Abstract
Cadmium exchange on clinoptilolite is performed and structurally studied for different durations of the ion exchange process (2 h, 24 h, 72 h, 168 h, 12 days, 22 days) at room temperature and 90 °C. The distribution of Cd2+ ions in all samples is elucidated after exchange on clinoptilolite using powder XRD data processed by Rietveld structural software. Clinoptilolite is not selective for cadmium cations, but at 90 °C the exchange is ~2.5 cations per unit cell. At RT it reaches ~1.25 cations per unit cell being twice as low. The obtained maximum exchanged sample for 22 days 90 °C was structurally refined in order to find the cadmium positions in the clinoptilolite voids. The structural refinements of the occupations of the incoming and outgoing cations give an idea of how the intracrystalline diffusion is processed. A good correlation between results obtained by structural refinement of the Cd-exchanged samples and the data of the EDS measurements was achieved.
1. Introduction
Natural zeolites possess attractive adsorption, cation-exchange, selective sorption, dehydration–rehydration, and catalysis properties, which contribute directly to their use in many fields including agriculture, environment protection, construction, pharmacy, petroleum technology, etc. [1,2].
In Bulgaria, the zeolitized pyroclastic rocks are related to the Late Eocene–Early Oligocene intermediate to acid volcanism in the Eastern Rhodopes [3,4,5]. One of the largest zeolite deposits explored so far in the Eastern Rhodopes is the clinoptilolite Beli Plast deposit. The average mineral composition of the zeolitized tuffs from the Beli Plast deposit (calculated on the base of 56 samples) is: 80% clinoptilolite, 6.2% montmorillonite, 2.9% illite and celadonite, 1% opal-CT, 0.6% calcite, 10.5% crystal- and litho-clasts [5]. According to Yanev et al. [4], the zeolitized pyroclastics contain zeolitized glass shards with zones of platy clinoptilolite crystals and idiomorphic clinoptilolite crystals.
Clinoptilolite is the most abundant natural zeolite of HEU-type (heulandite-clinoptilolite series). Heulandite and clinoptilolite are isostructural minerals of monoclinic symmetry—the space group C2/m is the maximum symmetry which may be lowered to C2, Cm, , and C1 [6]. The specific microporous HEU-type crystal structure is characterized by two-dimensional system of large channels built of 8- and 10-membered SiO44− and AlO45− tetrahedral rings. Skeletal charge-compensating cations (Na+, K+, Ca2+, Mg2+, and Ba2+) and coordinating H2O molecules are located in the channels [6,7,8]. According to the Zeolite Subcommittee of the Commission on New Minerals and Mineral Names of the International Mineralogical Association [9], heulandite is defined as the zeolite mineral series having the distinctive framework topology of heulandite and the ratio Si/Al < 4, while clinoptilolite is defined as the series with the same framework topology and Si/Al > 4.
Clinoptilolite as a low-cost sorbent has been intensively investigated for the removal of heavy metals including cadmium [10]. Environmental pollution has been assessed as one of the most important problems, which raises serious concerns about human health and the state of ecosystems. The presence of cadmium in the environment is mainly due to its use in different industries such as metallurgy, battery manufacturing, electroplating, smelting, cadmium-rich phosphate fertilizers, ceramics, plastics, pigments, etc. Cadmium is one of the most toxic heavy metals even at low concentrations. A wide range of methods has been developed for the removal of heavy metals from water and contaminated soils to decrease their impact on the environment. The ion-exchange method is generally considered to be a simple, relatively low-cost, and effective method for the removal of hazardous metals.
The published studies describe various aspects of clinoptilolite application as an ion exchanger for cadmium. The wide variability of the cation exchange properties of clinoptilolite dependent on its original extraframework cationic composition [11,12].
Stefanova et al. [13] used three chemical varieties of natural clinoptilolite samples (clinoptilolite-Na, clinoptilolite-K, and clinoptilolite-Ca) to study the equilibrium and kinetics of sorption of zinc and cadmium on zeolite rocks from different localities in Bulgaria. It has been found that clinoptilolite-Ca may be used as a sorbent for zinc and cadmium.
Kesraoui-Ouki et al. [14] investigated natural and sodium modified clinoptilolite for cadmium removal and demonstrated that Na-form improves significantly the exchange capacity. Similar studies on clinoptilolite-bearing rocks from Metaxades (Thrace, Greece) have been carried out by Misaelides et al. [15] and an improvement of Cd uptake ability was observed in the case of NaCl-pretreated material.
The study of Langela et al. [11] dealt with the exchange equilibria of the pre-exchanged Na-form of the Sardinian clinoptilolite with some hazardous cations including cadmium. Cation exchange features confirmed the low selectivity for Cd2+.
Mier et al. [16] described the interactions of Pb(II), Cd(II), and Cr(VI) competing for ion-exchange sites in natural clinoptilolite and the presence of phenol as organic co-contaminant and the effect of pH on the performance of multi-component ionic exchange were also investigated.
Vasylechko et al. [17] presented results of the investigation of adsorption of trace amounts of cadmium(II) ions from aqueous solutions on acid-modified clinoptilolite of the Ukrainian Transcarpathian region under dynamic conditions. The conditions of formation of clinoptilolite H-forms and the influence of preliminary thermal treatment of adsorbent on the concentration of cadmium have been investigated. It has been determined that the optimum concentration of cadmium(II) is the H-form of clinoptilolite obtained without considerable dealumination.
A spectroscopic study of cadmium sorption on natural Na-clinoptilolite has also been performed [18]. Changes in the IR spectra in the pseudo-lattice region are connected mainly with the ion-exchange process (the exchange of non-tetrahedral cations).
The relationships among soil microbial biomass, pH, and availability of heavy metal fractions including cadmium in long-term contaminated soils with the amendment of natural clinoptilolite were evaluated by Mühlbachová et al. [19].
Wingenfelder et al. [20] studied the influence of pH and grain size on the efficiency of clinoptilolite to remove heavy metals from synthetic mine waters, with the emphasis on the zeolite’s behavior toward divalent cations in competition with each other and found that the uptake of Cd decreased with low pH and high iron concentrations.
Arámbula-Villazana et al. [21] considered the cadmium retention behavior on Mexican zeolitic rich tuff as a function of temperature. The maximum cadmium adsorption capacity by the zeolitic material was at 318 K.
Berber-Mendoza et al. [22] published the results of the effect of pH and temperature on the ion-exchange equilibrium of Pb(II) and Cd(II) on clinoptilolite from different sources and found that the zeolite was much more selective toward Pb2+ than Cd2+ as the solution pH was reduced.
The study of heavy metal (Ni2+, Cu2+, Pb2+, and Cd2+) uptake from single- and multicomponent synthetic aqueous solutions by raw and pretreated forms of the natural clinoptilolite under static conditions was reported by Sprynskyy et al. [23]. A slight difference between the adsorption capacity of the clinoptilolite toward cadmium from single- and multi-component solutions was observed.
