Occurrence and Leaching Behavior of Chromium in Synthetic Stainless Steel Slag Containing FetO
Abstract
1. Introduction
2. Experimental
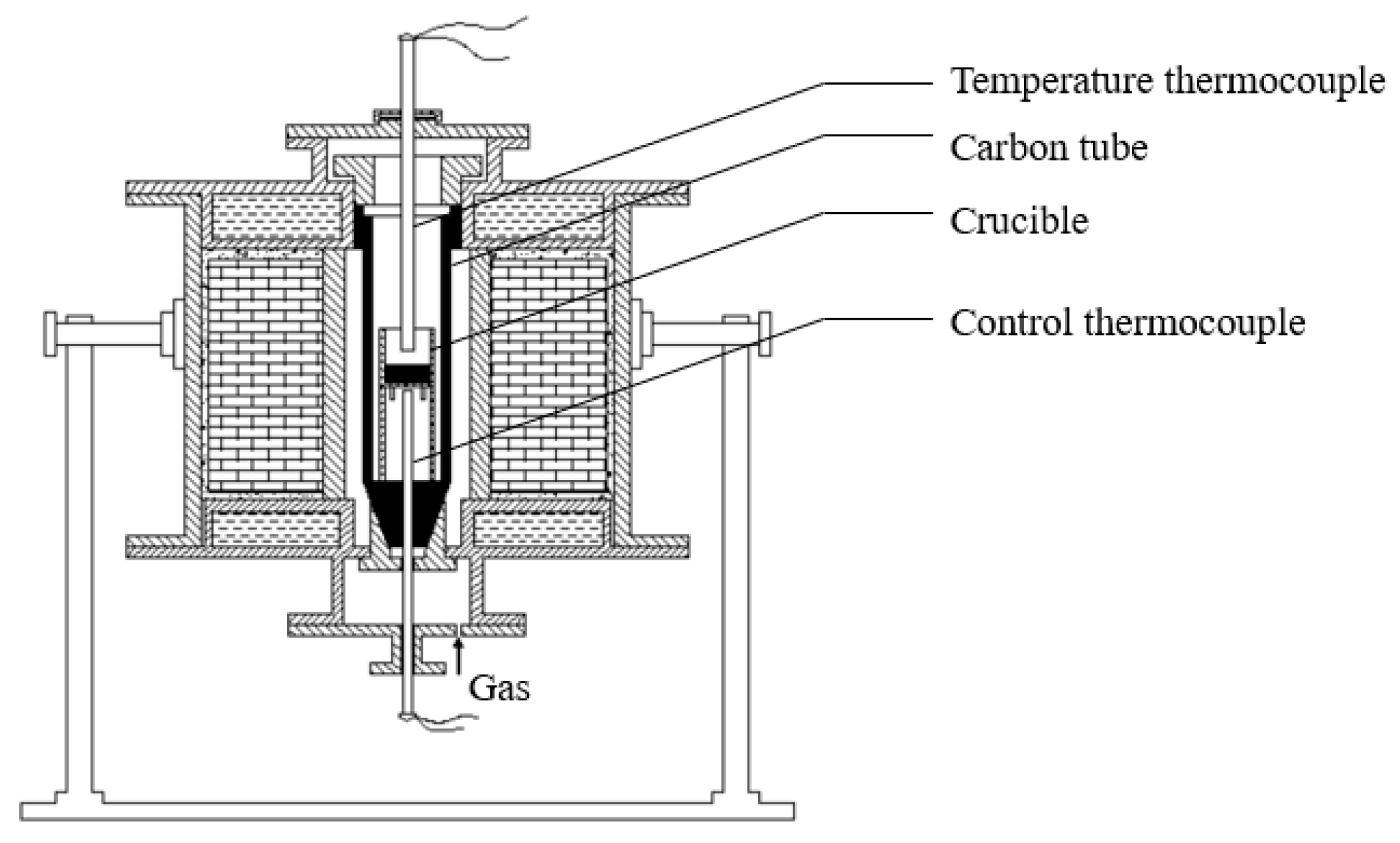
2.1. Samples Preparation
2.2. Leaching Test
2.3. Thermodynamic Calculation
- (1)
- Units: temperature/°C, pressure/atm, mass/g.
- (2)
- Databases: FactPS, Ftoxid.
- (3)
- Compounds: ideal-pure solids.
- (4)
- Solutions: FToxid-(SLAGA, SPINA, MeO_A, bC2SA, aC2SA, Mel_A, CaSpinel).
