Removal of Pb from Water: The Effectiveness of Gypsum and Calcite Mixtures
Abstract
:1. Introduction
2. Experimental Methods
3. Results
3.1. Chemical Evolution of the Liquid Phase
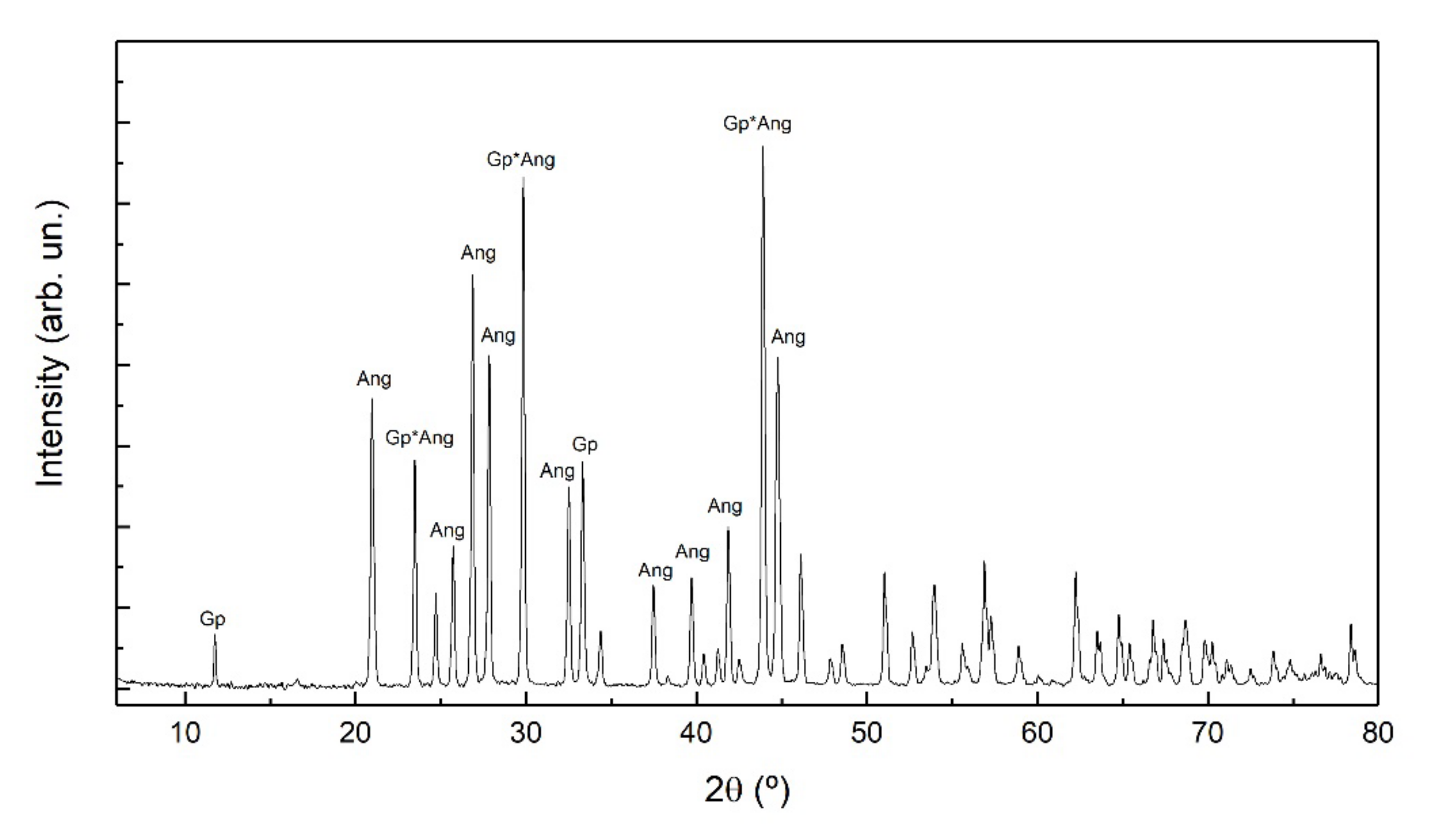
3.2. Newly Formed Phases: Nature and Morphology
4. Discussion
4.1. Effectiveness of Gypsum as Pb Scavenger
4.2. Effectiveness of Calcite as Pb Scavenger
4.3. Effectiveness of Gypsum + Calcite as Pb Scavenger
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bradl, H. Heavy Metals in the Environment: Origin, Interaction and Remediation; Elsevier: Amsterdam, The Netherlands, 2005; Volume 6, ISBN 0-08-045500-X. [Google Scholar]
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nat. Cell Biol. 1988, 333, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Thevenon, F.; Graham, N.D.; Chiaradia, M.; Arpagaus, P.; Wildi, W.; Pote-Wembonyama, J. Local to regional scale industrial heavy metal pollution recorded in sediments of large freshwater lakes in central Europe (lakes Geneva and Lucerne) over the last centuries. Sci. Total Environ. 2011, 412, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachara, J.; Cowan, C.; Resch, C. Sorption of divalent metals on calcite. Geochim. Cosmochim. Acta 1991, 55, 1549–1562. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.E.; Ireson, A.; Kovats, S.; Mojumder, S.K.; Khusru, A.; Rahman, A.; Vineis, P. Drinking Water Salinity and Maternal Health in Coastal Bangladesh: Implications of Climate Change. Environ. Health Perspect. 2011, 119, 1328–1332. [Google Scholar] [CrossRef]
- Charlesworth, S.; De Miguel, E.; Ordóñez, A. A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environ. Geochem. Health 2011, 33, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Von Storch, H.; Hagner, C. Controlling Lead Concentrations in Human Blood by Regulating the Use of Lead in Gasoline. Ambio A J. Hum. Environ. 2004, 33, 126–132. [Google Scholar] [CrossRef]
- Chattopadhyay, G.; Lin, K.C.-P.; Feitz, A. Household dust metal levels in the Sydney metropolitan area. Environ. Res. 2003, 93, 301–307. [Google Scholar] [CrossRef]
- Al Hejami, A.; Davis, M.; Prete, D.; Lu, J.; Wang, S. Heavy metals in indoor settled dusts in Toronto, Canada. Sci. Total Environ. 2020, 703, 134895. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-C.; Townsend, T.G. Leaching of Lead from Computer Printed Wire Boards and Cathode Ray Tubes by Municipal Solid Waste Landfill Leachates. Environ. Sci. Technol. 2003, 37, 4778–4784. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef]
- Tukker, A.; Buist, H.; Van Oers, L.; Van Der Voet, E. Risks to health and environment of the use of lead in products in the EU. Resour. Conserv. Recycl. 2006, 49, 89–109. [Google Scholar] [CrossRef]