Castaldi et al. [24] used X-ray powder diffraction to investigate the changes of clinoptilolite structure caused by the exchange with cadmium and concluded that the incorporation of Cd2+ into the zeolite frameworks do not change significantly the lattice parameters. The authors noticed that the X-ray peaks of the zeolite samples exchanged with cadmium are broadened per se beyond the instrumental conditions. This broadening was associated with the simultaneous effects from a reduced size of the microcrystals (crystallites) and to an increased amount of lattice disorder (microstrain).
The increase in Cd removal capacity was discussed by Gedik and Imamoglu [25] in terms of the progressive conversion of clinoptilolite to its homoionic Na-form, as well as the presence of other removal mechanisms accompanying ion exchange.
Faghihian and Nejati-Yazdinejad [26] have studied the sorption of Cd(II) and Pb(II) ions by clinoptilolite under different experimental conditions in order to ascertain their potential as sorbents for the removal of these metal ions from aqueous solutions. The authors found that the removal of Pb(II) ions was much greater than that of Cd(II) ions under identical experimental conditions.
Cadmium removal from aqueous solution on natural clinoptilolite and modified Na- and NH4-forms has been reported by Ruiz-Serrano and Ramírez-Bon [27]. The results showed that Cd removed from the aqueous solution and the amount of Cd loaded in clinoptilolite are higher for the modified Na- and NH4-forms in the complete range of Cd concentrations analyzed. In contrast, Stefanova et al. [13] noticed that the ion exchange of cadmium is strongly hindered by the ammonium cations.
Many researchers used different clinoptilolite modified forms in order to enhance its sorption properties for cadmium uptake: incorporation of magnetite nanoparticles and surface modification with cysteine [28]; sodium- and acid-modified forms [29]; acid-modified form [30]; Na-modified synthetic clinoptilolite [31]; pentetic acid-clinoptilolite nanoparticles adsorbent [32]; sonochemically modified clinoptilolite [33].
For ion exchange applications mainly clinoptilolite is used because this mineral is available in large quantities. However, for single-crystal diffraction experiments, heulandite is applicable due to the large size of the crystals. A single-crystal study of fully Cd-exchanged heulandite was published by Stolz et al. [34]. Crystal structure refinements were performed in the space groups C2/m, C2, Cm, C–1, and C1, of which only the Cm model yielded reasonable agreement with the observed diffraction data in contrast to most other natural and cation-exchanged heulandites that commonly show C2/m symmetry. Symmetry lowering was attributed to Si/Al ordering in the tetrahedral framework as well as to the asymmetrical distribution of Cd2+ ions. Subsequently, Doebelin and Armbruster [6] studied the dehydration mechanisms of a Cd-exchanged heulandite as a function of temperature. The crystal structures of the 50–200 °C data sets were refined in space group Cm, while the 250 °C data were refined in C2/m.
The Rietveld X-ray powder diffraction method is a full-profile approach based on the least-squares fit between the calculated diffraction pattern and the observed experimental data. This method gives the possibility not only to refine the crystal structural parameters such as unit cell parameters, atomic coordinates, site occupancies, etc., but also permits additional information for the microstructural characteristics (crystallite size and strain) to be extracted from the powder diffraction pattern.
Various techniques have been used to improve the selective properties of clinoptilolite by reducing the size of the crystallites and increasing the surface area of the zeolite material. Mechanical tribo- and thermo-chemical treatment are the most often used methods to lower the size of the crystallites in the nanoscale range [35,36,37]. Iazdani and Nezamzadeh-Ejhieh [38,39] estimated, using the Scherrer equation, the average crystallite size of the pretreated via the ball-mill method clinoptilolite nanoparticles and FeO- and Ni-modified clinoptilolite samples. The value of the crystallite size obtained for the pretreated natural clinoptilolite is 56 nm. Acid treatment of clinoptilolite followed by incorporation of Ce or Ce and Pd was used for a significant change of the samples’ morphology to nanoscale [40,41,42].
The aim of the present study is to elucidate the distribution of Cd ions after the exchange on purified microcrystalline clinoptilolite in different time intervals both at room temperature and 90 °C using powder XRD data processed by Rietveld structural software. This approach is helpful to follow the internal diffusion of Cd2+ ions and H2O molecules as well as the outgoing original cations from the clinoptilolite structure, which is of special microstructural importance.
2. Materials and Methods
2.1. Materials
The studied material is clinoptilolite tuff from Beli Plast deposit (Eastern Rhodopes, Bulgaria) rich in clinoptilolite (80 wt%, proved by quantitative XRD analysis). After grinding and sieving the fraction with grain size 0.032–0.016 0.016–0.032 mm (richest in clinoptilolite) was used for further experiments. The minerals from the clinoptilolite tuff were removed by heavy liquid separation yielding a clinoptilolite sample with only a small amount (6–7 wt%) of opal-C. The removal of opal-C is performed by using careful chemical treatment with a solution of NaOH. All the above stages are described in detail by Dimowa et al. [43]. Purified natural clinoptilolite of (Na1.01Ca1.77K1.11Mg0.49)Al6.39Si29.43O72 20.69 H2O composition was used for experiments.
2.2. Cation Exhange
The cadmium exchange experiments were performed using 8 g of purified clinoptilolite ground to below 20 μm in 240 mL 0.5 M solution of Cd(NO3)2 in sealed test glass tubes for two temperature regimes—room temperature (RT) and 90 °C. For the exchange at 90 degrees, we used muffle furnace with temperature control. The duration of ion exchange is 22 days. The solutions were changed by fresh ones at 48 h, 96 h, and 192 h. Samples for analyses were taken at 2 h, 24 h, 72 h, 168 h, 12 days, and finally on the 22nd day. Each obtained sample was carefully washed with distilled water.
A temperature of 90 degrees was used to enhance the cadmium exchange. The experiment for 22 days was performed to reach a complete exchange of cadmium on clinoptilolite and almost a plateau was achieved between the 12th and 22nd day. This reaction time was proved to be sufficient for reaching equilibrium. Our trials for more days of exchange gave the same results.
2.3. SEM/EDS
In order to obtain the chemical composition of each Cd exchanged sample EDS/SEM analyses were performed by scanning electron microscope (SEM) JEOL JSM6390 (JEOL technics Ltd., Tokyo, Japan) coupled with an Oxford Instruments energy-dispersive X-ray (EDX) analyzer (Oxford Instruments Analytical Limited, High Wycombe, Buckinghamshire, England), with an acceleration voltage of 20 kV.
Pellets from the powder of purified natural and Cd-exchanged clinoptilolite were prepared and coated with carbon for EDS analyses. The reported concentrations for each sample are average values from the ten measurements carried out on the pellet.
2.4. X-ray Diffraction Studies
Powder XRD measurements were performed using a PANanalytical EMPYREAN Diffractometer system with a goniometer radius of 240 mm, operating at 40 kV and 30 mA, Theta–Theta geometry, with HDD Cu Kα radiation, equipped with 3Dpixel detector. XRD patterns were recorded in Bragg–Brentano geometry at room temperature from 3 to 100 °2θ with a scanning rate of 0.013°/80 s.
