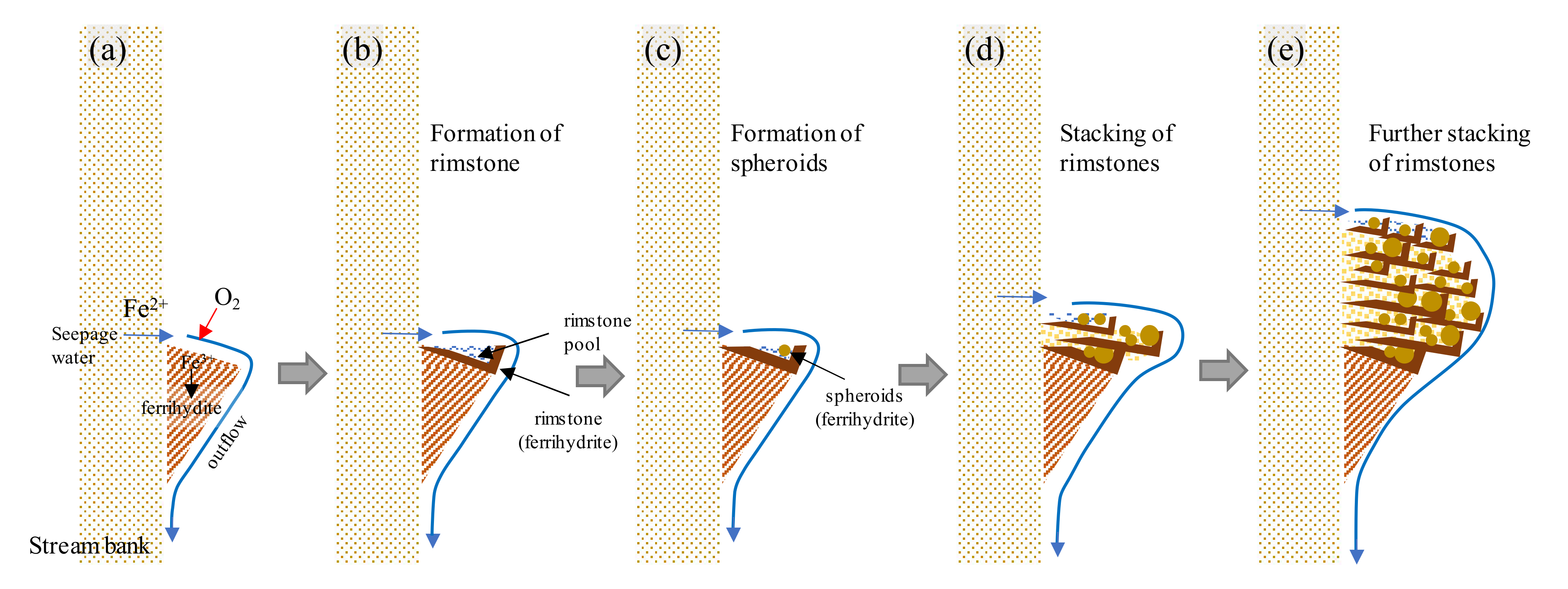
4.1. Formation of Ocherous Aggregate
4.2. As and Sb Uptake by Ocherous Aggregate
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarty, D.K.; Moore, J.N.; Marcus, W.A. Mineralogy and trace element association in an acid mine drainage iron oxide precipitate; comparison of selective extractions. Appl. Geochem. 1998, 13, 165–176. [Google Scholar] [CrossRef]
- Filella, M.; Philippo, S.; Belzile, N.; Chen, Y.; Quentel, F. Natural attenuation processes applying to antimony: A study in the abandoned antimony mine in Goesdorf, Luxembourg. Sci. Total Environ. 2009, 407, 6205–6216. [Google Scholar] [CrossRef] [PubMed]
- Baleeiro, A.; Fiol, S.; Otero-Fariña, A.; Antelo, J. Surface chemistry of iron oxides formed by neutralization of acidic mine waters: Removal of trace metals. Appl. Geochem. 2018, 89, 129–137. [Google Scholar] [CrossRef]
- Johnston, S.G.; Karimian, N.; Burton, E.D. Seasonal temperature oscillations drive contrasting arsenic and antimony mobilization in a mining-impacted river system. Water Resour. Res. 2020, 56, e2020WR028196. [Google Scholar] [CrossRef]
- Webster, J.G.; Swedlund, P.J.; Webster, K.S. Trace Metal Adsorption onto an Acid Mine Drainage Iron (III) Oxy Hydroxy Sulfate. Environ. Sci. Technol. 1998, 32, 1361–1368. [Google Scholar] [CrossRef]
- Fukushi, K.; Sasaki, M.; Sato, T.; Yanase, N.; Amano, H.; Ikeda, H. A natural attenuation of arsenic in drainage from an abandoned arsenic mine dump. Appl. Geochem. 2003, 18, 1267–1278. [Google Scholar] [CrossRef]
- Manaka, M.; Yanase, N.; Sato, T.; Fukushi, K. Natural attenuation of antimony in mine drainage water. Geochem. J. 2007, 41, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Ratié, G.; Vantelon, D.; Kalahroodi, E.L.; Bihannic, I.; Pierson-Wickmann, A.C.; Davranche, M. Iron speciation at the riverbank surface in wetland and potential impact on the mobility of trace metals. Sci. Total Environ. 2019, 651, 443–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elghali, A.; Benzaazoua, M.; Bussière, B.; Kennedy, C.; Parwani, R.; Graham, S. The role of hardpan formation on the reactivity of sulfidic mine tailings: A case study at Joutel mine (Québec). Sci. Total Environ. 2019, 654, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Metal Mining Agency of Japan. Geologic Map of Kasama District, 1:25,000; Metal Mining Agency of Japan: Tokyo, Japan, 1987. (In Japanese) [Google Scholar]
- Yoshioka, T.; Takizawa, F.; Takahashi, M.; Miyazaki, K.; Banno, Y.; Yanagisawa, Y.; Takahashi, Y.; Kubo, K.; Seki, Y.; Komazawa, M.; et al. Geological Map of Japan 1:200,000, Mito, Quadrangle Series 1:200,000, Mito NJ-54-24; Geological Survey of Japan: Tsukuba, Japan, 2001; (In Japanese with English abstract). [Google Scholar]
- Dong, G.; Morrison, G.; Jaireth, S. Quartz textures in epithermal veins, Queensland; classification, origin and implication. Econ. Geol. 1995, 90, 1841–1856. [Google Scholar] [CrossRef]
