Powder XRD Study of Changes of Cd2+ Modified Clinoptilolite at Different Stages of the Ion Exchange Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cation Exhange
2.3. SEM/EDS
2.4. X-ray Diffraction Studies
3. Results
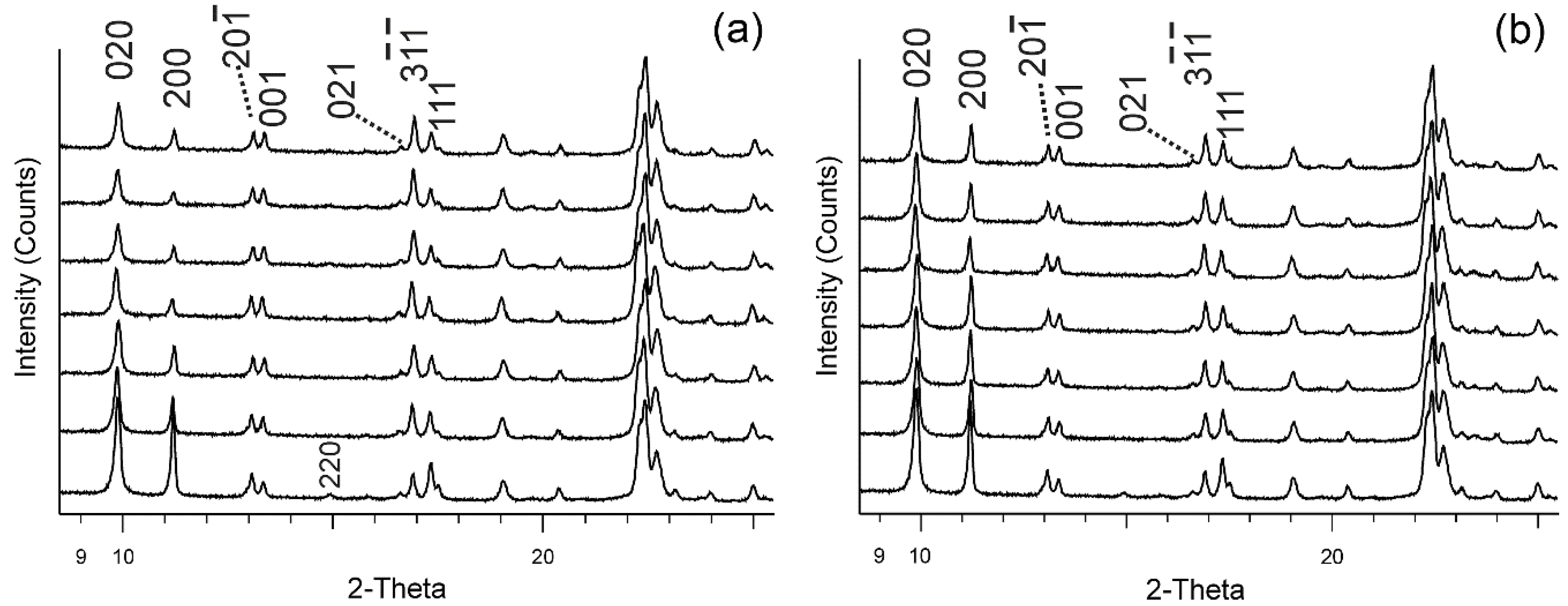
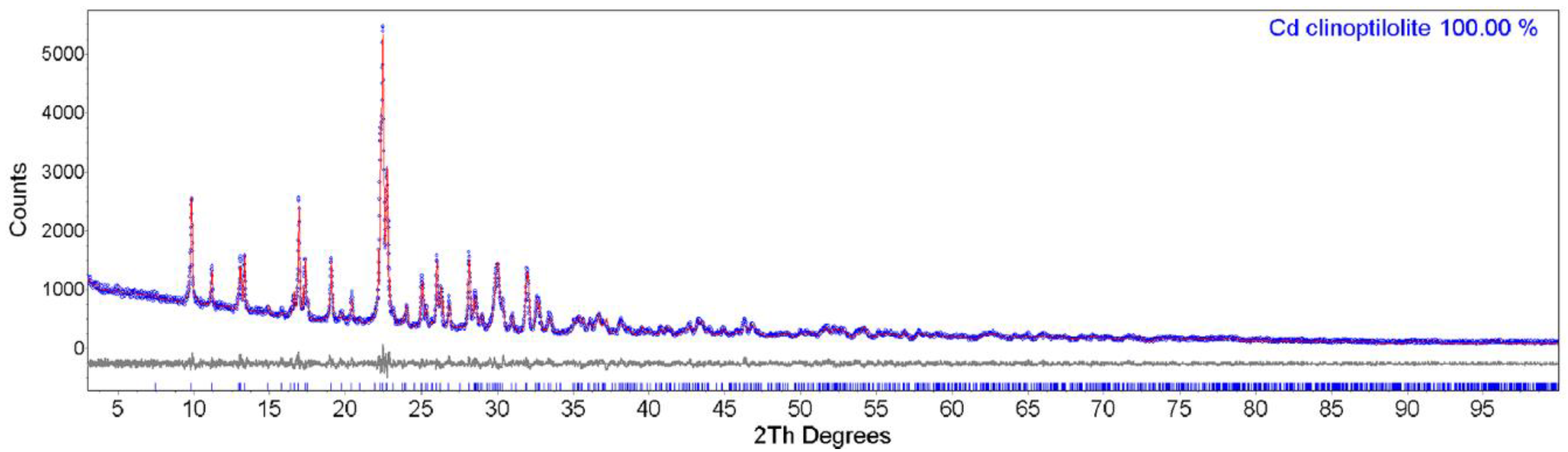
3.1. Powder XRD and EDS Data of Cd-Exchanged Clinoptilolite
3.2. Rietveld Structural Analyses of Cd2+ Exchange on Clinoptilolite with Time and Temperature
4. Discussion
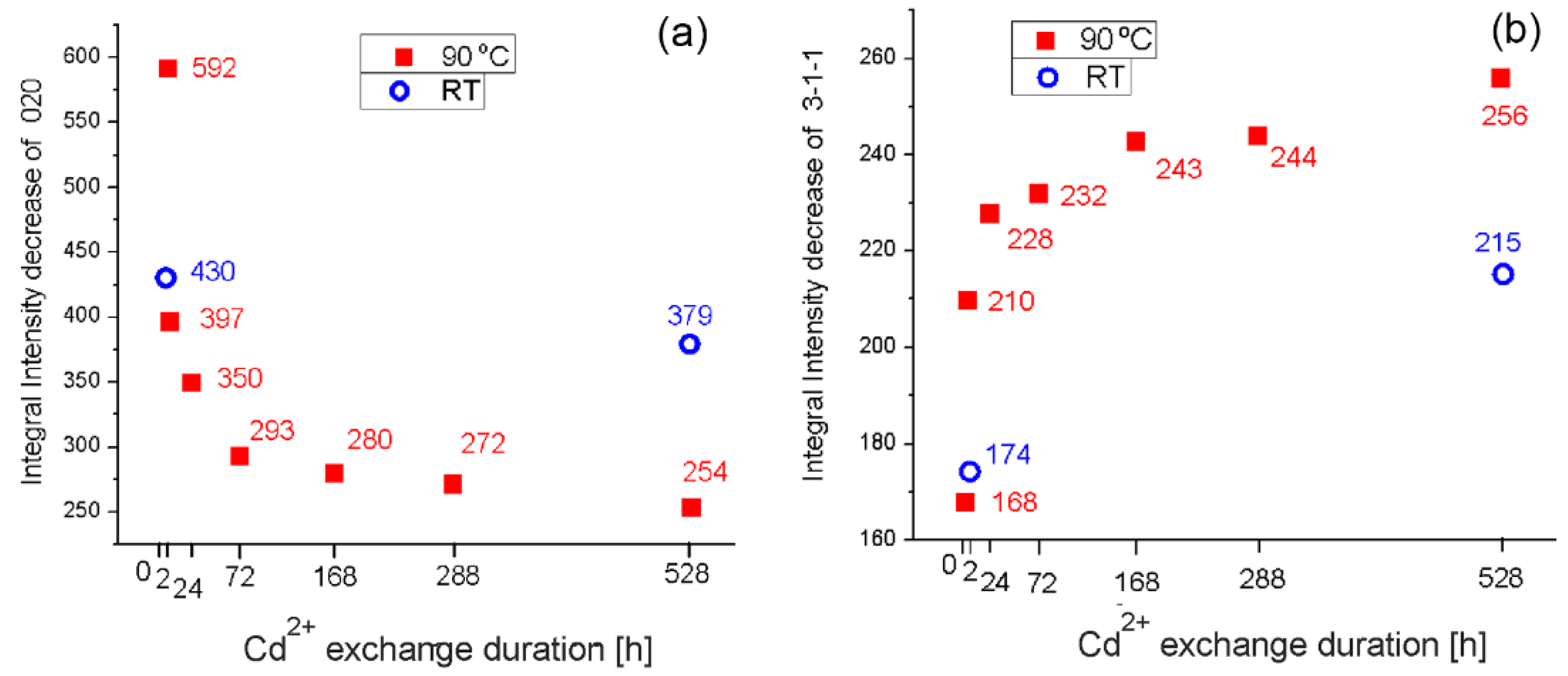
4.1. Powder XRD and EDS Data Interpretation of Cd-Exchanged Clinoptilolite
4.2. Rietveld Structural Interpretation of Natural and Cd2+ Exchanged Clinoptilolite with Time and Temperature
4.3. Movement of H2O Molecules and Cd2+ Cations in the Clinoptilolite Structure during Cadmium Exchange
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [Green Version]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189. [Google Scholar]
- Aleksiev, B.; Djourova, E.G. On the origin of zeolite rocks. Comptes Rendus Acad. Bulg. Sci. 1975, 28, 517–520. [Google Scholar]
- Yanev, Y.; Cocheme, J.-J.; Ivanova, R.; Grauby, O.; Burlet, E.; Pravchanska, R. Zeolites and zeolitization of acid pyroclastic rocks from paroxysmal Paleogene volcanism, Eastern Rhodopes, Bulgaria. Neues Jahrb. Mineral. Abh. J. Mineral. Geochem. 2006, 182, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Yanev, Y.; Ivanova, R. Post-conference field trip guidebook Eastern Rhodopes, South Bulgaria. In Proceedings of the Zeolite 2010—8th International Conference of the Occurrence, Properties, and Utilization of Natural Zeolites, Sofia, Bulgaria, 10–18 July 2010; p. 34. [Google Scholar]
- Doebelin, N.; Armbruster, T. Stepwise dehydration and change of framework topology in Cd-exchanged heulandite. Micropor. Microporous Mesoporous Mater. 2003, 61, 85–103. [Google Scholar] [CrossRef]
- Alberti, A. The crystal structure of two clinoptilolites. Tschermaks Mineral. Petrogr. Mitt. 1975, 22, 25–37. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Balzer, R.; Pergher, S. Microporous and mesoporous materials from natural and inexpensive sources. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Khatisov, B.I., Eds.; Springer: Basel, Switzerland, 2017; pp. 1–22. [Google Scholar]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral. 1997, 35, 1571–1606. [Google Scholar]
- Godelitsas, A.; Armbruster, T. HEU-type zeolites modified by transition elements and lead. Microporous Mesoporous Mater. 2003, 61, 3–24. [Google Scholar] [CrossRef]
- Langella, A.; Pansini, M.; Cappelletti, P.; de Gennaro, B.; De Gennaro, M.; Colella, C. NH4+, Cu2+, Zn2+, Cd2+ and Pb2+ exchange for Na+ in a sedimentary clinoptilolite, North Sardinia, Italy. Microporous Mesoporous Mater. 2000, 37, 337–343. [Google Scholar] [CrossRef]
- Armbruster, T. Clinoptilotite-heulandite: Applications and basic research. In Studies in Surface Science and Catalysis; Galarneau, A., Fajula, F., Di Renzo, F., Vedrine, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 135, pp. 13–27. [Google Scholar]
- Stefanova, I.; Djurova, E.; Gradev, G. Sorption of zinc and cadmium on zeolite rocks. J. Radioanal. Nucl. Chem. 1988, 128, 367–375. [Google Scholar] [CrossRef]
- Kesraoui-Ouki, S.; Cheeseman, C.; Perry, R. Effects of conditioning and treatment of chabazite and clinoptilolite prior to lead and cadmium removal. Environ. Sci. Technol. 1993, 27, 1108–1116. [Google Scholar] [CrossRef]