The unit-cell parameters, atomic positions, and site occupancies of cations and H2O molecules in the clinoptilolite structure at different stages of the exchangeable process were obtained by the Rietveld method [44] implemented in the Topas software [45]. The background was fitted by a Chebyshev polynomial with 20 coefficients. Topas software use classical Scherrer equation for crystallite size. The average crystallite size of the initial and Cd2+ exchanged samples were calculated by the integral breadth method by fitting experimental profiles to pseudo-Voigt functions and without instrumental correction. The obtained crystallite size varies between 175 and 180 nm.
For all Cd-exchanged clinoptilolite samples, the refinement started with the clinoptilolite structural models of Cappelletti et al. [46] ICSD #87846.
3. Results
3.1. Powder XRD and EDS Data of Cd-Exchanged Clinoptilolite
Figure 1 shows all the diffraction patterns of the exchanged samples at 90 °C and at RT. Comparing the patterns of exchanged samples from 2 h and the next taken at 24 h, 72 h, 168 h, 12 days, and 22 days, we observed significant intensity changes of some peaks in diffraction patterns, namely—peaks 020 and 200 decrease, while increases. There are other peaks slightly affected during ion-exchange procedures such as , 220, and 111, which decrease, and 001 which increases.
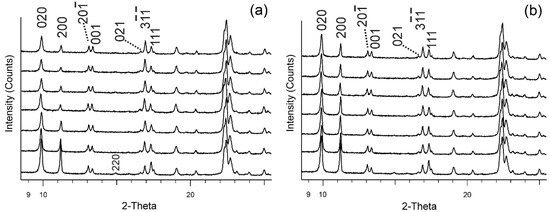
Figure 1.
Powder XRD patterns of the purified clinoptilolite (bottom—left and right) and the Cd-exchange series of clinoptilolite at 90 °C (a) and RT (b) (from second pattern to top—2 h, 24 h, 72 h, 168 h, 12 days, and 22 days of ion exchange process).
Comparing the intensity changes of the peaks 020 and in sample RT 22 day with powder patterns of Cd exchange series at 90 °C, we notice that sample RT 22 day is similar to 2 h and 24 h ones at 90 °C (Figure 2).
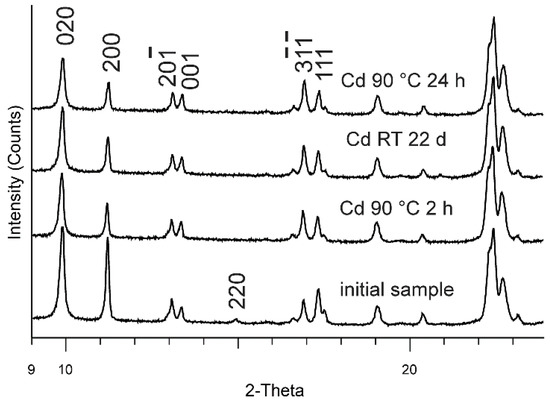
Figure 2.
Selected powder XRD patterns of Cd-exchanged clinoptlolite.
The performed EDS analyses give numeric data (Table 1 and Table 2) for cadmium composition during the exchange procedures, which show specific trends of exchange.

Table 1.
Chemical composition (EDS, wt%) and structural formulae of Cd-clinoptilolite (RT) based on 72 oxygen atoms.

Table 2.
Chemical composition (EDS, wt%) and structural formulae of Cd-clinoptilolite (90°) based on 72 oxygen atoms.
3.2. Rietveld Structural Analyses of Cd2+ Exchange on Clinoptilolite with Time and Temperature
The maximum Cd2+ exchanged clinoptilolite sample (90 °C, 22 days) is structurally studied by using the Rietveld method. The final refinement converged at acceptable reliability factors (Table 3) where all the factors of the Cd exchange series at 90 °C are presented. Data from Table 3 shows that the unit cell parameters of all studied Cd-exchanged samples are very similar except slight increase of the volume, V, from 2114.2 Å3 (sample 90 °C, 2 h) to 2115.5 Å3 (sample 90 °C, 22 days).

Table 3.
Reliability factors, crystallite size, and unit cell parameters of initial and Cd-clinoptilolite from the ion exchange sequence at 90 °C.
Topas software uses the classical Scherrer equation for crystallite size determination. The average crystal size of the initial and Cd2+-exchanged samples was calculated using the pseudo-Voigt function for profile fitting and integral breadth method. The crystallite size varies between 175 nm and 180 nm.
The difference powder XRD plot of sample Cd-clinoptilolite (90 °C, 22 days) after the final refinement stage is shown in Figure 3.
Figure 3.
Difference plot of the powder XRD experimental and calculated pattern of Cd-clinoptilolite (90 °C, 22 days).
Finishing the structural refinements of all Cd-exchanged clinoptilolite we obtain the values for the structural parameters. For the samples (90 °C, 22 days) and (90 °C, 2 h) these values are given in Table 4 and the interatomic distances are shown in Table 5.

Table 4.
Atomic coordinates, Wyckoff positions, and occupancy factors of samples Cd-clinoptilolite (90 °C, 22 days) and Cd-clinoptilolite (90 °C, 2 h).

Table 5.
Interatomic distances of the extra-framework cations and H2O molecules of samples Cd-clinoptilolite (90 °C, 22 days) and Cd-clinoptilolite (90 °C, 2 h).
4. Discussion
4.1. Powder XRD and EDS Data Interpretation of Cd-Exchanged Clinoptilolite
The data from the EDS analysis shows that cadmium is better exchanged in the 90 °C series (Figure 4). The maximum exchanged sample is at 22 days with 2.55 Cd ions per unit cell. In the series exchanged at room temperature for 22 days, cadmium reached a value of 1.23 per unit cell. The ion exchange process in both series begins relatively intensively, especially during the interval up to 24 h and continues like that until the 72-nd hour and then slows down with time, mostly after the 168th hour. The values of cadmium in the RT series in all samples are half and lower than those of the 90 °C series.
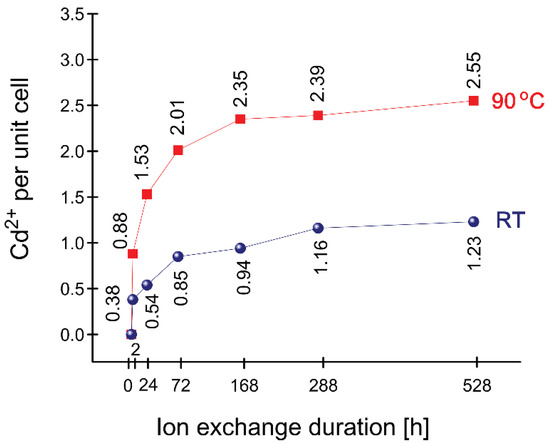
Figure 4.
Exchanged Cd2+ for different duration times and temperatures (EDS data).
