Effect of Dodecane-Oleic Acid Collector Mixture on the Evolution of Wetting Film between Air Bubble and Low-Rank Coal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
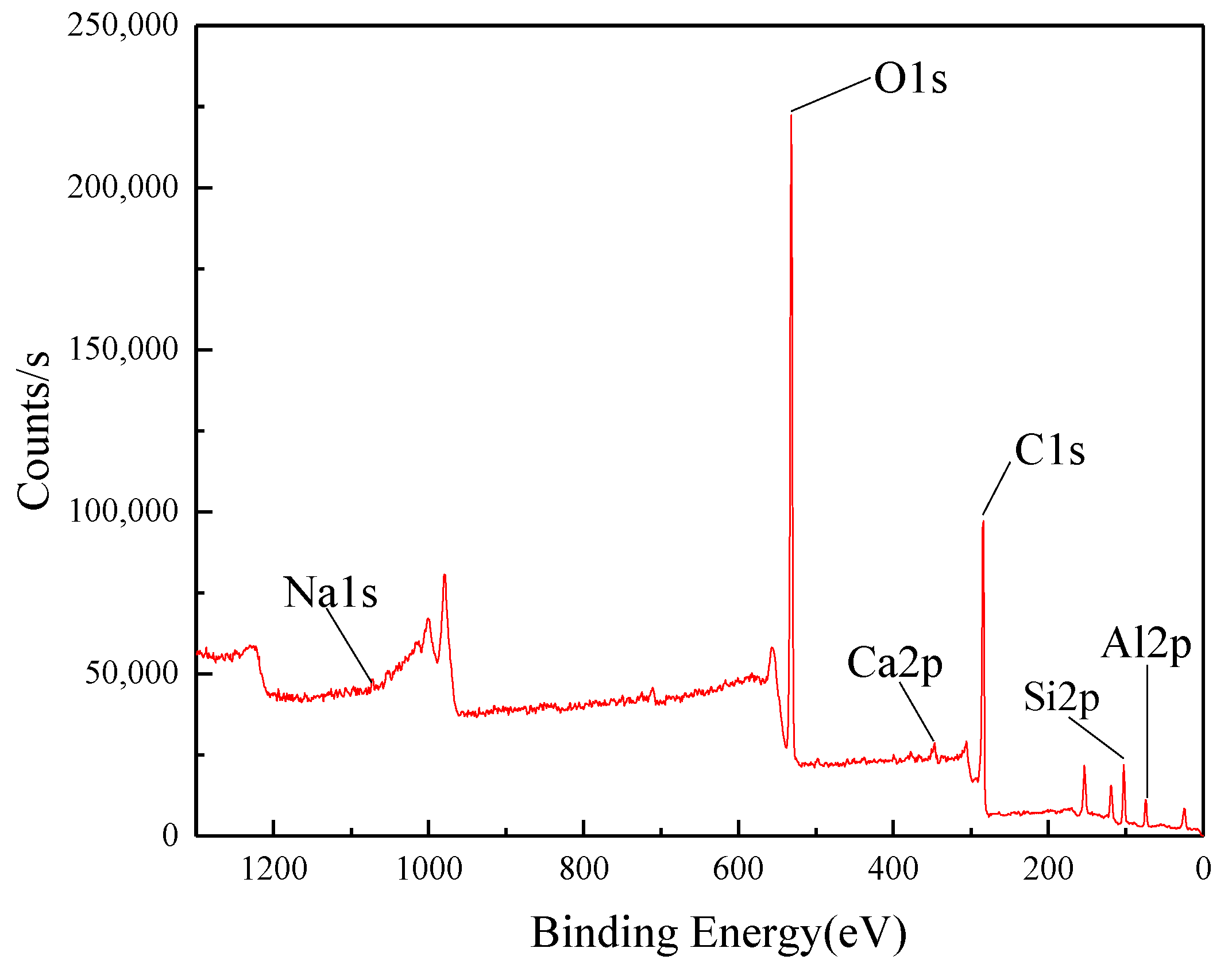
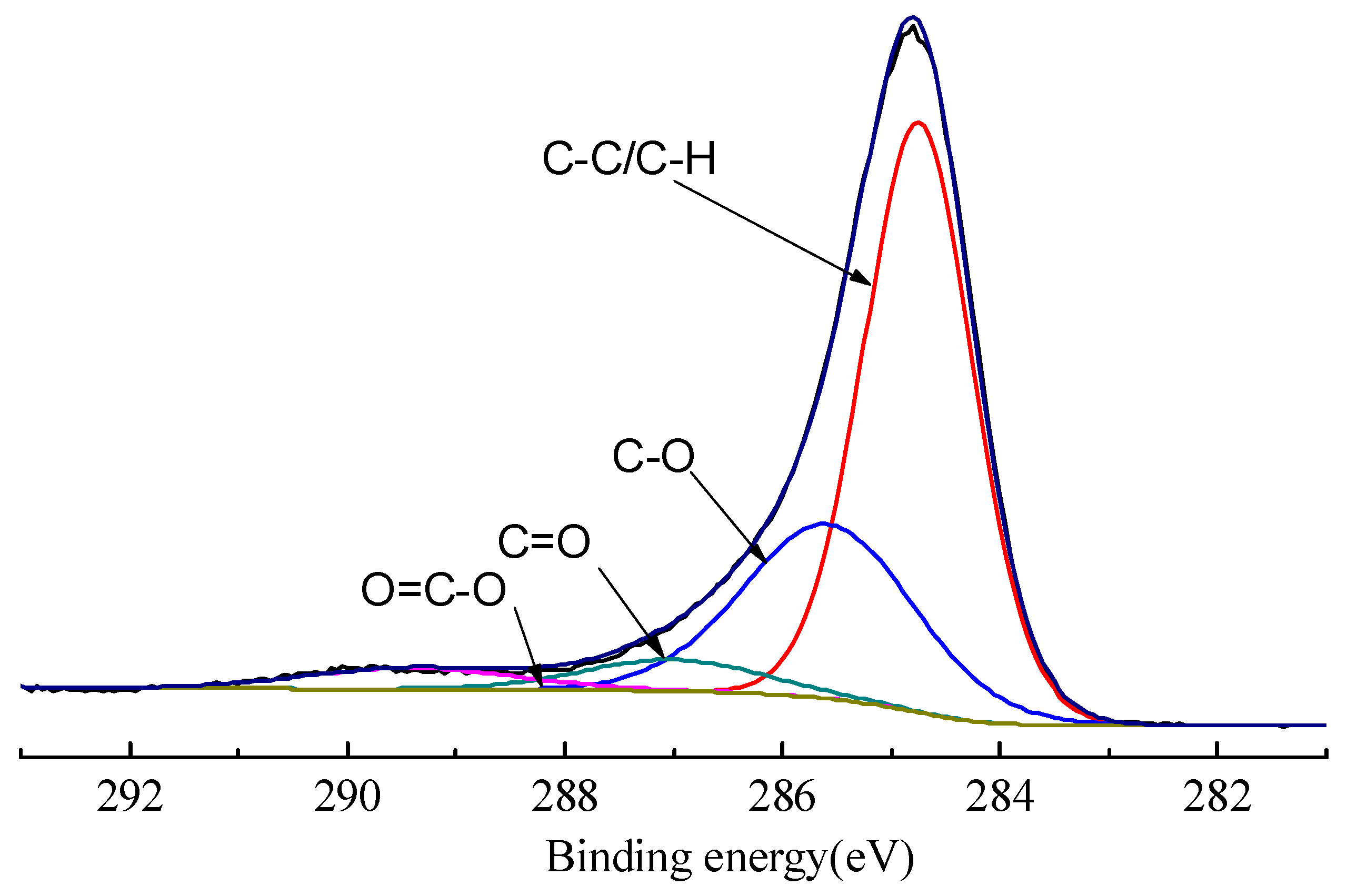
2.2. XPS Measurement
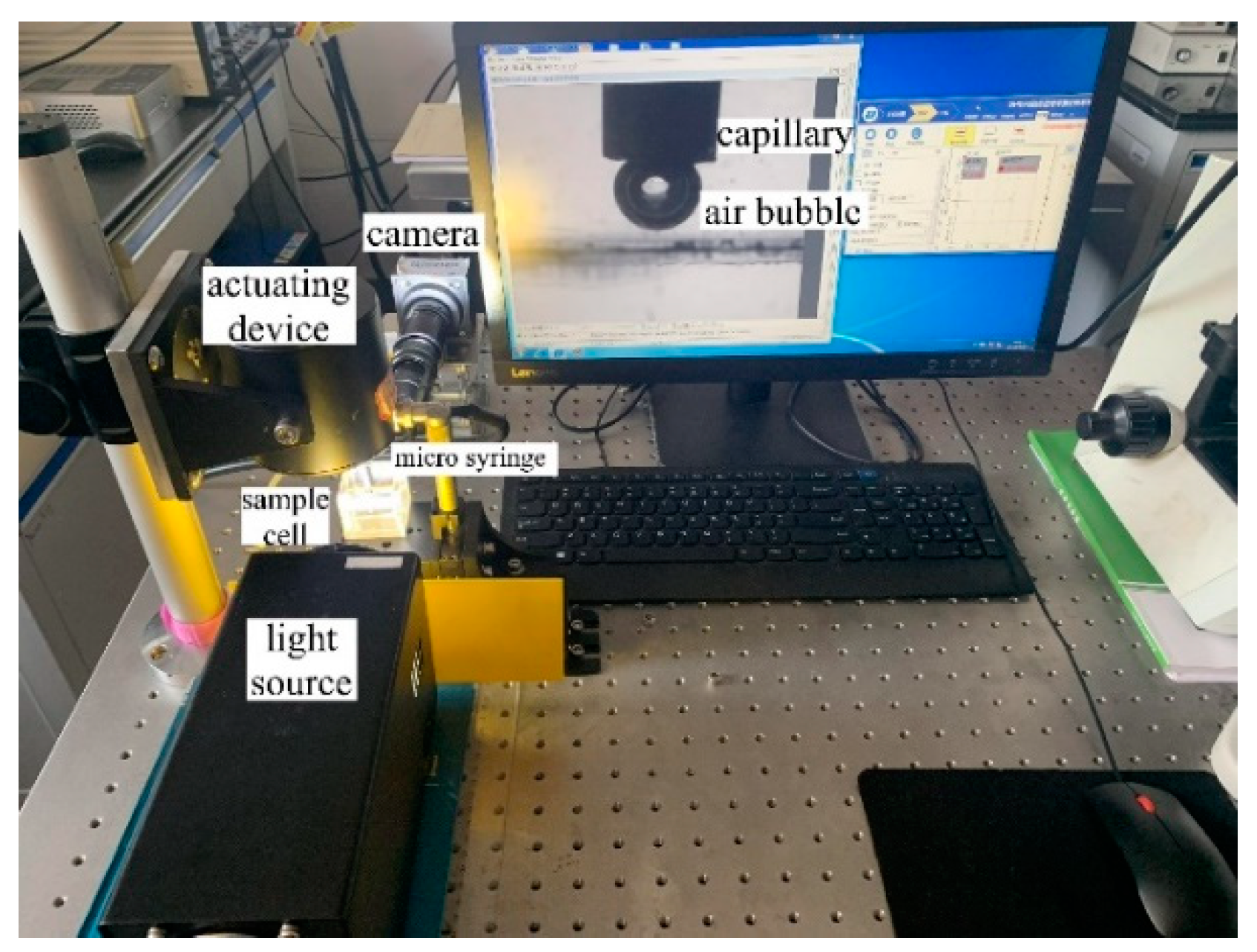
2.3. Induction Time Measurement
2.4. Zeta Potential Measurement
2.5. The Interaction Energy Calculation
2.6. The Wetting Film Drainage Process Calculation
3. Results and Discussion
3.1. XPS Measurement Results
3.2. Induction Time Results
3.3. Zeta Potential Results
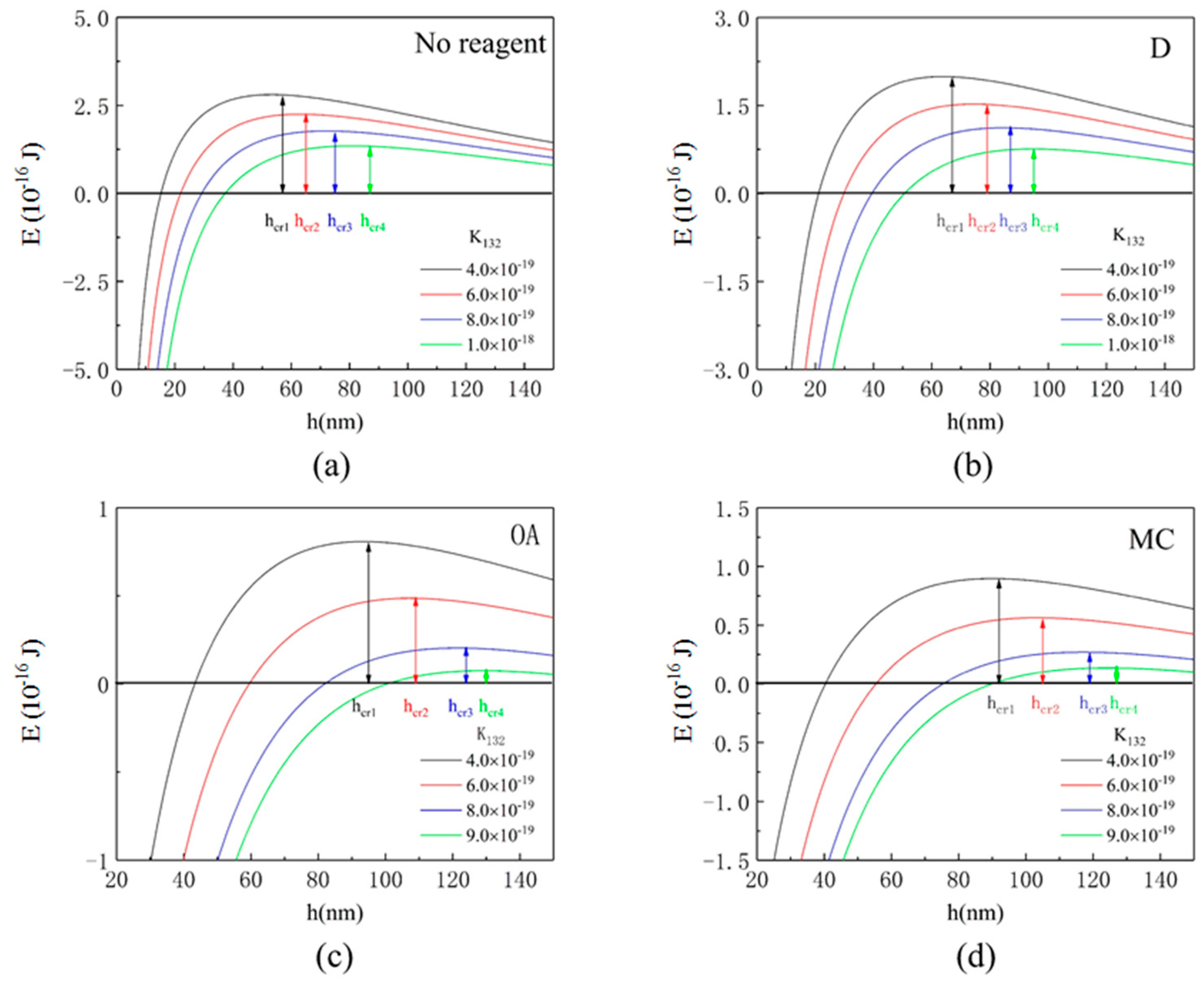
3.4. Interaction Energy Calculation Results
3.5. The Wetting Film Drainage Calculation Results
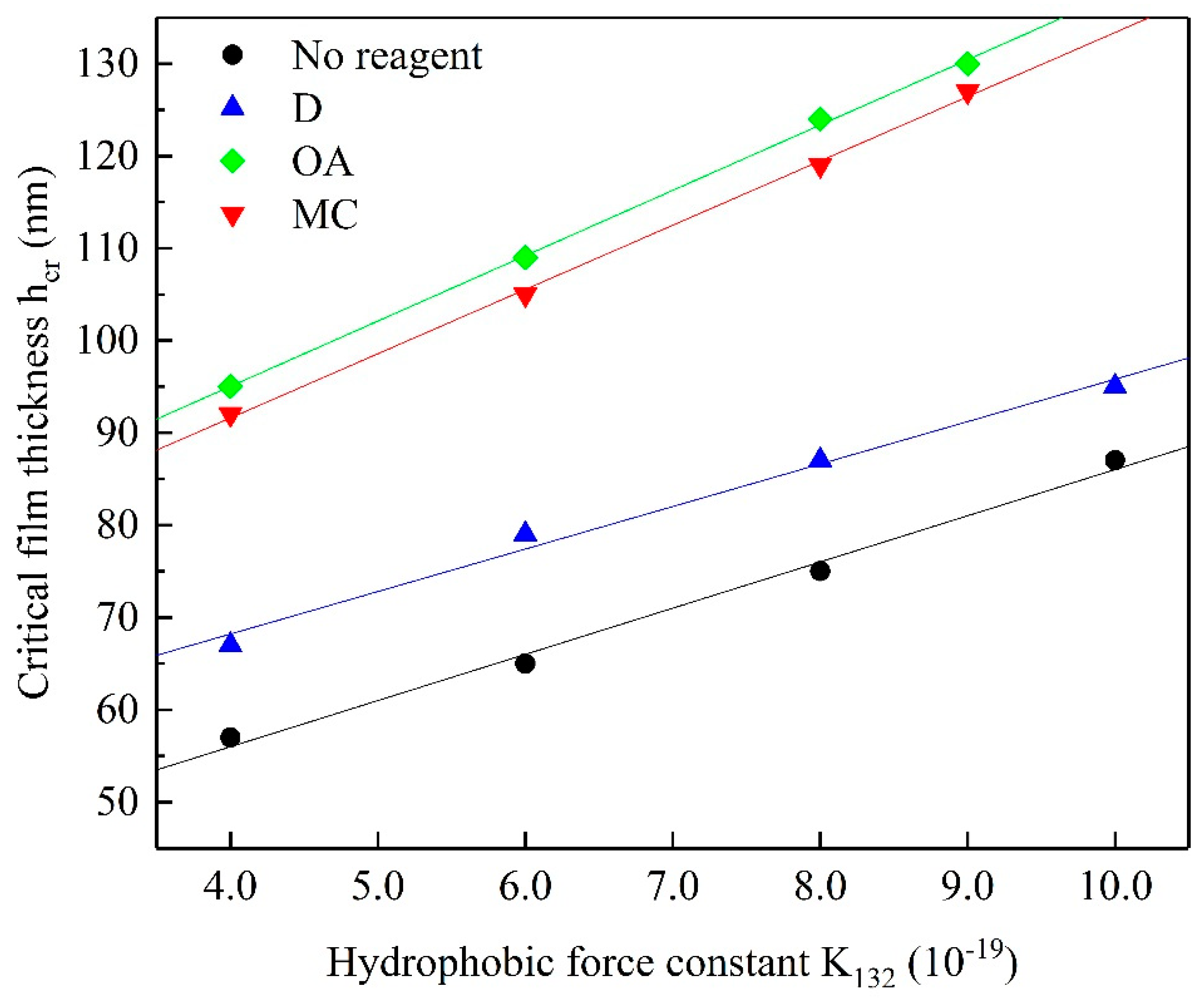
3.6. Back Calculation of Hydrophobic Force Constant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albijanic, B.; Ozdemir, O.; Nguyen, A.V.; Bradshaw, D. A review of induction and attachment times of wetting thin films between air bubbles and particles and its relevance in the separation of particles by flotation. Adv. Colloid Interface Sci. 2010, 159, 1–21. [Google Scholar] [CrossRef]
- Hubbard, A.; Colloidal Science of Flotation; Anh, V. Nguyen and Hans Joachim Schulze, Marcel Dekker, New York, 2004, 850 pages. Miner. Eng. 2004, 17, 839. [Google Scholar] [CrossRef]
- Huang, K.; Yoon, R.-H. Surface Forces in the Thin Liquid Films (TLFs) of Water Confined between n-Alkane Drops and Hydrophobic Gold Surfaces. Langmuir 2019, 35, 15681–15691. [Google Scholar] [CrossRef] [PubMed]