- Misaelides, P.; Godelitsas, A.; Charistos, V.; Ioannou, D.; Charistos, D. Heavy metal uptake by zeoliferous rocks from Metaxades, Thrace, Greece: An exploratory study. J. Radioanal. Nucl. Chem. 1994, 183, 159–166. [Google Scholar] [CrossRef]
- Mier, M.V.; Callejas, R.L.; Gehr, R.; Cisneros, B.E.J.; Alvarez, P.J.J. Heavy metal removal with Mexican clinoptilolite: Multi-component ionic exchange. Water Res. 2001, 35, 373–378. [Google Scholar] [CrossRef]
- Vasylechko, V.O.; Gryshchouk, G.V.; Kuz’ma, Y.B.; Zakordonskiy, V.P.; Vasylechko, L.O.; Lebedynets, L.O.; Kalytovs’ka, M.B. Adsorption of cadmium on acid-modified Transcarpathian clinoptilolite. Microporous Mesoporous Mater. 2003, 60, 183–196. [Google Scholar] [CrossRef]
- Mozgawa, W.; Bajda, T. Spectroscopic study of heavy metals sorption on clinoptilolite. Phys. Chem. Miner. 2005, 31, 706–713. [Google Scholar] [CrossRef]
- Mühlbachová, G.; Šimon, T.; Pechová, M. The availability of Cd, Pb and Zn and their relationships with soil pH and microbial biomass in soils amended by natural clinoptilolite. Plant Soil Environ. 2005, 51, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Wingenfelder, U.; Hansen, C.; Furrer, G.; Schulin, R. Removal of heavy metals from mine waters by natural zeolites. Environ. Sci. Technol. 2005, 39, 4606–4613. [Google Scholar] [CrossRef]
- Arámbula-Villazana, V.; Solache-Ríos, M.; Olguín, M.T. Sorption of cadmium from aqueous solutions at different temperatures by Mexican HEU-type zeolite rich tuff. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 229–236. [Google Scholar] [CrossRef]
- Berber-Mendoza, M.S.; Leyva-Ramos, R.; Alonso-Davila, P.; Mendoza-Barron, J.; Diaz-Flores, P.E. Effect of pH and temperature on the ion-exchange isotherm of Cd(II) and Pb(II) on clinoptilolite. J. Chem. Technol. Biotechnol. 2006, 81, 966–973. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef]
- Castaldi, P.; Santona, L.; Enzo, S.; Melis, P. Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. J. Hazard. Mater. 2008, 156, 428–434. [Google Scholar] [CrossRef]
- Gedik, K.; Imamoglu, I. Removal of cadmium from aqueous solutions using clinoptilolite: Influence of pretreatment and regeneration. J. Hazard. Mater. 2008, 155, 385–392. [Google Scholar] [CrossRef]
- Faghihian, H.; Nejati-Yazdinejad, M. A comparative study of the sorption of Cd(II) and Pb(II) ions from aqueous solution by local bentonite and clinoptilolite. Adsorpt. Sci. Technol. 2009, 27, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Serrano, D.; Ramírez-Bon, R. Cd sorption from aqueous solutions by natural clinoptilolite and its modified forms clinoptilolite-Na and clinoptilolite-NH4. J. Miner. Metal Mater. Eng. 2016, 2, 23–29. [Google Scholar]
- Sharifi, M.; Baghdadi, M. Enhanced selectivity and capacity of clinoptilolite for Cd2+ removal from aqueous solutions by incorporation of magnetite nanoparticles and surface modification with cysteine. Water Sci. Technol. 2016, 73, 2284–2293. [Google Scholar] [CrossRef] [PubMed]
- Abatal, M.; Olguin, M.T.; Abdellaoui, Y.; EL Bouari, A. Sorption of Cd(II), Ni(II) and Zn(II) on natural, sodium-, and acid-modified clinoptilolite-rich tuff. Environ. Prot. Eng. 2018, 44, 42–59. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Olguin, M.T.; Abatal, M.; Bassam, A.; Giácoman-Vallejoa, G. Relationship between Si/Al ratio and the sorption of Cd(II) by natural and modified clinoptilolite-rich tuff with sulfuric acid. Desalination Water Treat. 2019, 150, 157–165. [Google Scholar] [CrossRef]