In comparison to the sample of initial clinoptilolite, the intensity changes of some peaks are observed in the samples from 2 h to 22 days in both series 90 °C and RT (Figure 1). In the series at 90 °C (Figure 1a), the decreased peaks are 020, 200, , 111 as well as 220, which completely disappeared. The intensity of peak increases significantly and some others such as 001 and 021 increase less. The series at RT exchange is shown in Figure 1b, with the initial sample again at the bottom. Here, the intensity changes are weak compared to the series at 90 °C but are visible even in the second hour. The pair of peaks and 111 reacts to Cd uptake by changing their intensities visibly equalizing after two hours in cadmium solution. Comparing the Cd samples with the initial non-exchanged one it is observed that the peak is higher than the 111 peak. After different hours of exchange for both series, these peaks reversed their ratio and with the progress of the exchanged process and the peak becomes visibly higher than the 111 one. In general, peak intensity changes resulting from cadmium uptake are relatively weaker in the series at RT than in that at 90 °C.
The exchange of clinoptilolite with heavy cations reduced the intensity of 020 and 200. This remarkable intensity decrease in the peaks 020 and 200 was observed and commented on by Petrov et al. [47]. A detailed explanation was given by Petrov [48]. The author presented crystal chemical calculations for the intensity change of the 020 peak, theoretically showing the change of the structural factor F2020 value related to different compositions of the exchangeable cationic complex in clinoptilolite, modeling the content with Li+, Na+, Ca2+, K+, Ba2+, Cs+, and Tl+ ions in the plane of symmetry of the structure.
A more precise study than visual comparison of diffraction patterns was performed and the values of the integral intensities of the peaks 020 and were measured in order to make an assumption about the degree of cadmium exchange in the 22 days sample at RT (Figure 5).
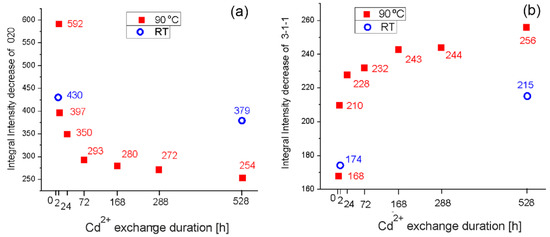
Figure 5.
Integral intensities of 020 (a) and (b) peaks of Cd-clinoptilolite for different exchange times and temperatures.
Figure 5 shows that the peak integral intensity of 020 in the sample at 22 days RT is lower (379) than that at 2 h 90 °C (397), which means a higher degree of cadmium exchange in the sample at 22 days RT. Moreover, the integral intensity of the peak has a value of 215, which is between samples 2 h and 24 h 90 °C. The peak increases as cadmium exchange progresses and this means that Cd content is slightly higher at 22 days RT in comparison to the sample at 2 h 90 °C. These data suggest that the longest exchanged sample at RT (for 22 days) has cadmium content between samples at 2 h and 24 h (90 °C).
Figure 5 also shows the values of the integral intensities of peaks 020 and for the sample at 2 h RT. For the peak 020, there is a decrease in the value of the integral intensity (430 compared to 592 of the initial non cadmium sample). For the peak, the value of the integral intensity slightly increased relative to the same one in the initial sample (174 versus 169). The intensity changes of the sample with the shortest Cd2+ exchange time—2 h RT—show that small amounts of cadmium cations are present in the voids of clinoptilolite structure during the first 2 h of Cd2+ exchange process and slightly affect the above-commented peak intensities.
The integral intensities changes of the peaks sensitive to heavier cations with the progress of the ion uptake indicate that this process is realized successfully. In order to obtain specific data for ingoing (Cd2+) and outgoing (Na+, Ca2+, K+, and Mg2+) cations, it is necessary to use other methods.
4.2. Rietveld Structural Interpretation of Natural and Cd2+ Exchanged Clinoptilolite with Time and Temperature
The initial structural refinement was performed on the purified natural clinoptilolite. The C2/m space group was confirmed and the refined atomic positions correspond well to the available published data [7,46,49].
The structurally refined Cd samples were taken at different hours of the current ion exchange for both series at 90 °C and RT. This approach allows specifying the changes of the occupation of Cd positions, as well as the outgoing cations during the exchange process. These changes of the position occupancy are related to the movement of the cations through the voids of the clinoptilolite porous structure and clarify the logistic and transport possibilities of Cd and outgoing cations. In the structure of natural clinoptilolite, four cationic positions (M1, M2, M3, and M4) were proved [49] to be occupied by the naturally occurring Na, Ca, K, and Mg, respectively (Figure 6). The negative charge of the framework comes mainly from the bridging oxygen atoms O1 of tetrahedron T2, keeping the extra-framework cations in the plane of symmetry, and the preferred sites are occupied by particular cations depending on their charge and size. Positions M1 and M2 accommodate cations with a radius of about 1.0 Å (Na+, Ca2+). Position M3 is occupied by cations with larger ionic radii such as K+ (1.31 Å) and Ba2+ (1.35 Å). Position M4 is the only one, which is not coordinated by framework oxygens and is usually occupied by Mg2+, or small divalent cations surrounded only by H2O molecules. The cation positions are usually partially occupied [50,51].
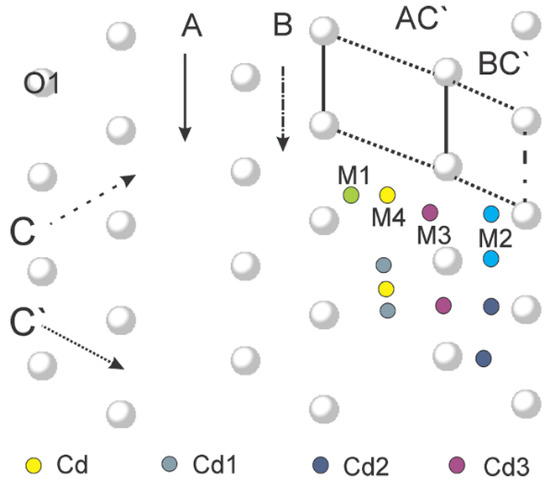
Figure 6.
Gallery representation of the structure of Cd-clinoptilolite: distribution of O1 atoms in the plane (020), with directions of lanes A, B, C, and C’ and location of the extra-framework cation positions and Cd positions.
The designed initial model of the Cd-clinoptilolite structure was based on the data of Cappelletti et al. [46] for natural clinoptilolite from volcanoclastites of North-Western Sardinia (monoclinic system, space group C2/m). The specific moment was to model the Cd populations—about 2.5 atoms per unit-cell according to the EDS data (Figure 3) at probable position M4 which is occupied by small divalent cations such as magnesium, zinc, and others. According to Dimowa et al. [35], zinc occupies M4 and also affects the intensity changes of the peaks 020 and in a similar way as in the cadmium form.