- Ralston, J.; Dukhin, S.; Mishchuk, N. Wetting film stability and flotation kinetics. Adv. Colloid Interface Sci. 2002, 95, 145–236. [Google Scholar] [CrossRef]
- Xie, L.; Shi, C.; Cui, X.; Zeng, H. Surface Forces and Interaction Mechanisms of Emulsion Drops and Gas Bubbles in Complex Fluids. Langmuir 2017, 33, 3911–3925. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Wang, J.; Yuan, D.; Shi, C.; Cui, X.; Zhang, H.; Liu, Q.; Liu, Q.; Zeng, H. Interaction Mechanisms between Air Bubble and Molybdenite Surface: Impact of Solution Salinity and Polymer Adsorption. Langmuir 2017, 33, 2353–2361. [Google Scholar] [CrossRef]
- Wang, J.; Teo, A.J.T.; Tan, S.H.; Evans, G.M.; Nguyen, N.T.; Nguyen, A.V. Influence of Interfacial Gas Enrichment on Controlled Coalescence of Oil Droplets in Water in Microfluidics. Langmuir 2019, 35, 3615–3623. [Google Scholar] [CrossRef] [Green Version]
- Yoon, R.-H. The role of hydrodynamic and surface forces in bubble–particle interaction. Int. J. Miner. Process. 2000, 58, 129–143. [Google Scholar] [CrossRef]
- Chan, D.Y.C.; Klaseboer, E.; Manica, R. Film drainage and coalescence between deformable drops and bubbles. Soft Matter 2010, 7, 2235–2264. [Google Scholar] [CrossRef]
- Zhou, Y.; Albijanic, B.; Tadesse, B.; Wang, Y.; Yang, J.; Zhu, X. The Stefan-Reynolds Model and the Modified Stefan-Reynolds Model for Studying Bubble-Particle Attachment Interactions in the Context of Flotation. Langmuir 2019, 35, 4278–4286. [Google Scholar] [CrossRef]
- Derjaguin, B.; Kussakov, M. Anomalous Properties of Thin Polymolecular Films. 1 V. Acta Physicochim. URSS 1939, 10, 26–45. [Google Scholar]