- Li, Y.; Bai, P.; Yan, Y.; Yan, W.; Shi, W.; Xu, R. Removal of Zn2+, Pb2+, Cd2+, and Cu2+ from aqueous solution by synthetic clinoptilolite. Microporous Mesoporous Mater. 2019, 273, 203–211. [Google Scholar] [CrossRef]
- Shafiof, M.a.S.; Nezamzadeh-Ejhieh, A. A comprehensive study on the removal of Cd(II) from aqueous solution on a novel pentetic acid-clinoptilolite nanoparticles adsorbent: Experimental design, kinetic and thermodynamic aspects. Solid State Sci. 2020, 99, 106071. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Pham, T.T.; Vranova, V.; Nguyen, H.T.H.; Nguyen, L.T.N.; Vuong, X.T.; Bui, Q.M. Removal of cadmium from aqueous solution using sonochemically modified clinoptilolite: Optimization and modeling. Environ. Technol. Innov. 2020, 20, 101166. [Google Scholar] [CrossRef]
- Stolz, J.; Yang, P.; Armbruster, T. Cd-exchanged heulandite: Symmetry lowering and site preference. Microporous Mesoporous Mater. 2000, 37, 233–242. [Google Scholar] [CrossRef]
- Petkova, V.; Serafimova, E.; Petrova, N.; Pelovski, Y. Thermochemistry of triboactivated natural and NH4-exchanged clinoptilolite mixed with Tunisian apatite. J. Therm. Anal. Calorim. 2011, 105, 535–544. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Kabiri-Samani, M. Effective removal of Ni(II) from aqueous solutions by modification of nano particles of clinoptilolite with dimethylglyoxime. J. Hazard. Mater. 2013, 260, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Anari-Anaraki, M.; Nezamzadeh-Ejhieh, A. Modification of an Iranian clinoptilolite nano-particles by hexadecyltrimethyl ammonium cationic surfactant and dithizone for removal of Pb(II) from aqueous solution. J. Colloid Interf. Sci. 2015, 440, 272–281. [Google Scholar] [CrossRef]
- Iazdani, F.; Nezamzadeh-Ejhieh, A. FeO-Clinoptilolite nanoparticles: Brief characterization and its photocatalytic kinetics towards 2,4-dichloroaniline. Chem. Phys. 2021, 550, 111305. [Google Scholar] [CrossRef]
- Iazdani, F.; Nezamzadeh-Ejhieh, A. Photocatalytic kinetics of 2,4-dichloroaniline degradation by NiO-clinoptilolite nanoparticles. Spectrochim. Acta. Mol. Biomol. Spectrosc. 2021, 250, 119228. [Google Scholar] [CrossRef]
- Asgari, N.; Haghighi, M.; Shafiei, S. Synthesis and physicochemical characterization of nanostructured Pd/ceriaclinoptilolite catalyst used for p-xylene abatement from waste gas streams at low temperature. J. Chem. Technol. Biotechnol. 2013, 88, 690–703. [Google Scholar] [CrossRef]
- Asgari, N.; Haghighi, M.; Shafiei, S. Synthesis and physicochemical characterization of nanostructured CeO2/clinoptilolite for catalytic total oxidation of xylene at low temperature. Environ. Prog. Sustain. Energy 2013, 32, 587–597. [Google Scholar] [CrossRef]
- Jamalzadeh, Z.; Haghighi, M.; Asgari, N. Synthesis and physicochemical characterizations of nanostructured Pd/carbon-clinoptilolite-CeO2 catalyst for abatement of xylene from waste gas streams at low temperature. J. Ind. Eng. Chem. 2014, 20, 2735–2744. [Google Scholar] [CrossRef]
- Dimowa, L.T.; Petrov, O.E.; Djourelov, N.I.; Shivachev, B.L. Structural study of Zn-exchanged natural clinoptilolite using powder XRD and positron annihilation data. Clay Miner. 2015, 50, 41–54. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction; Bruker AXS: Karlsruhe, Germany, 2009.