A lower symmetry (Cm) also has been reported for heulandite by Stolz et al. [34] and Dobelin and Armbruster [6]. There are no indications in our case for a lowering of the symmetry after the exchange with Cd2+. The reason for symmetry lowering, as the authors point out, is aluminum distribution in the tetrahedral framework as well as the asymmetrical distribution of Cd2+ ions.
After a number of refinement cycles of sample Cd-clinoptilolite (90 °C, 22 days) (Figure 6) we found that when refining the position M4 with placing Cd its occupancy increases from 0.4 Mg2+ for initial clinoptilolite (Table 6) to 0.415 Cd2+ (Table 4) or 0.83 Cd2+ per unit cell (Table 6) for 22 days at 90 °C. As we know from the chemical data (Table 2) in the sample 22 days 90 °C there is no magnesium, therefore in position M4, only cadmium may be refined. This is not the case for the previous samples (exchanged for a shorter time) where the EDS data show the presence of magnesium, which partially occupies the M4 position.

Table 6.
Number of atoms per unit cell in the cationic positions of initial and Cd-exchanged clinoptilolite (Cpt) at 90 °C.
In the initial sample, the M4 position is occupied by magnesium only. Cadmium ions gradually occupy M4 during the ion exchange process. When decoding occupancy of position M4 it is not possible to distinguish between magnesium or cadmium. Therefore, the results are somewhat inaccurate for the samples: 2 h, 24 h, 72 h, 168 h, 12 d at 90 °C, and all from the RT series.
In our study position M4 is named position Cd (Figure 6). The refined occupation for position Cd obtained by powder XRD data indicates less Cd2+ than the results given by the EDS data. Thus, it means that cadmium occupies other positions. During the refinement, another original position M2 increases its occupancy and indicates the presence of cadmium.
Assuming that cadmium cations occupy position M2 and refining their atomic coordinates, the reliability factors of the results were improved. This position is named Cd2 and it is situated very close to the calcium position M2 at a distance of 0.72 Å (Table 5). The position Cd2 is also sufficiently occupied 0.255 or 1.02 per unit cell of Cd2+ (Table 4 and Table 6).
According to the data from the EDS analysis, at least about 0.6 cadmium ions per unit cell should be additionally exchanged. During the refinement, there occurred positions, which increased their occupancy, and cadmium was refined in them. Using this approach, it is assumed that there could be two more cadmium positions that are less populated than the previous ones—Cd and Cd2. These positions are named Cd1 (at a distance 1.81 Å from M4 Table 5) and Cd3 (0.13 Å from M3) with occupancy of 0.073 and 0.092 Cd2+ or (0.29 and 0.37 Cd2+ per unit cell), respectively. Thus, the data obtained by the Rietveld structural analysis show a total amount of 2.51 cadmium ions per unit cell, which is very close to the data from the EDS analysis.
Our results show that during the ion-exchanged process the unit cell parameters remain almost similar. The same results were obtained by Castaldi et al. [24]. However, in our case, slight changes are observed for the unit cell parameters during the cadmium exchange. The tendency is a slight increase in the volume (2108.3–2116.6) and a decrease in the β angle (116.32–116.25) (Table 3). The measured size of the clinoptilolite crystallite is almost constant (~176(3) nm). This is an indication that the ion exchange process does not cause crystal defragmentation and nor increased size domain. No significant differences of apparent crystallite size between exchanged and initial clinoptilolite are observed. The high values of found crystallite sizes reflect no line broadening and is probably related to the well-formed crystals in the zones with platy and idiomorphic clinoptilolite [4].
The crystallite size in our work is greater in comparison with a similar study [24]—175–180 nm vs. 90–110 nm, which is due to a different degree of the zeolitization processes that have proceeded with time. Much lower values of the crystallite size are obtained by mechanical, thermal, and chemical pre-treatment of clinoptilolite—between 10 nm and 65 nm [35,36,37,38,39,40,41,42].
4.3. Movement of H2O Molecules and Cd2+ Cations in the Clinoptilolite Structure during Cadmium Exchange
In the voids of the clinoptilolite structure of the almost completely cadmium exchanged sample, nine H2O positions were specified and refined. Divalent cadmium compensates the charge from the framework with twice less Cd cations compared to the structure filling by monovalent ones in the voids. Thus, in Cd exchanged clinoptilolite the presence of more cation coordinating H2O molecules is expected. Table 4 shows the values of the atomic coordinates of the samples 2 h 90 °C and 22 days 90 °C. The lowest exchange for the 2 h sample is dominated by cation–H2O configurations similar to the initial non-cadmium sample, while in the sample exchanged for 22 days the cation–H2O configurations created by cadmium predominate. For this reason, many H2O molecules change their atomic coordinates as well as their occupations. There are some H2O positions that visibly do not coordinate any cations. Their role in the specific arrangement in the voids of the structure is difficult to elucidate due to the fact that X-ray diffraction averages the results, concealing the local asymmetry of the arrangement of cations and H2O molecules and also due to the impossibility to specify low occupied H2O and cation positions by powder X-ray diffraction data only. Such H2O molecules appear to “hang” in the space of the voids, but their role is most often associated with bridge connections [52]. In cadmium clinoptilolite, the H2O molecule which does not coordinate any cation is in position O11 (Table 4 and Table 5). Position O11 is close enough to M3 so they are mutually forbidden, but they have low occupancy. Position O11 does not particularly change its occupation during the times of cadmium exchange. Position O12 coordinates Na in the initial non-cadmium sample, but in the sample at 22 days 90 °C, cadmium exchange O12 H2O molecule is not coordinating, and it does not change its atomic coordinates and its occupancy decreases. At position O13, the H2O molecule coordinates calcium but also adapts to cadmium positions that are close to M2. During cadmium exchange, this H2O position changes slightly its atomic coordinates and occupancy (Table 4 and Table 5). Position O14 coordinates Ca and then Cd during the whole ion exchange process. The H2O molecule in position O15 coordinates magnesium in position M4 in the initial non-cadmium sample. Position O15 shifts slightly and is denoted as O15’ and in this case coordinates sodium and it is observed in the initial non-cadmium sample. During ion exchange, the occupancy of O15’ decreases due to the release of sodium, and O15 increases due to the presence of cadmium in position M4. Position O15’ changes its atomic coordinates in the sample at 22 days 90 °C, and probably coordinates position Cd1. Position O16 does not change its coordinates but increases its occupancy with the advance of ion exchange. In the initial sample, position O16 coordinates sodium but also coordinates cadmium in M4. Positions O17 and O18 change their atomic coordinates and increase their occupancy with advancing cadmium exchange. Position O17 coordinates Cd in M4 and position O18 is close to Cd1. Position O19 coordinates the potassium in the initial non-cadmium sample and probably its occupancy decreases with the release of some of the potassium cations. All these changes in the positions of H2O molecules during the ion exchange process indicate intra-space diffusion, together with exchangeable cations.
Figure 7 shows the mostly occupied by cadmium cations positions in this structure: Cd and Cd1 and surrounding H2O molecules. Around the position of cadmium in M4 in close distances of 2.08, 2.17, and 2.30 Å are presented in the picture those H2O molecules that are not mutually forbidden (these are positions O15, O16, and O17, respectively).