- Wang, S.; Albijanic, B.; Tao, X.; Fan, H. Thin liquid film drainage mechanism between air bubbles and low-rank coal particles in the presence of surfactant. Fuel Process. Technol. 2019, 186, 18–24. [Google Scholar] [CrossRef]
- Wang, L. Drainage and rupture of thin foam films in the presence of ionic and non-ionic surfactants. Int. J. Miner. Process. 2012, 102, 58–68. [Google Scholar] [CrossRef]
- Kosior, D.; Zawala, J.; Krasowska, M.; Malysa, K. Influence of n-octanol and α-terpineol on thin film stability and bubble attachment to hydrophobic surface. Phys. Chem. Chem. Phys. 2013, 15, 2586–2595. [Google Scholar] [CrossRef]
- Dey, S. Enhancement in hydrophobicity of low rank coal by surfactants—A critical overview. Fuel Process. Technol. 2012, 94, 151–158. [Google Scholar] [CrossRef]
- Wang, L.; Yoon, R.-H. Role of hydrophobic force in the thinning of foam films containing a nonionic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282, 84–91. [Google Scholar] [CrossRef]
- Wen, B.; Xia, W.; Sokolovic, J.M. Recent advances in effective collectors for enhancing the flotation of low rank/oxidized coals. Powder Technol. 2017, 319, 1–11. [Google Scholar] [CrossRef]
- Gui, X.; Xing, Y.; Wang, T.; Cao, Y.; Miao, Z.; Xu, M. Intensification mechanism of oxidized coal flotation by using oxygen-containing collector α-furanacrylic acid. Powder Technol. 2017, 305, 109–116. [Google Scholar] [CrossRef]