- Audry, S.; Schäfer, J.; Blanc, G.; Jouanneau, J.M. Fifty-year sedimentary record of heavy metal pollution (Cd, Zn, Cu, Pb) in the Lot River reservoirs (France). Environ. Pollut. 2004, 132, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Khanam, R.; Kumar, A.; Nayak, A.K.; Shahid; Tripathi, R.; Vijayakumar, S.; Bhaduri, D.; Kumar, U.; Mohanty, S.; Panneerselvam, P.; et al. Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci. Total Environ. 2020, 699, 134330. [Google Scholar] [CrossRef] [PubMed]
- Mapanda, F.; Mangwayana, E.; Nyamangara, J.; Giller, K. The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric. Ecosyst. Environ. 2005, 107, 151–165. [Google Scholar] [CrossRef]
- Oliver, M. Soil and human health: A review. Eur. J. Soil Sci. 1997, 48, 573–592. [Google Scholar] [CrossRef]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Z.; Van Der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef]
- Simon, M.; Ortiz, I.; García, I.; Fernández, E.; Dorronsoro, C.; Aguilar, J. Pollution of soils by the toxic spill of a pyrite mine (Aznalcollar, Spain). Sci. Total Environ. 1999, 242, 105–115. [Google Scholar] [CrossRef]
- Laidlaw, M.A.S.; Filippelli, G.M.; Sadler, R.C.; Gonzales, C.R.; Ball, A.S.; Mielke, H.W. Children’s Blood Lead Seasonality in Flint, Michigan (USA), and Soil-Sourced Lead Hazard Risks. Int. J. Environ. Res. Public Health 2016, 13, 358. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.C.; Thornton, I. Environmental contamination and seasonal variation of metals in soils, plants and waters in the paddy fields around a Pb-Zn mine in Korea. Sci. Total Environ. 1997, 198, 105–121. [Google Scholar] [CrossRef]
- Luo, X.-S.; Ding, J.; Xu, B.; Wang, Y.-J.; Li, H.-B.; Yu, S. Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Sci. Total Environ. 2012, 424, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.B.; Huet, N.; MacIntosh, D.L. Longitudinal investigation of exposure to arsenic, cadmium, and lead in drinking water. Environ. Health Perspect. 2000, 108, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.L.; Von Schirnding, Y.E.; Prapamontol, T. Environmental lead exposure: A public health problem of global dimensions. Bull. World Health Organ. 2000, 78, 1068–1077. [Google Scholar] [PubMed]
- Zheng, N.; Wang, Q.; Zheng, D. Health risk of Hg, Pb, Cd, Zn, and Cu to the inhabitants around Huludao Zinc Plant in China via consumption of vegetables. Sci. Total Environ. 2007, 383, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Njati, S.Y.; Maguta, M.M. Lead-based paints and children’s PVC toys are potential sources of domestic lead poisoning—A review. Environ. Pollut. 2019, 249, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Piekut, A.; Gut, K.; Ćwieląg-Drabek, M.; Domagalska, J.; Marchwińska-Wyrwał, E. The relationship between children’s non-nutrient exposure to cadmium, lead and zinc and the location of recreational areas-Based on the Upper Silesia region case (Poland). Chemosphere 2019, 223, 544–550. [Google Scholar] [CrossRef]