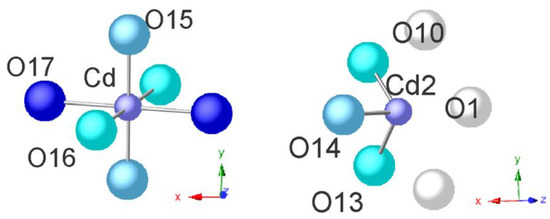
Figure 7.
Coordination spheres of the cadmium positions Cd and Cd2 in the structure of Cd-clinoptilolite (90 °C, 22 days).
Three cadmium cations could occupy the unit cell of clinoptilolite according to the Al content in the framework (6.23 Al atoms in our case). This allows more H2O molecules to occupy positions in the cavities and pores of the structure in comparison to the samples exchanged with monovalent cations. The model of Cappelletti et al. (ICSD #87846) [46], which we used for Rietveld analysis contains almost 27 H2O molecules, is suitable for the cadmium-exchanged sample in our study. Moreover, almost 20 H2O molecules per unit cell were identified in our Cd clinoptilolite by the Rietveld decoding procedure because powder diffraction methods are not suitable for detecting less populated H2O positions. Therefore, we additionally applied the TG method to obtain data for weight loss. By the TG method, the weight loss is 14.90% and after recalculation, it corresponds to 26.56 H2O molecules per unit cell for Cd clinoptilolite 22 days 90 °C. These data are similar to the results obtained by Doebelin and Armbruster [6] for initial cadmium heulandite where the formula is: Cd4.00Na0.01K<0.01Ca0.09[Al8.70Si27.30O71:75].29H2O. For comparison, the initially purified clinoptilolite (the sample of our experiments) shows about 20 H2O molecules per unit cell obtained by recalculated TG data analysis.
Cadmium is a divalent cation and in our case occupies mainly the position M4, which is in the center of the large tetrahedral channel A. In addition, around Cd2+ there are specified three H2O positions occupied by almost six H2O molecules. It can be assumed that the cadmium forms of clinoptilolite are similar to other monocationic exchanged clinoptilolites with divalent cations resulting in an increased amount of H2O molecules.
It may be commented that cadmium cation after the exchange configures in the pores of the structure-specific microstructural Cd2+–H2O configurations which in some cases my lower the symmetry (Cm) as reported by Stolz et al. [34] and Doebelin and Armbruster [6] for heulandite.
The structural data obtained from the applied Rietveld method for the total cadmium exchange show an intensive exchange up to 72 h at 90 °C; then, the exchange continues relatively less intensively up to 168 h, after which it slows down. (Figure 8) (Table 6). Figure 8a shows the trend of total cadmium exchange in the structure of clinoptilolite, which does not differ much from that obtained by EDS data. Figure 8b presents the data on the exchange of cadmium on structural positions. It is noteworthy, that up to the 2nd hour the positions have a more intense occupation with cadmium cations. After the 2nd hour, the occupation on the positions Cd and Cd2 follow the trend of total exchange, while in the other two positions Cd1 and Cd3 the occupation is very weak. Figure 8c shows the data for the occupancy of the positions of the leaving cations (Na, Ca, K). Again, we observe a relatively more intensive release up to the 2nd hour, after that the decrease is gradually up to 168 h and then the process almost subsides. No sodium was observed after 168 h but calcium and potassium do not leave the structure up to the 22nd day of exchange.
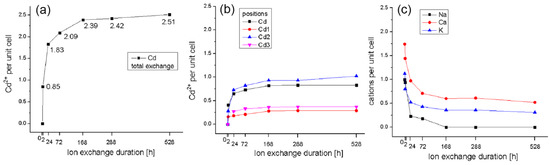
Figure 8.
Extent of Cd exchange (a), Cd sites (b) and outgoing cations (c) at 90 °C.
The results of the structural analysis of the series exchanged with cadmium at room temperature conditions show the exchange of cadmium ions with a lower value than the sample exchanged for 24 h at 90 °C (Figure 9a; Table 7). Cadmium exchange under these conditions is again more intense until the 2nd hour and then slows down without significantly decreasing until the 12th day, reaching the value of 1.27 Cd ions per unit cell, which is more than a half of the exchanged amount of cadmium during the first two hours. On the 22nd day, the value reaches 1.34. The position of cadmium in M4 is occupied relatively more intensely than the other two positions Cd1 and Cd2 (Figure 9b; Table 7) following, in general, the trend of total cadmium exchange. The position Cd1 is occupied more intensively up to the 24th hour, while the position Cd2, close to the calcium one, is occupied more intensively after the 24th hour. The outgoing cations leave the structure of the clinoptilolite relatively more intensively at the 2nd hour (Figure 9c). The leaving of all cations proceeds slowly and with a similar trend. In the case of calcium, the delay is between the 2nd and 24th hour, after which it is relatively more intense. It is noteworthy that sodium and potassium leave the clinoptilolite structure to some extent, although no cadmium positions are observed near their positions.
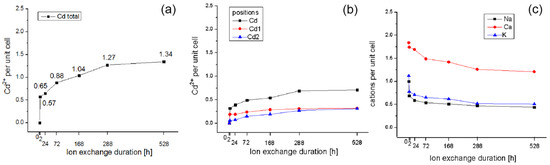
Figure 9.
Extent of Cd exchange (a), Cd sites (b), and outgoing cations (c) at RT.

Table 7.
Number of atoms per unit cell in the cationic positions of initial and Cd-exchanged clinoptilolite (Cpt) at RT.
5. Conclusions
Ion exchange of cadmium on clinoptilolite was studied at two temperature regimes: 90 °C and RT for a period up to 22 days. Clinoptilolite is not selective for cadmium cations, but at 90 °C the exchange is ~2.5 cations per unit cell. At RT it reaches ~1.25 cations per unit cell being twice as low. At high temperature, half of the amount of 2.5 Cd2+ is exchanged between the 2nd and 24th hour, and at room temperature between the 24th and 72nd hour. The obtained maximum exchanged sample for 22 days 90 °C was structurally refined in order to find the cadmium positions in the clinoptilolite voids. Two principle Cd positions have been specified: the first one is usually occupied by magnesium and another one is very close to the calcium position M2. The distribution of cadmium in structural positions is followed in samples taken at different stages of the 22-day ion-exchange regime. The major amount of exchanged cadmium cations accumulates at the two positions mentioned above. Some cadmium ions occupy other less populated positions, but the exchange there is much slower. For the entire period of 22 days, only sodium and magnesium leave the structure at 90 °C but not in the initial stages. It is noteworthy that K+ which is far from the cadmium positions is not inert and also partially leaves the structure. The positions of the H2O molecules, as well as their occupancies, are specified for certain stages of ion exchange. Most of the H2O molecules change their atomic coordinates and occupancies by adapting to the incoming cadmium cations or remain connected to the previous configurations. All these changes in the positions of H2O molecules during the ion exchange process show their diffusion, accompanied by the exchangeable motions of cations. Incoming Cd2+ prefers certain positions but small portions of its cations are dispersed in other less preferred locations. The charge and size of the cations are important but also the distribution of aluminum in the framework plays a significant role. Therefore, the cations do not completely fill their preferred positions, and some of them are distributed in more atypical positions.