3. Results and Discussion
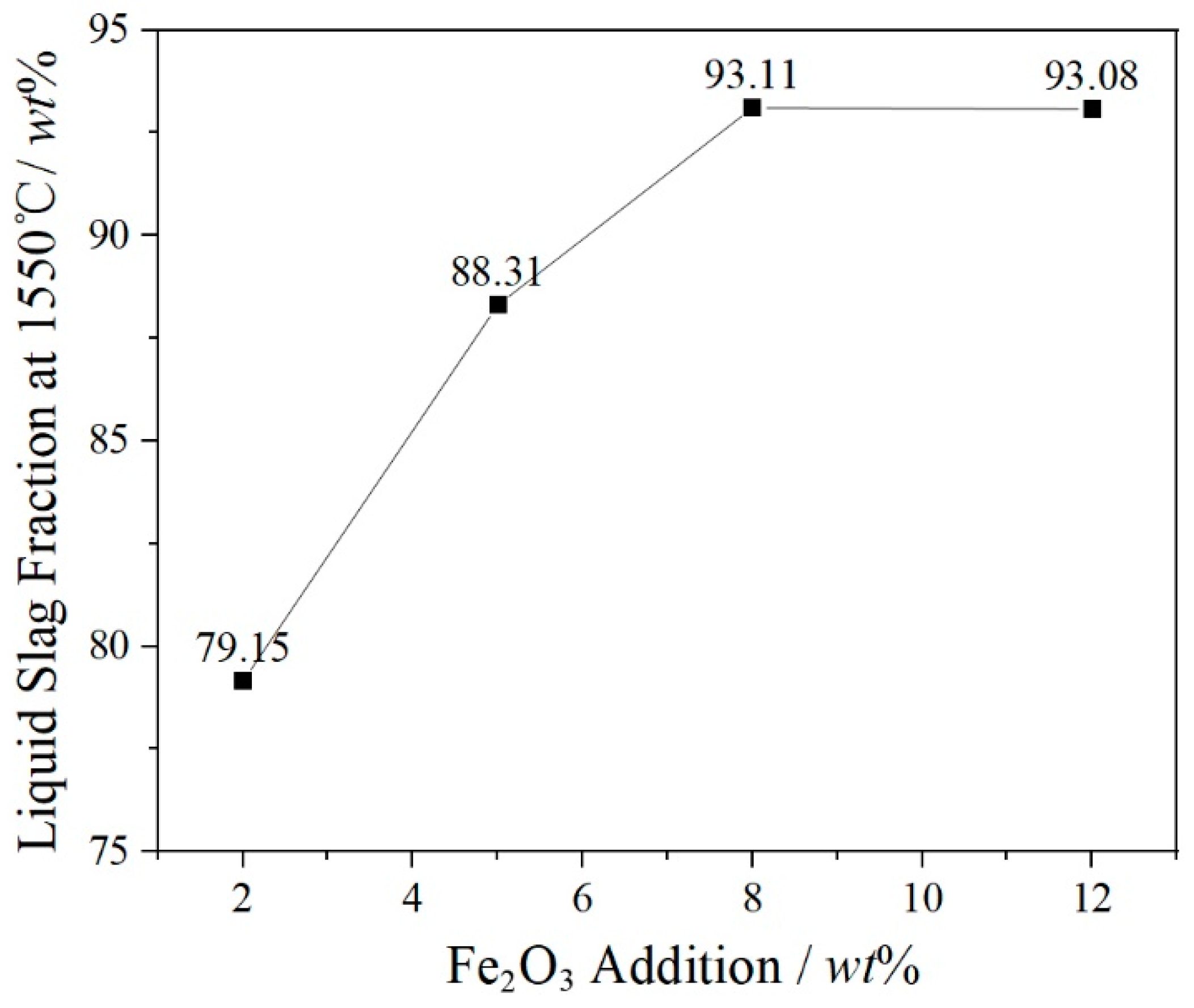
3.1. Calculation and Analysis of Slag Components
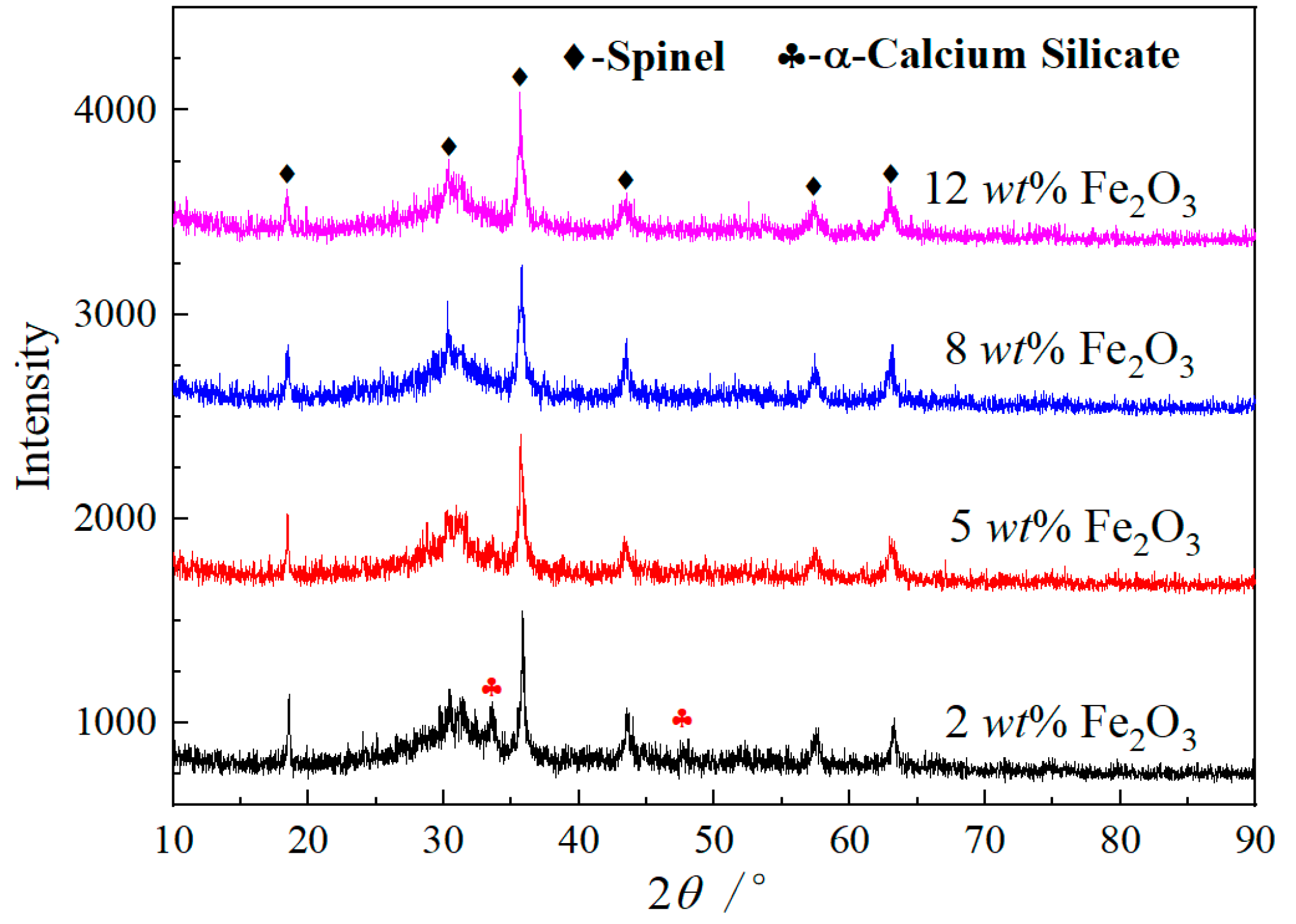
3.2. Microstructure
3.3. Leaching Toxicity
4. Conclusions
- (1)
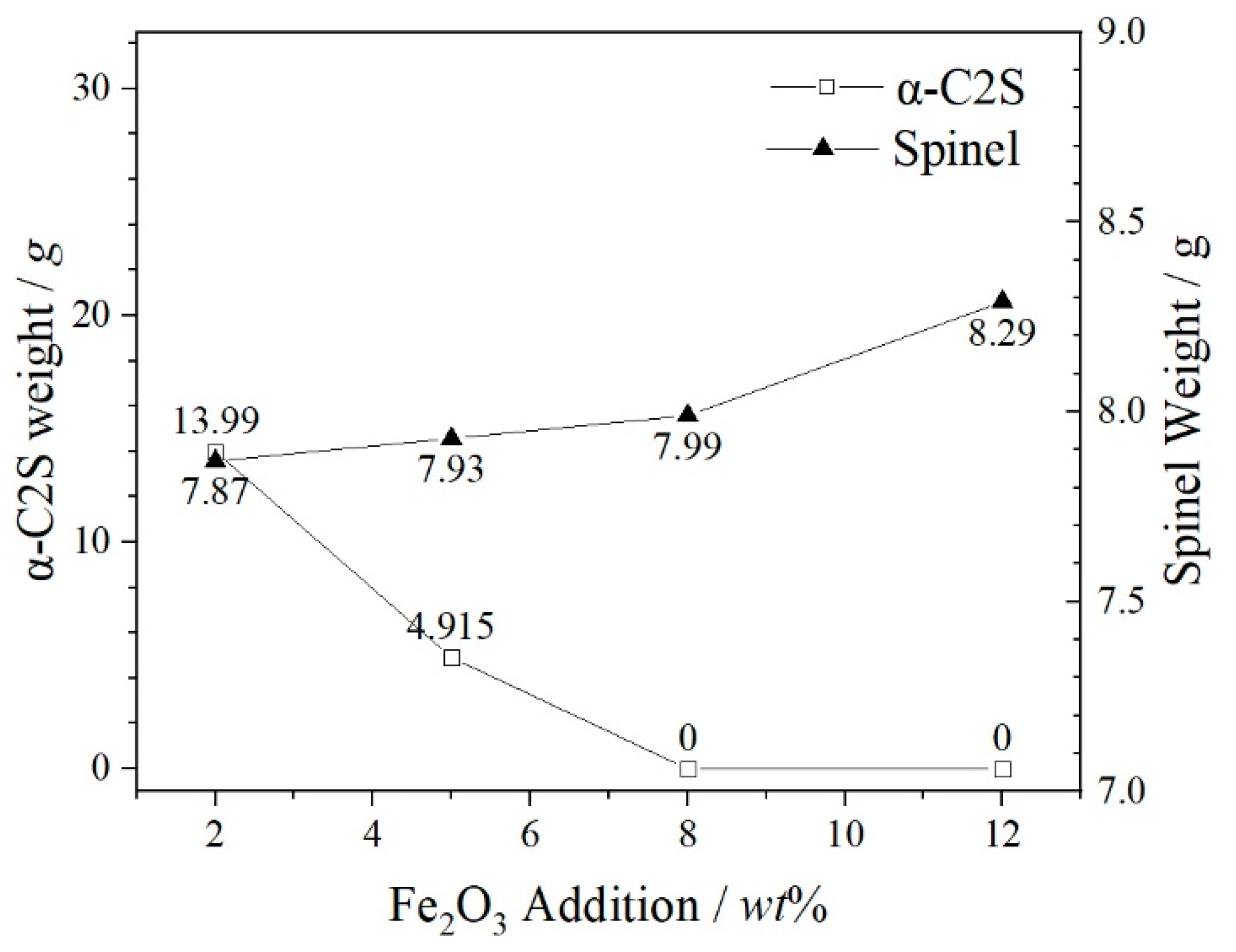
- The increase of Fe2O3 content can promote the precipitation