- Cappelletti, P.; Langella, A.; Cruciani, G. Crystal-chemistry and synchrotron Rietveld refinement of two different clinoptilolites from volcanoclastites of north-western Sardinia. Eur. J. Mineral. 1999, 11, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Petrov, O.E.; Filizova, L.D.; Kirov, G.N. Cation distribution in clinoptilolite structure: Ba-exchanged sample. Compt. Rend. Acad. Bulg. Sci. 1985, 38, 5, 603–606. [Google Scholar]
- Petrov, O.E. Cation exchange in clinoptilolite: An X-ray powder diffraction analysis. In Natural Zeolites ’93 Occurrence, Properties, Use; Ming, D.W., Mumpton, F.A., Eds.; ICNZ: New York, NY, USA, 1995; pp. 271–279. [Google Scholar]
- Koyama, K.; Takeuchi, Y. Clinoptilolite: The distribution of potassium atoms and its role in thermal stability. Z. Krist. 1977, 145, 216–239. [Google Scholar]
- Kirov, G.; Dimova, L.; Stanimirova, T. Gallery character of porous space and local extra-framework configurations in the HEU-type structure. Microporous Mesoporous Mater. 2020, 293, 109792. [Google Scholar] [CrossRef]
- Dimowa, L.; Tzvetanova, Y.; Petrov, O.; Piroeva, I.; Ublekov, F. Powder XRD structural study of Ba2+ modified clinoptilolite at different stages of the ion exchange process conducted at two temperature regimes—Room temperature and 90 °C. Minerals 2020, 10, 938. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.Y.; Park, G.B.; Heo, N.H.; Seff, K. Water molecules in Zeolite Y enhance the photoluminescent properties of its cesium lead bromide quantum dots, Na4Cs6PbBr48+. J. Phys. Chem. C 2021, 125, 5904–5918. [Google Scholar] [CrossRef]


| Samples Oxides | 2 h | 24 h | 72 h | 168 h | 12 Days | 22 Days |
|---|---|---|---|---|---|---|
| SiO2 | 63.04 | 72.39 | 65.89 | 62.61 | 62.89 | 54.38 |
| Al2O3 | 11.44 | 13.15 | 12.05 | 11.13 | 11.83 | 9.60 |
| MgO | 0.57 | 0.66 | 0.60 | 0.51 | 0.51 | 0.43 |
| CaO | 3.45 | 3.75 | 2.85 | 2.48 | 2.45 | 1.79 |
| Na2O | 0.62 | 0.56 | 0.50 | 0.47 | 0.46 | 0.31 |
| K2O | 1.23 | 1.38 | 1.21 | 1.10 | 1.05 | 0.85 |
| CdO | 1.74 | 2.84 | 4.05 | 4.25 | 5.30 | 4.81 |
| Σ | 82.09 | 94.73 | 87.15 | 82.55 | 84.49 | 72.17 |
| Atoms per formula unit (apfu) | ||||||
| Si | 29.66 | 29.65 | 29.62 | 29.76 | 29.46 | 29.80 |
| Al | 6.34 | 6.35 | 6.38 | 6.24 | 6.53 | 6.20 |
| Mg | 0.40 | 0.40 | 0.40 | 0.36 | 0.35 | 0.35 |
| Ca | 1.73 | 1.65 | 1.37 | 1.26 | 1.23 | 1.05 |
| Na | 0.57 | 0.44 | 0.44 | 0.43 | 0.42 | 0.33 |
| K | 0.74 | 0.72 | 0.69 | 0.68 | 0.63 | 0.59 |