Author Contributions
Conceptualization, L.D.; methodology, L.D. and Y.T.; software, L.D. and Y.T.; investigation, L.D. and Y.T.; writing—original draft preparation, L.D. and Y.T.; writing—review and editing, L.D. and Y.T.; visualization, L.D. and Y.T.; funding acquisition, L.D. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BULGARIAN NATIONAL SCIENCE FUND, grant number KП-06-H44/3/27.11.2020.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the technical support from the project PERIMED BG05M2OP001-1.002-0005/29.03.2018 (2018–2023) and are thankful to the reviewers for their constructive comments and suggestions, which helped us to improve considerably the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [Green Version]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189. [Google Scholar]
- Aleksiev, B.; Djourova, E.G. On the origin of zeolite rocks. Comptes Rendus Acad. Bulg. Sci. 1975, 28, 517–520. [Google Scholar]
- Yanev, Y.; Cocheme, J.-J.; Ivanova, R.; Grauby, O.; Burlet, E.; Pravchanska, R. Zeolites and zeolitization of acid pyroclastic rocks from paroxysmal Paleogene volcanism, Eastern Rhodopes, Bulgaria. Neues Jahrb. Mineral. Abh. J. Mineral. Geochem. 2006, 182, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Yanev, Y.; Ivanova, R. Post-conference field trip guidebook Eastern Rhodopes, South Bulgaria. In Proceedings of the Zeolite 2010—8th International Conference of the Occurrence, Properties, and Utilization of Natural Zeolites, Sofia, Bulgaria, 10–18 July 2010; p. 34. [Google Scholar]
- Doebelin, N.; Armbruster, T. Stepwise dehydration and change of framework topology in Cd-exchanged heulandite. Micropor. Microporous Mesoporous Mater. 2003, 61, 85–103. [Google Scholar] [CrossRef]
- Alberti, A. The crystal structure of two clinoptilolites. Tschermaks Mineral. Petrogr. Mitt. 1975, 22, 25–37. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Balzer, R.; Pergher, S. Microporous and mesoporous materials from natural and inexpensive sources. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Khatisov, B.I., Eds.; Springer: Basel, Switzerland, 2017; pp. 1–22. [Google Scholar]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral. 1997, 35, 1571–1606. [Google Scholar]
- Godelitsas, A.; Armbruster, T. HEU-type zeolites modified by transition elements and lead. Microporous Mesoporous Mater. 2003, 61, 3–24. [Google Scholar] [CrossRef]
- Langella, A.; Pansini, M.; Cappelletti, P.; de Gennaro, B.; De Gennaro, M.; Colella, C. NH4+, Cu2+, Zn2+, Cd2+ and Pb2+ exchange for Na+ in a sedimentary clinoptilolite, North Sardinia, Italy. Microporous Mesoporous Mater. 2000, 37, 337–343. [Google Scholar] [CrossRef]
- Armbruster, T. Clinoptilotite-heulandite: Applications and basic research. In Studies in Surface Science and Catalysis; Galarneau, A., Fajula, F., Di Renzo, F., Vedrine, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 135, pp. 13–27. [Google Scholar]
- Stefanova, I.; Djurova, E.; Gradev, G. Sorption of zinc and cadmium on zeolite rocks. J. Radioanal. Nucl. Chem. 1988, 128, 367–375. [Google Scholar] [CrossRef]
- Kesraoui-Ouki, S.; Cheeseman, C.; Perry, R. Effects of conditioning and treatment of chabazite and clinoptilolite prior to lead and cadmium removal. Environ. Sci. Technol. 1993, 27, 1108–1116. [Google Scholar] [CrossRef]
- Misaelides, P.; Godelitsas, A.; Charistos, V.; Ioannou, D.; Charistos, D. Heavy metal uptake by zeoliferous rocks from Metaxades, Thrace, Greece: An exploratory study. J. Radioanal. Nucl. Chem. 1994, 183, 159–166. [Google Scholar] [CrossRef]
- Mier, M.V.; Callejas, R.L.; Gehr, R.; Cisneros, B.E.J.; Alvarez, P.J.J. Heavy metal removal with Mexican clinoptilolite: Multi-component ionic exchange. Water Res. 2001, 35, 373–378. [Google Scholar] [CrossRef]
- Vasylechko, V.O.; Gryshchouk, G.V.; Kuz’ma, Y.B.; Zakordonskiy, V.P.; Vasylechko, L.O.; Lebedynets, L.O.; Kalytovs’ka, M.B. Adsorption of cadmium on acid-modified Transcarpathian clinoptilolite. Microporous Mesoporous Mater. 2003, 60, 183–196. [Google Scholar] [CrossRef]
- Mozgawa, W.; Bajda, T. Spectroscopic study of heavy metals sorption on clinoptilolite. Phys. Chem. Miner. 2005, 31, 706–713. [Google Scholar] [CrossRef]
- Mühlbachová, G.; Šimon, T.; Pechová, M. The availability of Cd, Pb and Zn and their relationships with soil pH and microbial biomass in soils amended by natural clinoptilolite. Plant Soil Environ. 2005, 51, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Wingenfelder, U.; Hansen, C.; Furrer, G.; Schulin, R. Removal of heavy metals from mine waters by natural zeolites. Environ. Sci. Technol. 2005, 39, 4606–4613. [Google Scholar] [CrossRef]
- Arámbula-Villazana, V.; Solache-Ríos, M.; Olguín, M.T. Sorption of cadmium from aqueous solutions at different temperatures by Mexican HEU-type zeolite rich tuff. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 229–236. [Google Scholar] [CrossRef]
- Berber-Mendoza, M.S.; Leyva-Ramos, R.; Alonso-Davila, P.; Mendoza-Barron, J.; Diaz-Flores, P.E. Effect of pH and temperature on the ion-exchange isotherm of Cd(II) and Pb(II) on clinoptilolite. J. Chem. Technol. Biotechnol. 2006, 81, 966–973. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef]
- Castaldi, P.; Santona, L.; Enzo, S.; Melis, P. Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. J. Hazard. Mater. 2008, 156, 428–434. [Google Scholar] [CrossRef]
- Gedik, K.; Imamoglu, I. Removal of cadmium from aqueous solutions using clinoptilolite: Influence of pretreatment and regeneration. J. Hazard. Mater. 2008, 155, 385–392. [Google Scholar] [CrossRef]
- Faghihian, H.; Nejati-Yazdinejad, M. A comparative study of the sorption of Cd(II) and Pb(II) ions from aqueous solution by local bentonite and clinoptilolite. Adsorpt. Sci. Technol. 2009, 27, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Serrano, D.; Ramírez-Bon, R. Cd sorption from aqueous solutions by natural clinoptilolite and its modified forms clinoptilolite-Na and clinoptilolite-NH4. J. Miner. Metal Mater. Eng. 2016, 2, 23–29. [Google Scholar]