- Qu, J.; Tao, X.; He, H.; Zhang, X.; Xu, N.; Zhang, B. Synergistic Effect of Surfactants and a Collector on the Flotation of a Low-Rank Coal. Int. J. Coal Prep. Util. 2015, 35, 14–24. [Google Scholar] [CrossRef]
- Chander, S.; Polat, H.; Mohal, B. Flotation and wettability of a low-rank coal in the presence of surfactants. Min. Met. Explor. 1994, 11, 55–61. [Google Scholar] [CrossRef]
- Polat, M.; Polat, H.; Chander, S. Physical and chemical interactions in coal flotation. Int. J. Miner. Process. 2003, 72, 199–213. [Google Scholar] [CrossRef] [Green Version]
- Polat, H.; Chander, S. Interaction between physical and chemical variables in the flotation of low rank coals. Min. Met. Explor. 1998, 15, 41–47. [Google Scholar] [CrossRef]
- Jena, M.; Biswal, S.; Rudramuniyappa, M. Study on flotation characteristics of oxidised Indian high ash sub-bituminous coal. Int. J. Miner. Process. 2008, 87, 42–50. [Google Scholar] [CrossRef]
- Sis, H.; Ozbayoglu, G.; Sarikaya, M. Comparison of non-ionic and ionic collectors in the flotation of coal fines. Miner. Eng. 2003, 16, 399–401. [Google Scholar] [CrossRef]
- Sis, H.; Ozbayoglu, G.; Sarikaya, M. Utilization of Fine Coal Tailings by Flotation Using Ionic Reagents. Energy Sources 2004, 26, 941–949. [Google Scholar] [CrossRef]
- Xia, W.; Ni, C.; Xie, G. Effective Flotation of Lignite Using a Mixture of Dodecane and 4-Dodecylphenol (DDP) as a Collector. Int. J. Coal Prep. Util. 2016, 36, 262–271. [Google Scholar] [CrossRef]