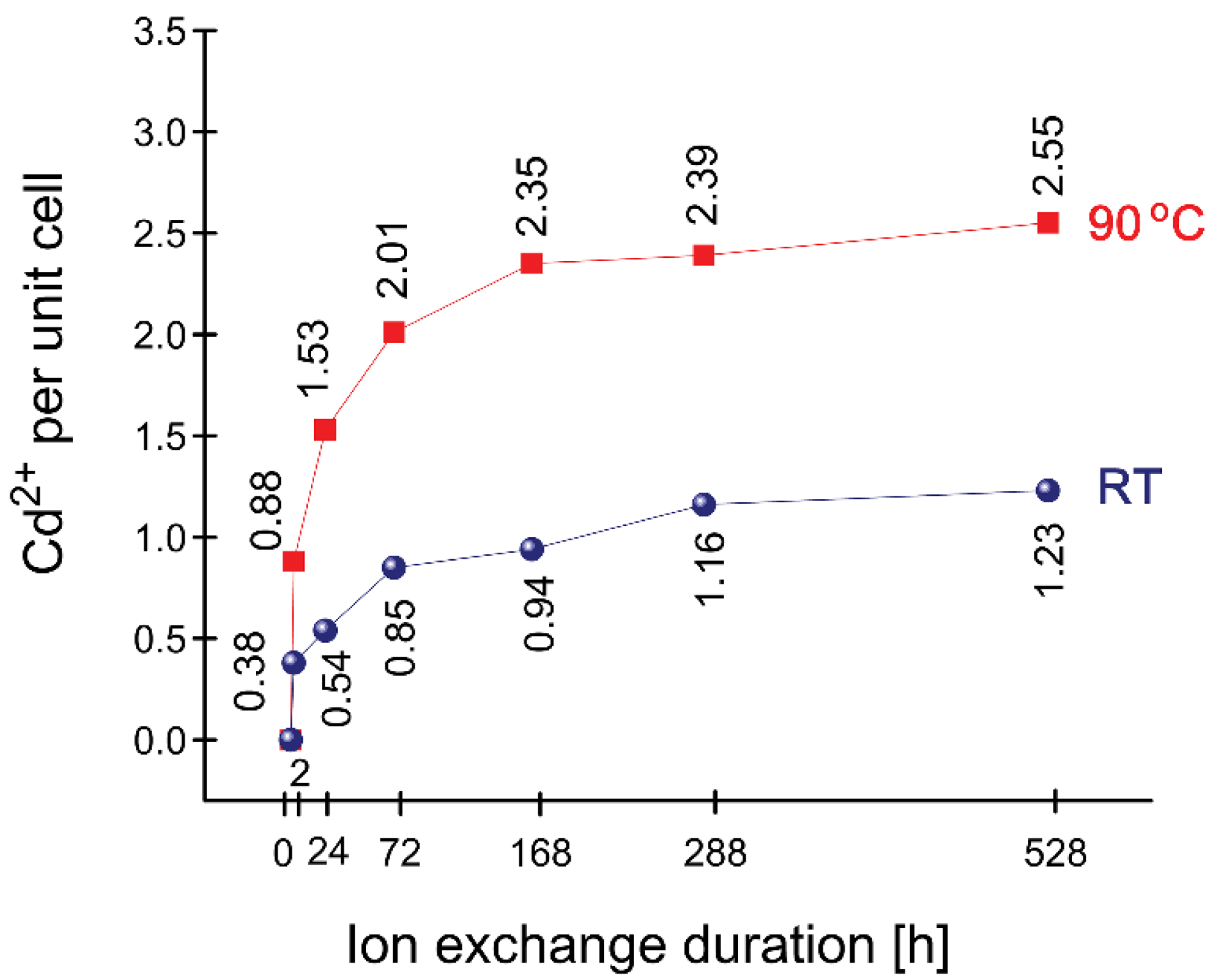
| Cd | 0.38 | 0.54 | 0.85 | 0.94 | 1.16 | 1.23 |
| Samples Oxides | 2 h | 24 h | 72 h | 168 h | 12 Days | 22 Days |
|---|---|---|---|---|---|---|
| SiO2 | 61.48 | 64.18 | 62.31 | 62.17 | 56.39 | 55.52 |
| Al2O3 | 11.16 | 11.54 | 11.36 | 11.30 | 10.25 | 10.03 |
| MgO | 0.56 | 0.47 | 0.37 | 0.21 | 0.17 | - |
| CaO | 2.87 | 1.89 | 1.12 | 0.91 | 0.80 | 0.72 |
| Na2O | 0.31 | 0.26 | 0.22 | - | - | - |
| K2O | 0.83 | 0.82 | 0.76 | 0.69 | 0.60 | 0.56 |
| CdO | 3.92 | 7.05 | 9.05 | 10.55 | 9.73 | 10.21 |
| Σ | 77.21 | 86.21 | 85.19 | 85.83 | 77.94 | 77.04 |
| Atoms per formula unit (apfu) | ||||||
| Si | 29.66 | 29.70 | 29.63 | 29.65 | 29.65 | 29.68 |
| Al | 6.34 | 6.29 | 6.37 | 6.35 | 6.35 | 6.32 |
| Mg | 0.40 | 0.32 | 0.26 | 0.15 | 0.13 | - |
| Ca | 1.48 | 0.94 | 0.57 | 0.47 | 0.45 | 0.41 |
| Na | 0.29 | 0.23 | 0.20 | - | - | - |
| K | 0.51 | 0.48 | 0.46 | 0.42 | 0.40 | 0.38 |
| Cd | 0.88 | 1.53 | 2.01 | 2.35 | 2.39 | 2.55 |
| Header Samples Parameters | Initial Cpt | 2 h | 24 h | 72 h | 168 h | 12 Days | 22 Days |
|---|---|---|---|---|---|---|---|
| Rexp | 4.89 | 4.97 | 4.95 | 4.97 | 5.00 | 5.05 | 4.97 |
| Rwp | 7.33 | 6.37 | 6.42 | 6.16 | 6.24 | 6.33 | 6.20 |
| Rp | 5.54 | 4.84 | 4.87 | 4.62 | 4.70 | 4.80 | 4.67 |
| GOF | 1.50 | 1.28 | 1.30 | 1.24 | 1.25 | 1.25 | 1.25 |
| RB | 1.41 | 1.20 | 1.46 | 1.03 | 1.31 | 1.45 | 1.14 |
| Crystallite size [nm] | 175.00(15) | 172.82(18) | 177.82(15) | 175.77(16) | 173.44(18) | 179.12(15) | 180.26(17) |
| α (Å) | 17.6773(8) | 17.6898(7) | 17.6884(9) | 17.6838(9) | 17.6805(7) | 17.6783(8) | 17.6850(6) |
| b (Å) | 17.9470(8) | 17.9777(6) | 17.9807(8) | 17.9898(8) | 17.9915(8) | 17.9943(8) | 17.9958(8) |
| c (Å) | 7.4146(5) | 7.4167(3) | 7.4161(4) | 7.4151(4) | 7.4154(3) | 7.4145(4) | 7.4154(3) |
| β(°) | 116.324(2) | 116.297(2) | 116.290(3) | 116.272(3) | 116.262(3) | 116.259(3) | 116.250(2) |
| V (Å3) | 2108.3(3) | 2114.6(2) | 2114.7(2) | 2115.3(2) | 2115.3(2) | 2115.3(3) | 2116.6(2) |
| Site | Wp | x | y | z | Atom | Occ 2 h | Occ 22 Days |
|---|---|---|---|---|---|---|---|
| Si/Al 1 | 8 j | 0.1783(9) | 0.1704(7) | 0.0956(3) | Si4+ | 1 | 1 |
| Si/Al 2 | 8 j | 0.2119(9) | 0.4118(6) | 0.5002(2) | Si4+ | 1 | 1 |
| Si/Al 3 | 8 j | 0.2098(8) | 0.1914(6) | 0.2828(4) | Si4+ | 1 | 1 |
| Si/Al 4 | 8 j | 0.0686(8) | 0.2980(7) | 0.5887(2) | Si4+ | 1 | 1 |
| Si/Al 5 | 4 g | 0.0 | 0.2185(3) | 0.0 | Si4+ | 1 | 1 |