of spinel phases and effectively inhibit the formation and precipitation of α-C2S. Fe2O3 promotes the spinel crystal precipitations as a result of the increase of FeCr2O4, MgFe2O4, MgAl2O4 and FeAl2O4 in the spinel solid solution by FactSage 7.3. MgFe2O4 and Fe2O3 play a key role in the formation of the spinel solid solution (Mg, Fe) (Cr, Fe, Al)2O4.
- (2)
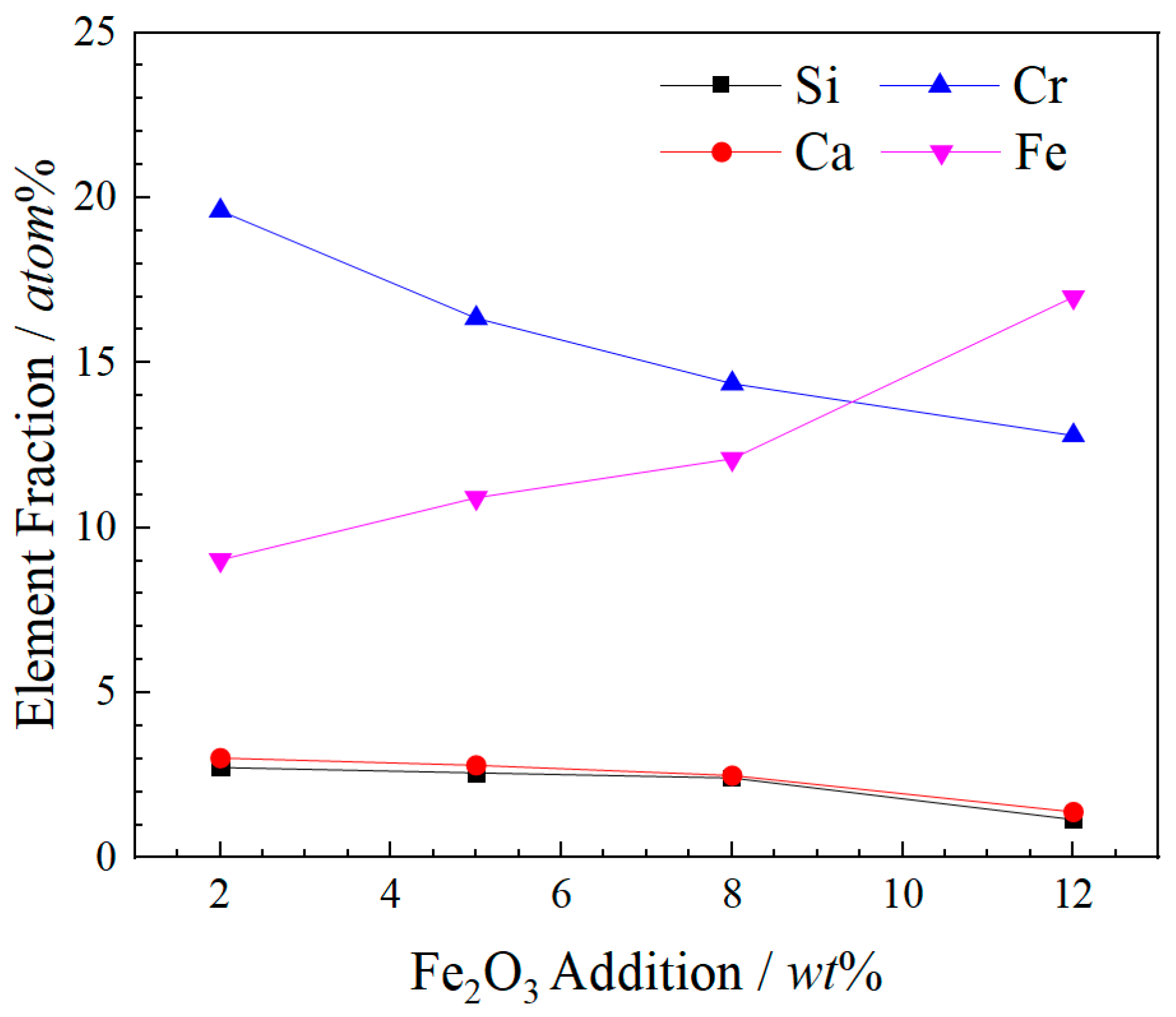
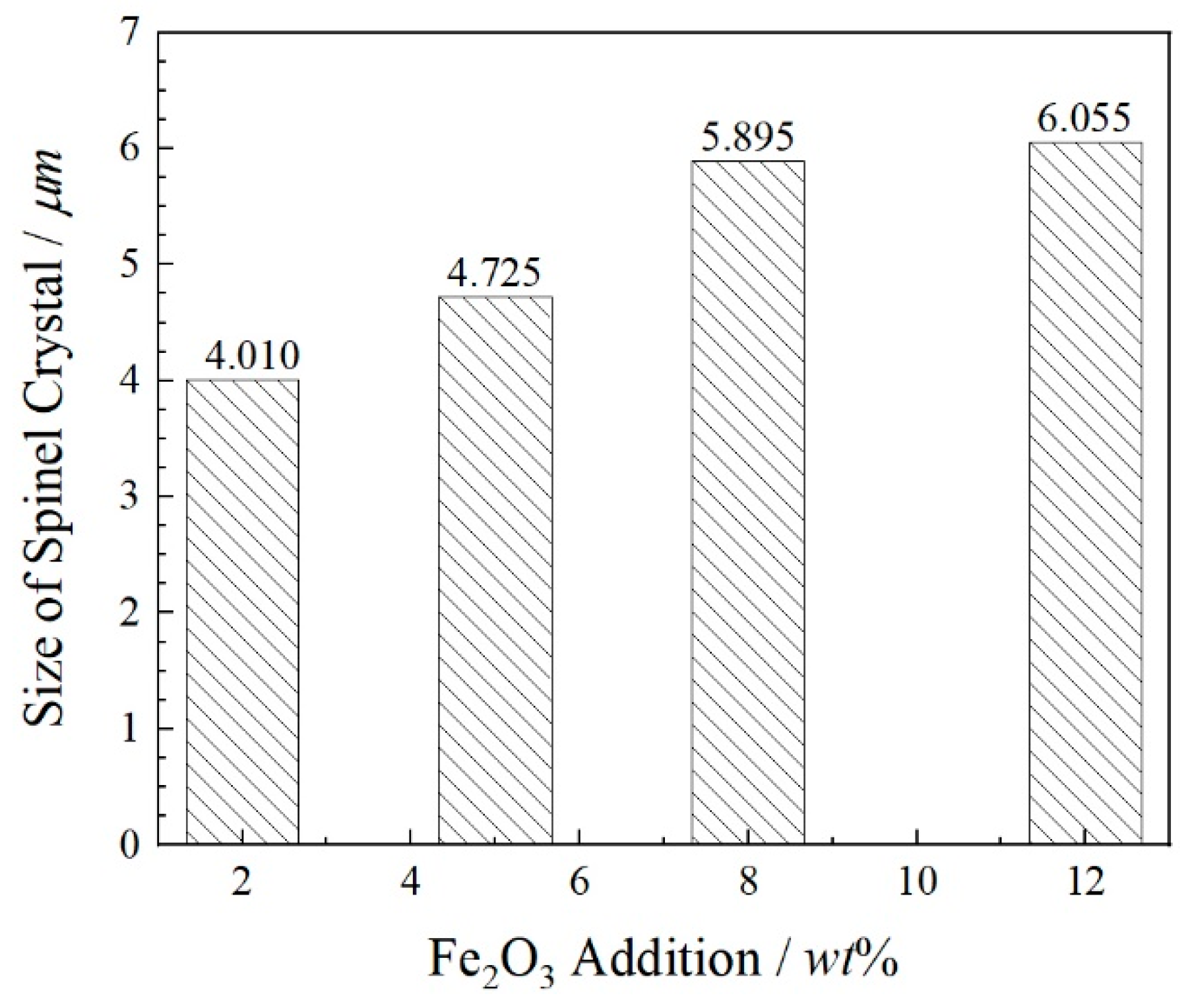
- Fe2O3 contents increase from 0 wt% to 12 wt%, the sizes of spinel crystals increase from 4.01 μm to 6.06 μm and the growth rate is 51.12% compared with these two contents of Fe2O3. The atomic ratios Fe and Cr are from 26.48 at% to 29.78 at%. The combined valence of Fe in FetO is +2.75, +2.71 and +2.26, respectively, in the 2 wt%–12 wt% Fe2O3 samples. The enhancement in the proportion of Fe in the cationic of spinel solid solution promotes the enrichment of Cr in spinel phases and reduces the leaching risk of Cr6+.