- Pirkle, J.L.; Kaufmann, R.B.; Brody, D.J.; Hickman, T.; Gunter, E.W.; Paschal, D.C. Exposure of the U.S. population to lead, 1991–1994. Environ. Health Perspect. 1998, 106, 745–750. [Google Scholar] [CrossRef]
- Zheng, N.; Liu, J.; Wang, Q.; Liang, Z. Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, Northeast of China. Sci. Total Environ. 2010, 408, 726–733. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Traina, S.J.; Logan, T.J.; Ryan, J.A. In Situ lead immobilization by apatite. Environ. Sci. Technol. 1993, 27, 1803–1810. [Google Scholar] [CrossRef]
- Traina, S.J.; Laperche, V. Contaminant bioavailability in soils, sediments, and aquatic environments. Proc. Natl. Acad. Sci. USA 1999, 96, 3365–3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, J.K. Evaporites: Sediments, Resources and Hydrocarbons; Springer: Berlin, Germany, 2006; ISBN 3-540-32344-9. [Google Scholar]
- Sposito, G. Distinguishing Adsorption from Surface Precipitation; American Chemical Society (ACS): Washington, DC, USA, 1987; pp. 217–228. [Google Scholar]
- Prieto, M.; Cubillas, P.; Fernández-Gonzalez, Á. Uptake of dissolved Cd by biogenic and abiogenic aragonite: A comparison with sorption onto calcite. Geochim. Cosmochim. Acta 2003, 67, 3859–3869. [Google Scholar] [CrossRef]
- Brown, G.; Parks, G.; O’Day, P. Mineral Surfaces; Vaughan, D.J., Pattrick, R.A.D., Eds.; Chapman & Hall: New York, NY, USA, 1995. [Google Scholar]
- Pérez-Garrido, C.; Fernández-Díaz, L.; Pina, C.M.; Prieto, M. In Situ AFM observations of the interaction between calcite surfaces and Cd-bearing aqueous solutions. Surf. Sci. 2007, 601, 5499–5509. [Google Scholar] [CrossRef] [Green Version]
- Dyer, A. Mineral Surfaces; Vaughan, D.J., Pattrick, R.A.D., Eds.; Chapman Hall: London, UK, 1995; pp. 333–354. [Google Scholar]
- Putnis, A.; Putnis, C.V. The mechanism of reequilibration of solids in the presence of a fluid phase. J. Solid State Chem. 2007, 180, 1783–1786. [Google Scholar] [CrossRef]
- Putnis, A. Mineral Replacement Reactions. Rev. Miner. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Agudo, E.R.; Putnis, C.V.; Putnis, A. Coupled dissolution and precipitation at mineral–fluid interfaces. Chem. Geol. 2014, 383, 132–146. [Google Scholar] [CrossRef]
- Pollok, K.; Putnis, C.V.; Putnis, A. Mineral replacement reactions in solid solution-aqueous solution systems: Volume changes, reactions paths and end-points using the example of model salt systems. Am. J. Sci. 2011, 311, 211–236. [Google Scholar] [CrossRef]
- Putnis, C.V.; Geisler, T.; Schmid-Beurmann, P.; Stephan, T.; Giampaolo, C. An experimental study of the replacement of leucite by analcime. Am. Miner. 2007, 92, 19–26. [Google Scholar] [CrossRef]