| O1 | 4 i | 0.1941(2) | 0.5 | 0.4597 (4) | O2- | 1 | 1 |
| O2 | 8 j | 0.2316(2) | 0.1242(7) | 0.6019(2) | O2- | 1 | 1 |
| O3 | 8 j | 0.1842(2) | 0.1575(8) | 0.8819(3) | O2- | 1 | 1 |
| O4 | 8 j | 0.2323(3) | 0.1046(2) | 0.2493(2) | O2- | 1 | 1 |
| O5 | 4 h | 0 | 0.3275(2) | 0.5 | O2- | 1 | 1 |
| O6 | 8 j | 0.0785(3) | 0.1590(3) | 0.0599(3) | O2- | 1 | 1 |
| O7 | 8 j | 0.1265(2) | 0.2342(2) | 0.5573(2) | O2- | 1 | 1 |
| O8 | 8 j | 0.0114(1) | 0.2769(9) | 0.1830(3) | O2- | 1 | 1 |
| O9 | 8 j | 0.2113(2) | 0.2526(2) | 0.1843(2) | O2- | 1 | 1 |
| O10 | 8 j | 0.1169(3) | 0.3764 (9) | 0.4214(3) | O2- | 1 | 1 |
| Na(M1) | 4 i | 0.1427(24) | 0.0 | 0.6670(18) | Na+ | 0.235(21) | 0 |
| Ca(M2) | 4 i | 0.0470(27) | 0.5 | 0.1960(35) | Ca2+ | 0.359(23) | 0.130(12) |
| K(M3) | 4 i | 0.2182(31) | 0.5 | 1.0039 (25) | K+ | 0.204(14) | 0.077(12) |
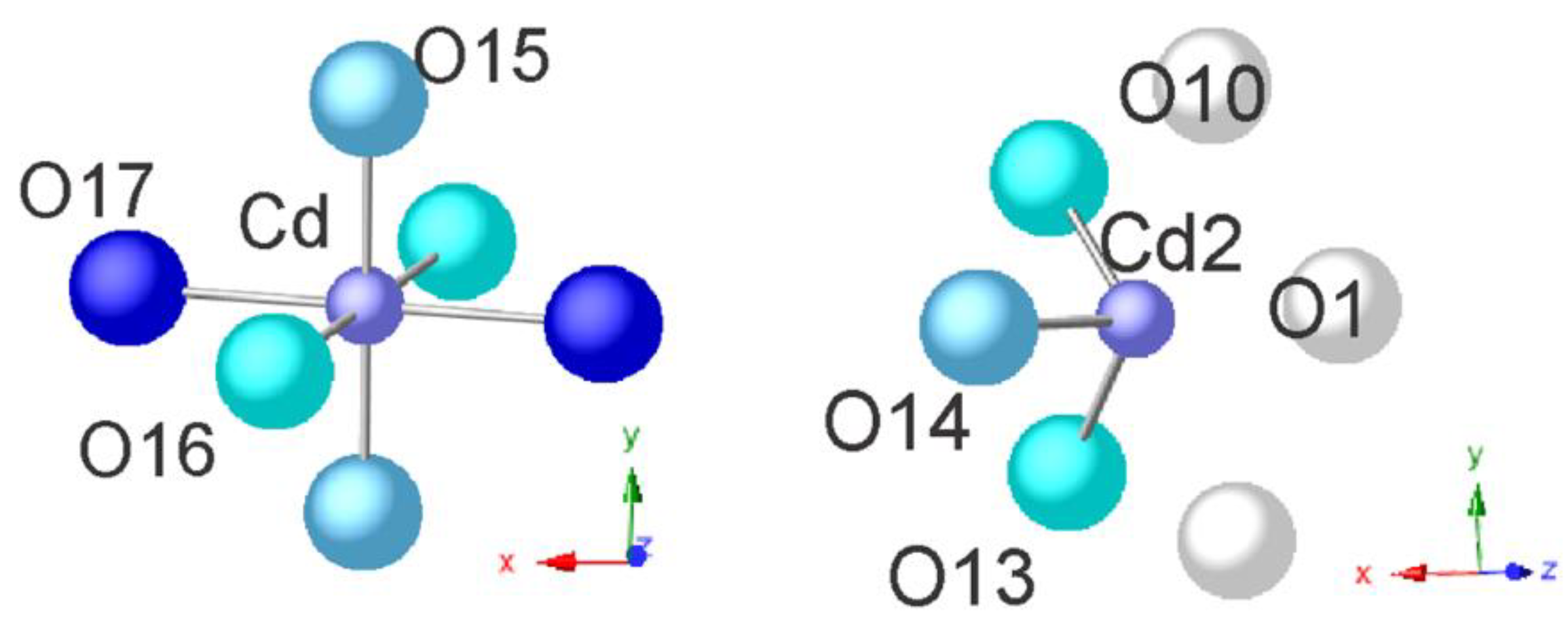
| Cd | 2 c | 0 | 0 | 0.5 | Cd2+ | 0.206(15) | 0.415(11) |
| Cd1 | 4 i | 0.0080(21) | 0 | 0.7530(21) | Cd2+ | 0.042(48) | 0.073(21) |
| Cd2 | 4 i | 0.0191(38) | 0.5 | 0.2442(23) | Cd2+ | 0.069(31) | 0.255(30) |
| Cd3 | 4 i | 0.2189(35) | 0.5 | 0.0220(38) | Cd2+ | 0 | 0.092(24) |
| O11 | 4 i | 0.321(16) | 0.0 | 0.0730(14) | O2- | 0.301(20) | 0.318(28) |
| O12 | 4 i | 0.075(22) | 0.0 | 0.891(16) | O2- | 0.306(30) | 0.102(29) |
| O13 2 h O13 22 days | 8 j | 0.081(32) 0.079(34) | 0.414(21) 0.420(26) | 0.961(39) 0.960(34) | O2- | 0.700(21) | 0.686(22) |
| O14 | 2 d | 0.5 | 0.0 | 0.5 | O2- | 1 | 1 |
| O15 | 4 h | 0.0 | 0.1151(30) | 0.5 | 0.2426(25) | 0.365(31) | |
| O15’ 2 h O15’ 22 days | 8 j 8 j | 0.0391(38) 0.0190(33) | 0.0980(36) 0.1101(26) | 0.5297(34) 0.4231(26) | O2- | 0.2187(30) | 0.125(34) |
| O16 | 4 i | 0.0616(26) | 0.0 | 0.3039(41) | O2- | 0.241(21) | 0.448(19) |
| O17 2 h O17 22 days | 4 i | 0.0850(43) 0.140(33) | 0.0 | 0.7040(16) 0.730(25) | O2- | 0.209(50) | 0.637(29) |
| O18 2 h O18 22 days | 4 i | 0.0510(40) 0.0680(35) | 0.0 | 0.0990(41) 0.1041(33) | O2- | 0.445(4) | 0.757(28) |
| O19 2 h O19 22 days | 4 i | 0.1400(38) 0.1501(42) | 0.0 | 0.6240(63) 0.689(35) | O2- | 0.366(32) | 0.225(30) |
| Sample | Atom 1 | Atom 2 | d(Å) | Sample | Atom 1 | Atom 2 | d(Å) |
|---|---|---|---|---|---|---|---|
| 2 h | Cd | 2×O17 | 1.591(18) | 2 h/22 days | K | O4 | 3.04(10) |
| 22 days | 2 × O17 | 2.306(22) | 2 h/22 days | Ca | Cd2 | 0.72(12) | |
| 2 h | 2 × Cd1 | 1.818(16) | 2 h/22 days | O1 | 2.46(10) | ||
| 2 h | 4 × O15’ | 1.870(44) | 2 h | 2 × O13 | 2.55(12) | ||