- (3)
- Cr2O3 is a nucleating agent to promote the nucleation of the spinel solid solution phase. The increase of the concentration of FetO in the liquid phase promotes the concentration gradient of Fe2O3 and FeO components on the liquid side of the interface, and the amount of liquid phase is increased. The diffusion conditions of particles are improved, and the structure of spinel phase is a Cr-rich center and an Fe-rich edge.
- (4)
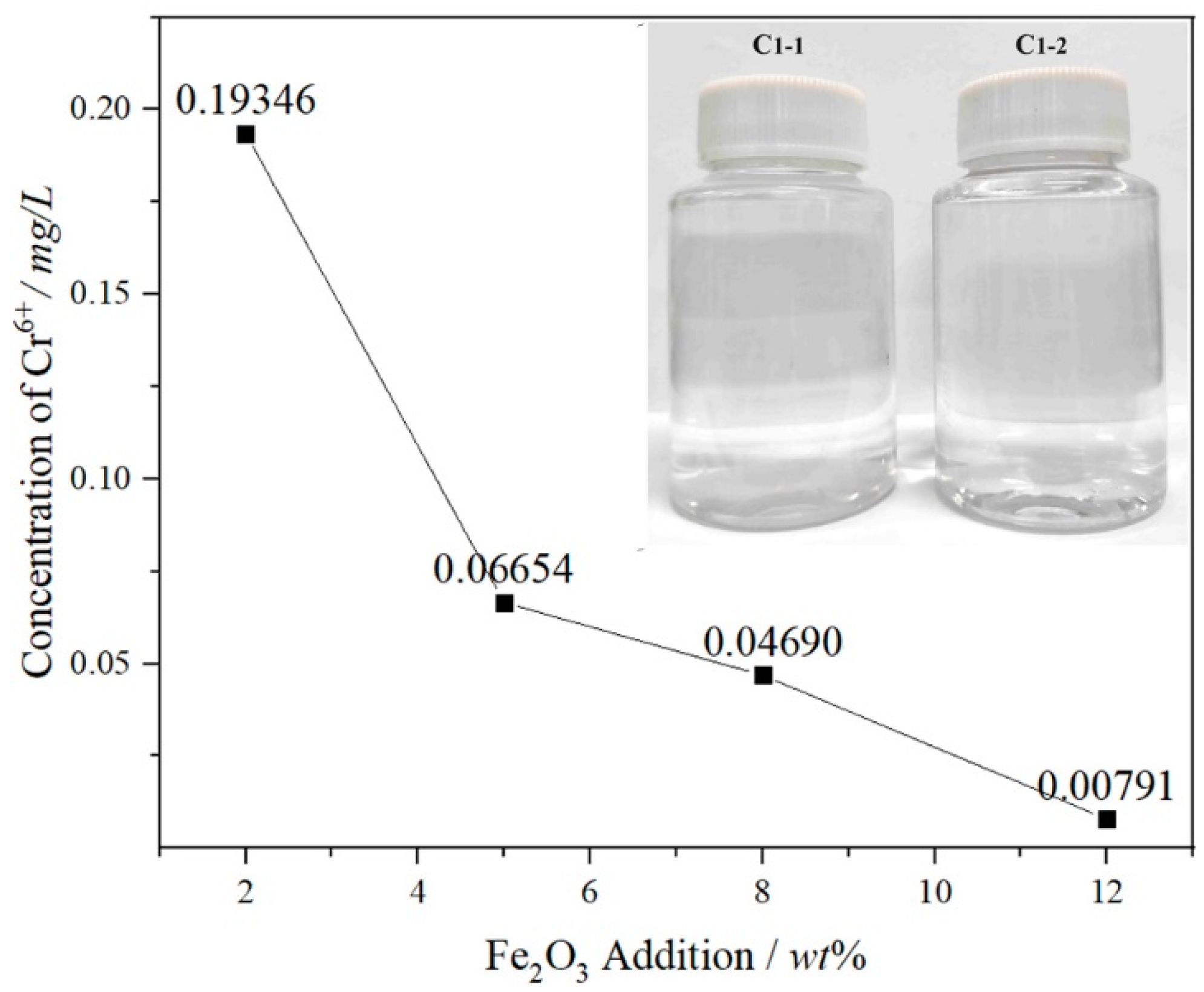
- The leaching amount of Cr6+ in the FetO samples is far lower than the standard limit under the acid leaching conditions by the TRGS 613 standard or HJ/T 299-2007 standard, and the color of the leaching solution is transparent, with the maximum leaching concentration of Cr6+ at 0.19346 mg/L in the 2 wt% Fe2O3 sample.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, Y.; Nassaralla, C.L. Formation of hexavalent chromium by reaction between slag and magnesite-chrome refractory. Met. Mater. Trans. B 1998, 29, 405–410. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Li, Y. Solidification/stabilization of stainless steel pickling residue with aluminum potassium sulfate amended fly ash. J. Clean. Prod. 2019, 234, 400–409. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espada, J.J.; Gallardo, M.; Molina, R.; López-Muñoz, M.J. Life cycle assessment and techno-economic evaluation of alternatives for the treatment of wastewater in a chrome-plating industry. J. Clean. Prod. 2018, 172, 2351–2362. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.W.; Zhao, M.; Zhang, M.X. Growth of diopside crystals in CMAS glass-ceramics using Cr2O3 as a nucleating agent. J. Am. Ceram. Soc. 2018, 101, 3968–3978. [Google Scholar] [CrossRef]
- Nath, M.; Song, S.; Xu, T.; Wu, Y.; Li, Y. Effective inhibition of Cr (VI) in Al2O3-CaO-Cr2O3 refractory castables system through silica-gel assisted in-situ secondary phase tuning. J. Clean. Prod. 2019, 233, 1038–1046. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Zeng, Y.; Wang, Z. Toxicity assessment and geochemical model of chromium leaching from AOD slag. Chemosphere 2016, 114, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.M.; Ubios, Á.M. Alterations in odontogenesis and tooth eruption resulting from exposure to hexavalent chromium in suckling animals. Int. J. Paediatr. Dent. 2020, 30, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Wang, Z.; Zeng, Y.; Ren, Q. Long-term leaching characterization and geochemical modeling of chromium released from AOD slag. Environ. Sci. Pollut. Res. 2020, 27, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Nassaralla, C.L. Minimization of Hexavalent chromium in magnesite-chrome refractory. Met. Mater. Trans. B 1997, 5, 855–859. [Google Scholar] [CrossRef]