- Agudo, E.R.; Putnis, C.V.; Rodriguez-Navarro, C.; Putnis, A. Mechanism of leached layer formation during chemical weathering of silicate minerals. Geology 2012, 40, 947–950. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; King, H.E.; Patiño-López, L.D.; Putnis, C.V.; Geisler, T.; Rodriguez-Navarro, C.; Putnis, A. Control of silicate weathering by interface-coupled dissolution-precipitation processes at the mineral-solution interface. Geology 2016, 44, 567–570. [Google Scholar] [CrossRef]
- Altree-Williams, A.; Pring, A.; Ngothai, Y.; Brugger, J. Textural and compositional complexities resulting from coupled dissolution–reprecipitation reactions in geomaterials. Earth-Sci. Rev. 2015, 150, 628–651. [Google Scholar] [CrossRef]
- Altree-Williams, A.; Brugger, J.; Pring, A.; Bedrikovetsky, P. Coupled reactive flow and dissolution with changing reactive surface and porosity. Chem. Eng. Sci. 2019, 206, 289–304. [Google Scholar] [CrossRef]
- Prieto, M.; Astilleros, J.M.; Fernández-Díaz, L. Environmental Remediation by Crystallization of Solid Solutions. Elements 2013, 9, 195–201. [Google Scholar] [CrossRef]
- Morales, J.; Astilleros, J.M.; Jiménez, A.; Göttlicher, J.; Steininger, R.; Fernández-Díaz, L. Uptake of dissolved lead by anhydrite surfaces. Appl. Geochem. 2014, 40, 89–96. [Google Scholar] [CrossRef]
- Wang, L.; Putnis, C.V.; Ruiz-Agudo, E.; King, H.E.; Putnis, A. Coupled Dissolution and Precipitation at the Cerussite-Phosphate Solution Interface: Implications for Immobilization of Lead in Soils. Environ. Sci. Technol. 2013, 47, 13502–13510. [Google Scholar] [CrossRef]
- Pina, C.; Fernández-Díaz, L.; Prieto, M.; Putnis, A. In Situ atomic force microscope observations of a dissolution-crystallisation reaction: The phosgenite-cerussite transformation. Geochim. Cosmochim. Acta 2000, 64, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Roncal-Herrero, T.; Astilleros, J.M.; Bots, P.; Rodríguez-Blanco, J.D.; Prieto, M.; Benning, L.G.; Fernández-Díaz, L. Reaction pathways and textural aspects of the replacement of anhydrite by calcite at 25 °C. Am. Miner. 2017, 102, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Pastor, N.; Pina, C.M.; Fernández-Díaz, L. A combined in situ AFM and SEM study of the interaction between celestite (001) surfaces and carbonate-bearing aqueous solutions. Surf. Sci. 2007, 601, 2973–2982. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Garrido, C.; Astilleros, J.M.; Fernández-Díaz, L.; Prieto, M. In Situ AFM study of the interaction between calcite {1 0 1− 4} surfaces and supersaturated Mn2+-CO32− aqueous solutions. J. Cryst. Growth 2009, 311, 4730–4739. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Cametti, G.; Vanhecke, D.; Churakov, S.V. The Role of Interfaces in Controlling Pb2+ Removal by Calcium Carbonate Minerals. Cryst. Growth Des. 2020, 20, 6157–6169. [Google Scholar] [CrossRef]