| 22 days | 4 × O15’ | 2.128(25) | 22 days | 2 × O13 | 2.51(13) | ||
| 2 h/22 days | 4 × O15 | 2.085(30) | 2 h/22 days | Ca | 2.63(11) | ||
| 2 h/22 days | 2 × O16 | 2.17(11) | 2 h/22 days | 2 × O10 | 2.72(12) | ||
| 2 h | 2 × O19 | 2.22(12) | 2 h/22 days | O14 | 2.72(11) | ||
| 22 days | 2 × O19 | 2.38(14) | 2 h/22 days | Cd2 | 2.94(13) | ||
| 2 h/22 days | 2 × Na | 2.26(12) | 2 h | O11 | 2 × O13 | 2.41(13) | |
| 2 h | 2 × O12 | 2.26(13) | 22 days | 2 × O13 | 2.37(12) | ||
| 22 days | 2 × O12 | 2.60(11) | 2 h/22 days | 2 × O4 | 3.08(13) | ||
| 2 h/22 days | Cd1 | O16 | 1.11(12) | 2 h | O12 | O17 | 1.47(11) |
| 2 h/22 days | O12 | 1.17(14) | 22 days | O17 | 1.99(12) | ||
| 2 h | O18 | 1.81(19) | 2 h | O18 | 1.77(11) | ||
| 22 days | O18 | 2.04(14) | 22 days | O18 | 2.54(10) | ||
| 2 h | O18 | 2.32(13) | 2 h/22 days | O16 | 2.19(12) | ||
| 22 days | O18 | 2.34(12) | 2 h | O19 | 2.69(13) | ||
| 2 h | O15’ | 2.57(18) | 22 days | O19 | 2.46(10) | ||
| 22 days | O15’ | 2.30(11) | 2 h | 2 × O15’ | 3.02(12) | ||
| 2 h/22 days | Na | 2.72(15) | 22 days | 2 × O15’ | 2.94(12) | ||
| 2 h | O15 | 2.63(11) | 2 h | O13 | O8 | 2.88(10) | |
| 22 days | O15 | 2.75(12) | 22 days | O8 | 2.97(11) | ||
| 2 h/22 days | Cd2 | Ca | 0.72(12) | 2 h | O13 | 3.09(12) | |
| 2 h/22 days | O14 | 2.06(12) | 22 days | O13 | 2.87(13) | ||
| 2 h | 2 × O13 | 2.33(14) | 2 h | O15 | 2 × O15’ | 0.70(10) | |
| 22 days | 2 × O13 | 2.24(20) | 22 days | 2 × O15’ | 0.78(11) | ||
| 2 h/22 days | 2 × O10 | 2.76(13) | 2 h | 2 × O16 | 3.00(10) | ||
| 2 h/22 days | O1 | 2.78(10) | 22 days | 2 × O16 | 2.99(10) | ||
| 22 days | Cd3 | K | 0.13(11) | 2 h | O15’ | O15’ | 0.75(11)/1.25(10) |
| 22 days | O11 | 0.74(14) | 22 days | O15’ | 0.78(12)/1.56(12) | ||
| 22 days | O17 | 2.34(13) | 2 h | O17 | 2.12(13) | ||
| 22 days | O19 | 2.39(13) | 22 days | O17 | 3.06(11) | ||
| 22 days | Na | 2.51(12) | 2 h | O12 | 3.02(13) | ||
| 2 h | Na | O19 | 0.30(10) | 22 days | O12 | 2.94(12) | |
| 2 h | O17 | 1.46(11) | 2 h | O16 | O18 | 1.44(13)/2.56(11) | |
| 2 h | Cd | 2.26(14) | 22 days | O18 | 1.53(11)/2.87(12) | ||
| 2 h | 2 × O15’ | 2.41(12) | 2 h | O19 | 2.15(13) | ||
| 2 h | O16 | 2.42(12) | 22 days | O19 | 2.52(12) | ||
| 2 h | O12 | 2.48(11) | 2 h | O17 | 2.56(10)/2.52(12) | ||
| 2 h | K | 2.58(15) | 22 days | O17 | 2.83(12) | ||
| 2 h | 2 × O2 | 2.89(14) | 2 h | O17 | O19 | 1.34(13) | |
| 2 h/22 days | K | O11 | 0.67(15) | 22 days | O19 | 1.47(13) | |