- Sunagawa, I. Crystals: Growth, Morphology, and Perfection; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Ishihara, S. On the main antimony ore deposits in Japan and their genetic problems. Shigen Chishitsu 2012, 62, 151–161, (In Japanese with English abstract). [Google Scholar]
- Moore, D.M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory; VCH Publishers: Weinheim, Germany, 1991. [Google Scholar]
- Yanase, N.; Sato, T.; Isobe, H.; Sekine, K. Selective extraction procedure and its importance in a natural analogue study—Case study at the Koongarra uranium deposit. Radioact. Waste Res. 1996, 2, 121–135, (In Japanese with English abstract). [Google Scholar]
- Tamm, O. Über die Oxalatemethode in der chemischen Bodenanalyse. Medd. Statens Skogsförsöksanstalt. 1932, 27, 1–20. [Google Scholar]
- Kodama, H.; Kotlyar, L.S.; Ripmeester, J.A. Quantification of crystalline and non-crystalline material in ground kaolinite by X-ray powder diffraction, infrared solid-state nuclear magnetic resonance and chemical dissolution analysis. Clays Clay Miner. 1989, 4, 364–370. [Google Scholar] [CrossRef]
- Dreybrodt, W.; Gabrovšek, F. Small-scale terraces and isolated rimstone pools on stalagmites in caves exhibit striking similarity to large-scale terrace landscapes at hot springs. Acta Carsol. 2009, 38, 19–26. [Google Scholar] [CrossRef]
- Jones, B. Cave Pearls—The Integrated Product of Abiogenic and Biogenic Processes. J. Sediment. Res. 2009, 79, 689–710. [Google Scholar] [CrossRef]
- Barthelmy, D. Mineralogy Database. 2014. Available online: http://www.webmineral.com (accessed on 14 May 2020).
- Kerr, G.; Pope, J.; Trumm, D.; Craw, D. Experimental metalloid mobilisation from a New Zealand orogenic gold deposit. Mine Water Environ. 2015, 34, 404–416. [Google Scholar] [CrossRef]
- Pierce, M.L.; Moore, C.B. Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Res. 1982, 16, 1247–1253. [Google Scholar] [CrossRef]
- Swedlund, P.J.; Webster, J.G. Arsenic removal from geothermal bore waters: The effect of monosilicic acid. In Water-Rock Interaction, Proceedings of the 9th International Symposium on Water-Rock Interaction, WRI-9, Taupo, New Zealand, 30 March–3 April 1998; Arehart, G.B., Hulston, J.R., Eds.; Balkema: Rotterdam, The Netherlands, 1998; pp. 947–950. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manaka, M. Morphology, Mineralogy, and Chemistry of Ocherous Precipitate Aggregates Downstream of an Abandoned Mine Site. Minerals 2021, 11, 32. https://doi.org/10.3390/min11010032
Manaka M. Morphology, Mineralogy, and Chemistry of Ocherous Precipitate Aggregates Downstream of an Abandoned Mine Site. Minerals. 2021; 11(1):32. https://doi.org/10.3390/min11010032
Chicago/Turabian StyleManaka, Mitsuo. 2021. "Morphology, Mineralogy, and Chemistry of Ocherous Precipitate Aggregates Downstream of an Abandoned Mine Site" Minerals 11, no. 1: 32. https://doi.org/10.3390/min11010032
APA StyleManaka, M. (2021). Morphology, Mineralogy, and Chemistry of Ocherous Precipitate Aggregates Downstream of an Abandoned Mine Site. Minerals, 11(1), 32. https://doi.org/10.3390/min11010032