- Wang, L.; Agudo, E.R.; Putnis, C.V.; Menneken, M.; Putnis, A. Kinetics of Calcium Phosphate Nucleation and Growth on Calcite: Implications for Predicting the Fate of Dissolved Phosphate Species in Alkaline Soils. Environ. Sci. Technol. 2012, 46, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Agudo, E.R.; Putnis, C.V.; Putnis, A. Posner’s cluster revisited: Direct imaging of nucleation and growth of nanoscale calcium phosphate clusters at the calcite-water interface. CrystEngComm 2012, 14, 6252–6256. [Google Scholar] [CrossRef]
- Guren, M.G.; Putnis, C.V.; Montes-Hernandez, G.; King, H.E.; Renard, F. Direct imaging of coupled dissolution-precipitation and growth processes on calcite exposed to chromium-rich fluids. Chem. Geol. 2020, 552, 119770. [Google Scholar] [CrossRef]
- Pérez, P.F.; Astilleros, J.M.; Fernández-Díaz, L. The Formation of Barite and Celestite through the Replacement of Gypsum. Minerals 2020, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.; Astilleros, J.M.; Fernández-Díaz, L.; Álvarez-Lloret, P.; Jiménez, A. Anglesite (PbSO4) epitactic overgrowths and substrate-induced twinning on anhydrite (CaSO4) cleavage surfaces. J. Cryst. Growth 2013, 380, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.D.; Jiménez, A.; Prieto, M.; Torre, L.; García-Granda, S. Interaction of gypsum with As (V)-bearing aqueous solutions: Surface precipitation of guerinite, sainfeldite, and Ca2NaH(AsO4)2 6H2O, a synthetic arsenate. Am. Miner. 2008, 93, 928–939. [Google Scholar] [CrossRef]
- Pinto, A.J.; Agudo, E.R.; Putnis, C.V.; Putnis, A.; Jiménez, A.; Prieto, M. AFM study of the epitaxial growth of brushite (CaHPO4 2H2O) on gypsum cleavage surfaces. Am. Miner. 2010, 95, 1747–1757. [Google Scholar] [CrossRef]
- Pinto, A.J.; Jimenez, A.; Prieto, M. Interaction of phosphate-bearing solutions with gypsum: Epitaxy and induced twinning of brushite (CaHPO4 2H2O) on the gypsum cleavage surface. Am. Miner. 2009, 94, 313–322. [Google Scholar] [CrossRef]
- Yu, L.; Daniels, L.M.; Mulders, J.J.; Saldi, G.D.; Harrison, A.L.; Liu, L.; Oelkers, E.H. An experimental study of gypsum dissolution coupled to CaCO3 precipitation and its application to carbon storage. Chem. Geol. 2019, 525, 447–461. [Google Scholar] [CrossRef]
- Godelitsas, A.; Astilleros, J.M.; Hallam, K.; Harissopoulos, S.; Putnis, A.; Harissopulos, S. Interaction of Calcium Carbonates with Lead in Aqueous Solutions. Environ. Sci. Technol. 2003, 37, 3351–3360. [Google Scholar] [CrossRef] [Green Version]
- Elzinga, E.J.; Rouff, A.A.; Reeder, R.J. The long-term fate of Cu2+, Zn2+, and Pb2+ adsorption complexes at the calcite surface: An X-ray absorption spectroscopy study. Geochim. Cosmochim. Acta 2006, 70, 2715–2725. [Google Scholar] [CrossRef]
- Rangel-Porras, G.; García-Magno, J.; González-Muñoz, M. Lead and cadmium immobilization on calcitic limestone materials. Desalination 2010, 262, 1–10. [Google Scholar] [CrossRef]