- Gencel, O.; Sutcu, M.; Erdogmus, E.; Koc, V.; Cay, V.V.; Gok, M.S. Properties of bricks with waste ferrochromium slag and zeolite. J. Clean. Prod. 2013, 59, 111–119. [Google Scholar] [CrossRef]
- OuYang, S.; Zhang, Y.; Chen, Y.; Zhao, Z.; Wen, M.; Li, B.; Shi, Y.; Zhang, M.; Liu, S. Preparation of Glass-ceramics Using Chromium-containing Stainless Steel Slag: Crystal Structure and Solidification of Heavy Metal Chromium. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Sheen, Y.-N.; Wang, H.-Y.; Sun, T.-H. Properties of green concrete containing stainless steel oxidizing slag resource materials. Constr. Build. Mater. 2014, 50, 22–27. [Google Scholar] [CrossRef]
- Li, W.; Xue, X. Effect of cooling regime on phase transformation and chromium enrichment in stainless-steel slag. Ironmak. Steelmak. 2019, 46, 642–648. [Google Scholar] [CrossRef]
- Shu, Q.; Luo, Q.; Wang, L.; Chou, K. Effects of MnO and CaO/SiO2 Mass Ratio on Phase Formations of CaO-Al2O3-MgO-SiO2-CrOx Slag at 1673 K and PO2 = 10−10 atm. Steel. Res. Int. 2015, 86, 391–399. [Google Scholar] [CrossRef]
- Idriss, K.A.; Sedaira, H.; Dardeery, S. Spectrophotometric Determination of Water-Soluble Hexavalent Chromium and Determination of Total Hexavalent Chromium Content of Portland Cement in the Presence of Iron (III) and Titanium (IV) Using Derivative Ratio Spectrophotometry. Am. J. Anal. Chem. 2013, 4, 653–660. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Chen, Z.; Shen, J.; Wang, B.; Fan, L. The enhancement effect of pre-reduction using zero-valent iron on the solidification of chromite ore processing residue by blast furnace slag and calcium hydroxide. Chemosphere 2015, 134, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Hwang, H.W.; Qureshi, T.I.; Kim, Y.J. Comparative study on the leaching characteristics of industrial sludge and fly ash using KSLP and TCLP techniques. J. Chem. Soc. 2010, 32, 538–643. [Google Scholar]
- Zhao, Q.; Liu, C.; Cao, L.; Zheng, X.; Jiang, M. Effect of Lime on Stability of Chromium in Stainless Steel Slag. Minerals 2018, 8, 424. [Google Scholar] [CrossRef]
- Wu, X.; Dong, X.; Wang, R.; Lv, H.; Cao, F.; Shen, X. Crystallization Behaviour of Chromium in Stainless Steel Slag: Effect of Feo and Basicity. J. Residuals Sci. Technol. 2016, 13, S57–S62. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.; Cao, L.; Zheng, X.; Jiang, M. Stability of Chromium in Stainless Steel Slag during Cooling. Minerals 2018, 8, 445. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, J.; Mou, Q.; Zhu, H.; Xue, Z. Effect of FeO on Spinel Crystallization and Chromium Stability in Stainless Steel-Making Slag. JOM 2019, 71, 2331–2337. [Google Scholar] [CrossRef]