- Erol, M.; Colduroglu, C.; Aktas, Z. The effect of reagents and reagent mixtures on froth flotation of coal fines. Int. J. Miner. Process. 2003, 71, 131–145. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Liu, J. Extended DLVO theory applied to coal slime-water suspensions. J. Cent. South Univ. 2012, 19, 3558–3563. [Google Scholar] [CrossRef]
- Liao, Y.; Hao, X.; An, M.; Yang, Z.; Ma, L.; Ren, H. Enhancing low-rank coal flotation using mixed collector of dodecane and oleic acid: Effect of droplet dispersion and its interaction with coal particle. Fuel 2020, 280, 118634. [Google Scholar] [CrossRef]
- Yan, G.; Bai, L.; Feng, J.; Zhang, Z. A Comparative Study on the Wettability of Two Coal Samples during Deep Burial Metamorphism. J. Chem. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Wang, S.; Li, L.; Qu, J.; Tao, X.; He, H. Exploration on the mechanism of enhancing low-rank coal flotation with cationic surfactant in the presence of oily collector. Fuel 2018, 227, 190–198. [Google Scholar] [CrossRef]
- Ulusoy, Æ.; Selma, S.; Cebeci, Y. Investigation of the effect of agglomeration time, pH and various salts on the cleaning of Zonguldak bituminous coal by oil agglomeration. Fuel 2002, 81, 1131–1137. [Google Scholar]
- Zou, W.; Zhao, J.; Sun, C. Adsorption of anionic polyacrylamide onto coal and kaolinite calculated from the extended DLVO theory using the van Oss-Chaudhury-Good theory. Polymers 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñeres, J.; Barraza, J.M. Energy barrier of aggregates coal particle–bubble through the extended DLVO theory. Int. J. Miner. Process. 2011, 100, 14–20. [Google Scholar] [CrossRef]