- Sharifi, M.; Baghdadi, M. Enhanced selectivity and capacity of clinoptilolite for Cd2+ removal from aqueous solutions by incorporation of magnetite nanoparticles and surface modification with cysteine. Water Sci. Technol. 2016, 73, 2284–2293. [Google Scholar] [CrossRef] [PubMed]
- Abatal, M.; Olguin, M.T.; Abdellaoui, Y.; EL Bouari, A. Sorption of Cd(II), Ni(II) and Zn(II) on natural, sodium-, and acid-modified clinoptilolite-rich tuff. Environ. Prot. Eng. 2018, 44, 42–59. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Olguin, M.T.; Abatal, M.; Bassam, A.; Giácoman-Vallejoa, G. Relationship between Si/Al ratio and the sorption of Cd(II) by natural and modified clinoptilolite-rich tuff with sulfuric acid. Desalination Water Treat. 2019, 150, 157–165. [Google Scholar] [CrossRef]
- Li, Y.; Bai, P.; Yan, Y.; Yan, W.; Shi, W.; Xu, R. Removal of Zn2+, Pb2+, Cd2+, and Cu2+ from aqueous solution by synthetic clinoptilolite. Microporous Mesoporous Mater. 2019, 273, 203–211. [Google Scholar] [CrossRef]
- Shafiof, M.a.S.; Nezamzadeh-Ejhieh, A. A comprehensive study on the removal of Cd(II) from aqueous solution on a novel pentetic acid-clinoptilolite nanoparticles adsorbent: Experimental design, kinetic and thermodynamic aspects. Solid State Sci. 2020, 99, 106071. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Pham, T.T.; Vranova, V.; Nguyen, H.T.H.; Nguyen, L.T.N.; Vuong, X.T.; Bui, Q.M. Removal of cadmium from aqueous solution using sonochemically modified clinoptilolite: Optimization and modeling. Environ. Technol. Innov. 2020, 20, 101166. [Google Scholar] [CrossRef]
- Stolz, J.; Yang, P.; Armbruster, T. Cd-exchanged heulandite: Symmetry lowering and site preference. Microporous Mesoporous Mater. 2000, 37, 233–242. [Google Scholar] [CrossRef]
- Petkova, V.; Serafimova, E.; Petrova, N.; Pelovski, Y. Thermochemistry of triboactivated natural and NH4-exchanged clinoptilolite mixed with Tunisian apatite. J. Therm. Anal. Calorim. 2011, 105, 535–544. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Kabiri-Samani, M. Effective removal of Ni(II) from aqueous solutions by modification of nano particles of clinoptilolite with dimethylglyoxime. J. Hazard. Mater. 2013, 260, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Anari-Anaraki, M.; Nezamzadeh-Ejhieh, A. Modification of an Iranian clinoptilolite nano-particles by hexadecyltrimethyl ammonium cationic surfactant and dithizone for removal of Pb(II) from aqueous solution. J. Colloid Interf. Sci. 2015, 440, 272–281. [Google Scholar] [CrossRef]
- Iazdani, F.; Nezamzadeh-Ejhieh, A. FeO-Clinoptilolite nanoparticles: Brief characterization and its photocatalytic kinetics towards 2,4-dichloroaniline. Chem. Phys. 2021, 550, 111305. [Google Scholar] [CrossRef]
- Iazdani, F.; Nezamzadeh-Ejhieh, A. Photocatalytic kinetics of 2,4-dichloroaniline degradation by NiO-clinoptilolite nanoparticles. Spectrochim. Acta. Mol. Biomol. Spectrosc. 2021, 250, 119228. [Google Scholar] [CrossRef]
- Asgari, N.; Haghighi, M.; Shafiei, S. Synthesis and physicochemical characterization of nanostructured Pd/ceriaclinoptilolite catalyst used for p-xylene abatement from waste gas streams at low temperature. J. Chem. Technol. Biotechnol. 2013, 88, 690–703. [Google Scholar] [CrossRef]
- Asgari, N.; Haghighi, M.; Shafiei, S. Synthesis and physicochemical characterization of nanostructured CeO2/clinoptilolite for catalytic total oxidation of xylene at low temperature. Environ. Prog. Sustain. Energy 2013, 32, 587–597. [Google Scholar] [CrossRef]
- Jamalzadeh, Z.; Haghighi, M.; Asgari, N. Synthesis and physicochemical characterizations of nanostructured Pd/carbon-clinoptilolite-CeO2 catalyst for abatement of xylene from waste gas streams at low temperature. J. Ind. Eng. Chem. 2014, 20, 2735–2744. [Google Scholar] [CrossRef]
- Dimowa, L.T.; Petrov, O.E.; Djourelov, N.I.; Shivachev, B.L. Structural study of Zn-exchanged natural clinoptilolite using powder XRD and positron annihilation data. Clay Miner. 2015, 50, 41–54. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction; Bruker AXS: Karlsruhe, Germany, 2009.
- Cappelletti, P.; Langella, A.; Cruciani, G. Crystal-chemistry and synchrotron Rietveld refinement of two different clinoptilolites from volcanoclastites of north-western Sardinia. Eur. J. Mineral. 1999, 11, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Petrov, O.E.; Filizova, L.D.; Kirov, G.N. Cation distribution in clinoptilolite structure: Ba-exchanged sample. Compt. Rend. Acad. Bulg. Sci. 1985, 38, 5, 603–606. [Google Scholar]
- Petrov, O.E. Cation exchange in clinoptilolite: An X-ray powder diffraction analysis. In Natural Zeolites ’93 Occurrence, Properties, Use; Ming, D.W., Mumpton, F.A., Eds.; ICNZ: New York, NY, USA, 1995; pp. 271–279. [Google Scholar]
- Koyama, K.; Takeuchi, Y. Clinoptilolite: The distribution of potassium atoms and its role in thermal stability. Z. Krist. 1977, 145, 216–239. [Google Scholar]
- Kirov, G.; Dimova, L.; Stanimirova, T. Gallery character of porous space and local extra-framework configurations in the HEU-type structure. Microporous Mesoporous Mater. 2020, 293, 109792. [Google Scholar] [CrossRef]
- Dimowa, L.; Tzvetanova, Y.; Petrov, O.; Piroeva, I.; Ublekov, F. Powder XRD structural study of Ba2+ modified clinoptilolite at different stages of the ion exchange process conducted at two temperature regimes—Room temperature and 90 °C. Minerals 2020, 10, 938. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.Y.; Park, G.B.; Heo, N.H.; Seff, K. Water molecules in Zeolite Y enhance the photoluminescent properties of its cesium lead bromide quantum dots, Na4Cs6PbBr48+. J. Phys. Chem. C 2021, 125, 5904–5918. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).