| 2 h | 2 × O13 | 2.77(13) | 2 h | O18 | O18 | 1.75(11) | |
| 22 days | c | 2.74(11) | 22 days | O18 | 2.21(10) | ||
| 2 h | O19 | 2.78(10) | 2 h | O19 | O2 | 2.80(10) | |
| 22 days | O19 | 2.46(13) | 22 days | O2 | 2.85(12) |
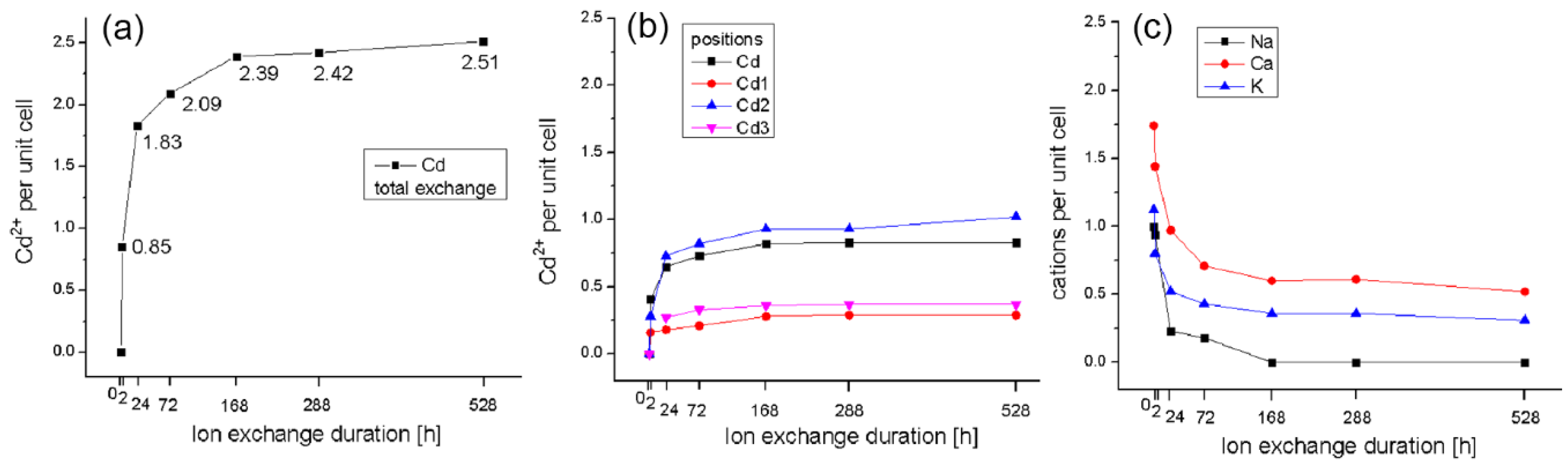
| Sample | M4 Cd | ~M4 Cd1 | ~M2 Cd2 | ~M3 Cd3 | M1 Na | M2 Ca | M3 K | M4 Mg | Total Cd | Charge |
|---|---|---|---|---|---|---|---|---|---|---|
| Initial Cpt | - | - | - | - | 1.00 | 1.74 | 1.12 | 0.40 | - | 6.39 |
| 2 h | 0.41 | 0.16 | 0.28 | - | 0.94 | 1.44 | 0.80 | 0.85 | 6.32 | |
| 24 h | 0.65 | 0.18 | 0.73 | 0.27 | 0.23 | 0.97 | 0.52 | 1.83 | 6.35 | |
| 72 h | 0.73 | 0.21 | 0.82 | 0.33 | 0.18 | 0.71 | 0.43 | 2.09 | 6.34 | |
| 168 h | 0.82 | 0.28 | 0.93 | 0.36 | 0.60 | 0.36 | 2.39 | 6.34 | ||
| 12 days | 0.83 | 0.29 | 0.93 | 0.37 | 0.61 | 0.36 | 2.42 | 6.42 | ||
| 22 days | 0.83 | 0.29 | 1.02 | 0.37 | - | 0.52 | 0.31 | - | 2.51 | 6.37 |
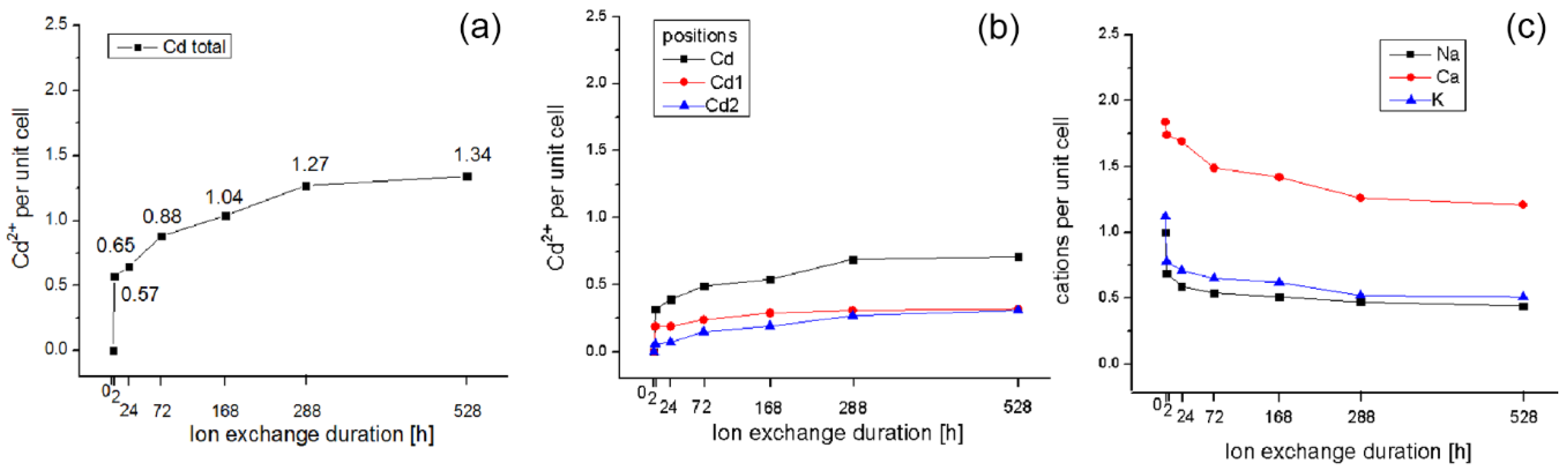
| Sample | M4 Cd | ~M4 Cd1 | ~M2 Cd2 | M1 Na | M2 Ca | M3 K | M4 Mg | Total Cd | Charge |
|---|---|---|---|---|---|---|---|---|---|
| Initial Cpt | - | - | - | 1.0 | 1.84 | 1.12 | 0.40 | - | 6.38 |
| 2 h | 0.32 | 0.19 | 0.06 | 0.69 | 1.74 | 0.78 | - | 0.57 | 5.90 |
| 24 h | 0.39 | 0.19 | 0.07 | 0.59 | 1.69 | 0.71 | - | 0.65 | 5.92 |
| 72 h | 0.49 | 0.24 | 0.15 | 0.54 | 1.49 | 0.65 | - | 0.88 | 5.93 |
| 168 h | 0.54 | 0.29 | 0.19 | 0.51 | 1.42 | 0.62 | - | 1.04 | 5.99 |
| 12 days | 0.69 | 0.31 | 0.27 | 0.47 | 1.26 | 0.52 | - | 1.27 | 6.05 |
| 22 days | 0.71 | 0.32 | 0.31 | 0.44 | 1.21 | 0.51 | - | 1.34 | 6.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimowa, L.; Tzvetanova, Y. Powder XRD Study of Changes of Cd2+ Modified Clinoptilolite at Different Stages of the Ion Exchange Process. Minerals 2021, 11, 1130. https://doi.org/10.3390/min11101130
Dimowa L, Tzvetanova Y. Powder XRD Study of Changes of Cd2+ Modified Clinoptilolite at Different Stages of the Ion Exchange Process. Minerals. 2021; 11(10):1130. https://doi.org/10.3390/min11101130
Chicago/Turabian StyleDimowa, Louiza, and Yana Tzvetanova. 2021. "Powder XRD Study of Changes of Cd2+ Modified Clinoptilolite at Different Stages of the Ion Exchange Process" Minerals 11, no. 10: 1130. https://doi.org/10.3390/min11101130
APA StyleDimowa, L., & Tzvetanova, Y. (2021). Powder XRD Study of Changes of Cd2+ Modified Clinoptilolite at Different Stages of the Ion Exchange Process. Minerals, 11(10), 1130. https://doi.org/10.3390/min11101130