- Li, C.; Somasundaran, P. Reversal of bubble charge in multivalent inorganic salt solutions—Effect of magnesium. J. Colloid Interface Sci. 1991, 146, 215–218. [Google Scholar] [CrossRef]
- Yang, C.; Dabros, T.; Li, D.; Czarnecki, J.; Masliyah, J.H. Measurement of the Zeta Potential of Gas Bubbles in Aqueous Solutions by Microelectrophoresis Method. J. Colloid Interface Sci. 2001, 243, 128–135. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Z.; Li, G.; Rowson, N. Attachment of solid particles to air bubbles in surfactant-free aqueous solutions. Chem. Eng. Sci. 2004, 59, 2639–2645. [Google Scholar] [CrossRef]
- Elmahdy, A.M.; Mirnezami, M.; Finch, J.A. Zeta potential of air bubbles in presence of frothers. Int. J. Miner. Process. 2008, 89, 40–43. [Google Scholar] [CrossRef]
- Sheludko, A. Thin liquid films. Adv. Colloid Interface Sci. 2002, 1, 391–464. [Google Scholar] [CrossRef]
- Attard, P.; Miklavcic, S.J. Effective Spring Constant of Bubbles and Droplets. Langmuir 2001, 17, 8217–8223. [Google Scholar] [CrossRef]
- Chau, T.; Bruckard, W.; Koh, P.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kerisit, S.; Schwenzer, B.; Vijayakumar, M. Effects of Oxygen-Containing Functional Groups on Supercapacitor Performance. J. Phys. Chem. Lett. 2014, 5, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yoon, R.-H. Measurement of hydrophobic forces in thin liquid films of water between bubbles and xanthate-treated gold surfaces. Miner. Eng. 2016, 98, 240–250. [Google Scholar] [CrossRef]
- Maršálek, R. The Influence of Surfactants on the Zeta Potential of Coals. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 31, 66–75. [Google Scholar] [CrossRef]