- Astilleros, J.; Godelitsas, A.; Rodriguez-Blanco, J.D.; Fernández-Díaz, L.; Prieto, M.; Lagoyannis, A.; Harissopulos, S. Interaction of gypsum with lead in aqueous solutions. Appl. Geochem. 2010, 25, 1008–1016. [Google Scholar] [CrossRef] [Green Version]
- Rouff, A.A.; Elzinga, E.J.; Reeder, R.J.; Fisher, N.S. The Effect of Aging and pH on Pb(II) Sorption Processes at the Calcite—Water Interface. Environ. Sci. Technol. 2006, 40, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Sturchio, N.C.; Chiarello, R.P.; Cheng, L.; Lyman, P.F.; Bedzyk, M.J.; Qian, Y.; You, H.; Yee, D.; Geissbuhler, P.; Sorensen, L.B.; et al. Lead adsorption at the calcite-water interface: Synchrotron X-ray standing wave and X-ray reflectivity studies. Geochim. Cosmochim. Acta 1997, 61, 251–263. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Ruiz-Agudo, C.; Churakov, S.V. The key effects of polymorphism during PbII uptake by calcite and aragonite. CrystEngComm 2019, 21, 6145–6155. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C. Description of input and examples for PHREEQC version 3: A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Tech. Methods 2013. [Google Scholar] [CrossRef] [Green Version]
- Gray, N.F. Drinking Water Quality: Problems and Solutions; John Wiley & Sons: Chichester, UK, 2008; ISBN 1-139-47041-8. [Google Scholar]
- Prieto, M.; Putnis, A.; Fernández-Díaz, L.; López-Andrés, S. Metastability in diffusing-reacting systems. J. Cryst. Growth 1994, 142, 225–235. [Google Scholar] [CrossRef]
- Prieto, M.; Putnis, A.; Fernández-Díaz, L. Crystallization of solid solutions from aqueous solutions in a porous medium: Zoning in (Ba,Sr)SO4. Geol. Mag. 1993, 130, 289–299. [Google Scholar] [CrossRef]
- Yuan, K.; Lee, S.S.; De Andrade, V.; Sturchio, N.C.; Fenter, P. Replacement of Calcite (CaCO3) by Cerussite (PbCO3). Environ. Sci. Technol. 2016, 50, 12984–12991. [Google Scholar] [CrossRef]
- Berner, R.A. Early Diagenesis: A Theoretical Approach; Princeton University Press: Princeton, NJ, USA, 1980; ISBN 0-691-08260-X. [Google Scholar]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roza Llera, A.; Jimenez, A.; Fernández-Díaz, L. Removal of Pb from Water: The Effectiveness of Gypsum and Calcite Mixtures. Minerals 2021, 11, 66. https://doi.org/10.3390/min11010066
Roza Llera A, Jimenez A, Fernández-Díaz L. Removal of Pb from Water: The Effectiveness of Gypsum and Calcite Mixtures. Minerals. 2021; 11(1):66. https://doi.org/10.3390/min11010066
Chicago/Turabian StyleRoza Llera, Ana, Amalia Jimenez, and Lurdes Fernández-Díaz. 2021. "Removal of Pb from Water: The Effectiveness of Gypsum and Calcite Mixtures" Minerals 11, no. 1: 66. https://doi.org/10.3390/min11010066
APA StyleRoza Llera, A., Jimenez, A., & Fernández-Díaz, L. (2021). Removal of Pb from Water: The Effectiveness of Gypsum and Calcite Mixtures. Minerals, 11(1), 66. https://doi.org/10.3390/min11010066