- Mou, Q.Q.; Li, J.L.; Zeng, Q.; Zhu, H.Y. Effect of Fe2O3 on the size and components of spinel crystals in the CaO-SiO2-MgO-Al2O3-Cr2O3 system. Int. J. Miner. Met. Mater. 2019, 26, 1113–1119. [Google Scholar] [CrossRef]
- Sørensen, P.M.; Pind, M.; Yue, Y.Z.; Rawlings, R.D.; Boccaccini, A.R.; Nielsen., E.R. Effect of the redox state and concentration of iron on the crystallization behavior of iron-rich aluminosilicate glasses. J. Non-Cryst. Solids 2005, 351, 1246–1253. [Google Scholar] [CrossRef]
- Rezvani, M.; Eftekhari-Yekta, B.; Solati-Hashjin, M.; Marghussian, V.K. Effect of Cr2O3 Fe2O3 and TiO2 nucleants on the crystallization behaviour of SiO2–Al2O3–CaO–MgO(R2O) glass-ceramics. Ceram. Int. 2005, 31, 75–80. [Google Scholar] [CrossRef]
- Han, C.; Jiao, Y.; Wu, Q.; Yang, W.; Yang, H.; Xue, X. Kinetics and mechanism of hexavalent chromium removal by basic oxygen furnace slag. J. Environ. Sci. 2016, 46, 63–71. [Google Scholar] [CrossRef] [PubMed]


| No. | CaO | SiO2 | MgO | Al2O3 | Cr2O3 | FeO | Fe2O3 | B |
|---|---|---|---|---|---|---|---|---|
| C1 | 46.67 | 33.33 | 8.00 | 6.00 | 6.00 | 8.00 | 2.00 | 1.40 |
| C2 | 46.67 | 33.33 | 8.00 | 6.00 | 6.00 | 8.00 | 5.00 | 1.40 |
| C3 | 46.67 | 33.33 | 8.00 | 6.00 | 6.00 | 8.00 | 8.00 | 1.40 |
| C4 | 46.67 | 33.33 | 8.00 | 6.00 | 6.00 | 8.00 | 12.00 | 1.40 |
| Activity | 2 wt% | 5 wt% | 8 wt% | 12 wt% |
|---|---|---|---|---|
| CaO | 0.0098 | 0.0096 | 0.0091 | 0.0081 |
| SiO2 | 0.0104 | 0.0111 | 0.0120 | 0.0138 |
| α-C2S | 0.8049 | 0.8141 | 0.7947 | 0.7319 |
| K | 7.58 × 105 | 6.88 × 105 | 6.05 × 105 | 4.70 × 105 |
| Fe2O3 | O | Mg | Al | Si | Ca | Cr | Fe |
|---|---|---|---|---|---|---|---|
| 2 wt% | 51.18 | 9.89 | 4.57 | 2.73 | 3.02 | 19.60 | 9.03 |
| 5 wt% | 54.37 | 9.46 | 3.56 | 2.57 | 2.80 | 16.35 | 10.91 |
| 8 wt% | 54.30 | 10.33 | 4.02 | 2.41 | 2.49 | 14.37 | 12.09 |
| 12 wt% | 54.01 | 9.85 | 3.83 | 1.15 | 1.39 | 12.79 | 16.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Li, J.; Yu, Y.; Zhu, H. Occurrence and Leaching Behavior of Chromium in Synthetic Stainless Steel Slag Containing FetO. Minerals 2021, 11, 1055. https://doi.org/10.3390/min11101055
Zeng Q, Li J, Yu Y, Zhu H. Occurrence and Leaching Behavior of Chromium in Synthetic Stainless Steel Slag Containing FetO. Minerals. 2021; 11(10):1055. https://doi.org/10.3390/min11101055
Chicago/Turabian StyleZeng, Qiang, Jianli Li, Yue Yu, and Hangyu Zhu. 2021. "Occurrence and Leaching Behavior of Chromium in Synthetic Stainless Steel Slag Containing FetO" Minerals 11, no. 10: 1055. https://doi.org/10.3390/min11101055
APA StyleZeng, Q., Li, J., Yu, Y., & Zhu, H. (2021). Occurrence and Leaching Behavior of Chromium in Synthetic Stainless Steel Slag Containing FetO. Minerals, 11(10), 1055. https://doi.org/10.3390/min11101055