- Schulze, H.; Stöckelhuber, K.W.; Wenger, A. The influence of acting forces on the rupture mechanism of wetting films—Nucleation or capillary waves. Colloids Surf. A Physicochem. Eng. Asp. 2001, 192, 61–72. [Google Scholar] [CrossRef]
- Kondrat’Ev, S.A.; Moshkin, N.P. Estimate of collecting force of flotation agent. J. Min. Sci. 2015, 51, 150–156. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Zhu, J.; Qiao, E. Effect of Oleic Acid on Froth Properties and Reverse Flotation Performance of Thermal Coal. Trans. Indian Inst. Met. 2018, 71, 1841–1846. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, Y.; Lai, Q.; An, M.; Liao, Y.; Wang, Y. Adsorption behavior of mixed dodecane/n-valeric acid collectors on low-rank coal surface: Experimental and molecular dynamics simulation study. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123840. [Google Scholar] [CrossRef]


| Ultimate Analysis (%) | ||||
|---|---|---|---|---|
| Cdaf | Hdaf | Odaf | Ndaf | St,d |
| 75.55 | 4.96 | 15.35 | 1.05 | 2.07 |
| Number | No Reagent | D | OA | MC | ||||
|---|---|---|---|---|---|---|---|---|
| InT | ZeP | InT | ZeP | InT | ZeP | InT | ZeP | |
| 1 | 1175 | −20.57 | 595 | −19.08 | 65 | −16.58 | 60 | −12.66 |
| 2 | 1065 | −35.45 | 495 | −18.39 | 105 | −10.36 | 55 | −15.32 |
| 3 | 1140 | −19.14 | 535 | −19.41 | 90 | −12.82 | 35 | −14.71 |
| 4 | 1095 | −18.93 | 575 | −22.19 | 95 | −11.33 | 65 | −13.97 |
| 5 | 1150 | −23.61 | 555 | −21.18 | 105 | −18.76 | 25 | −15.84 |
| AVE | 1125 | −23.54 | 551 | −20.05 | 92 | −13.97 | 48 | −14.50 |
| STDVE | 39.62 | 6.19 | 34.41 | 1.41 | 14.70 | 3.20 | 15.36 | 1.11 |
| 4.0 × 10−19 | 6.0 × 10−19 | 8.0 × 10−19 | 9.0 × 10−19 | 1.0 × 10−18 | |
|---|---|---|---|---|---|
| No reagent | 57 | 65 | 75 | - | 87 |
| D | 67 | 79 | 87 | - | 95 |
| OA | 95 | 109 | 124 | 130 | - |
| MC | 92 | 105 | 119 | 127 | - |
| 4.0 × 10−19 | 6.0 × 10−19 | 8.0 × 10−19 | 9.0 × 10−19 | 1.0 × 10−18 | |
|---|---|---|---|---|---|
| No reagent | 1.193 | 0.899 | 0.659 | - | 0.472 |
| D | 0.857 | 0.594 | 0.472 | - | 0.381 |
| OA | 0.384 | 0.267 | 0.182 | 0.156 | - |
| MC | 0.416 | 0.295 | 0.207 | 0.168 | - |
| Reagent | - | ||
|---|---|---|---|
| No reagent | 1.125 | 4.42 × 10−19 | |
| D | 0.551 | 6.94 × 10−19 | |
| OA | 0.092 | 1.11 × 10−18 | |
| MC | 0.048 | 1.34 × 10−18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Song, X.; An, M.; Yang, Z.; Hao, X.; Ren, H. Effect of Dodecane-Oleic Acid Collector Mixture on the Evolution of Wetting Film between Air Bubble and Low-Rank Coal. Minerals 2021, 11, 58. https://doi.org/10.3390/min11010058
Liao Y, Song X, An M, Yang Z, Hao X, Ren H. Effect of Dodecane-Oleic Acid Collector Mixture on the Evolution of Wetting Film between Air Bubble and Low-Rank Coal. Minerals. 2021; 11(1):58. https://doi.org/10.3390/min11010058
Chicago/Turabian StyleLiao, Yinfei, Xingwei Song, Maoyan An, Zhe Yang, Xiaodong Hao, and Hourui Ren. 2021. "Effect of Dodecane-Oleic Acid Collector Mixture on the Evolution of Wetting Film between Air Bubble and Low-Rank Coal" Minerals 11, no. 1: 58. https://doi.org/10.3390/min11010058




